Abstract
There is increasing evidence that green tea polyphenols can protect against myocardial damage. Recently, we showed that they bind to cardiac troponin C and alter myofilament Ca2+ sensitivity in cardiac muscle. In the present study, we examined whether green tea extract (GTE) could prevent the progressive remodeling seen in ischemic myocardium and improve cardiac function by modulation of the contractile apparatus utilizing a myocardial infarction (MI) model in the rat involving ligation of the left anterior descending branch. Using this model, severe myocardial injury was found, including altered cardiac performance and the appearance of extensive fibrosis and left ventricular (LV) enlargement. Supplementation with 400 mg/kg/day of GTE for 4, 18, or 46 days had beneficial effects in preventing the hemodynamic changes. Histopathological studies showed that GTE attenuated the progressive remodeling seen after myocardial injury. Echocardiography confirmed that GTE prevented LV enlargement and improved LV performance in post-MI rats. In addition, we showed that GTE supplementation for 18 or 46 days increased the myofilament Ca2+ sensitivity of the ischemic myocardium in post-MI rats. These results validate the novel action of green tea polyphenols in protecting against myocardial damage and enhancing cardiac contractility by modulating myofilament Ca2+ sensitivity in post-MI rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green tea-derived polyphenols [e.g., catechin and (−)-epigallocatechin-3-gallate (EGCg)] have attracted much interest in the prevention of cardiovascular diseases [17, 36, 37, 39, 47]. Epidemiological studies have shown that green tea consumption is associated with reduced mortality caused by cardiovascular diseases [15, 20, 21, 31, 33]. Experimental studies have suggested that the myocardial protective effect of green tea polyphenols is associated with their antioxidant properties of scavenging reactive oxygen radicals, modulating redox-sensitive transcription factors (e.g., NFκB, AP-1), reducing the activation of signal transducers and activators of transcription (STAT)-1 and the expression of Fas receptor, and increasing NO production [2, 14, 38, 44]. More recently, green tea-derived polyphenols have been shown to have positive inotropic [16] and anti-hypertropy [13] effects, presumably via activation of the Na+/H+ exchanger and the reverse mode of the Na+/Ca2+ exchanger [29] or a protein kinase-Cε-dependent signaling pathway [22]. However, limited information is available with regard to a direct action of green tea polyphenols on the cardiac contractile apparatus.
Ischemic heart disease is the leading cause of death worldwide. Progressive damage caused by myocardial ischemia leads to decreased contractility during heart failure, which may account for the high mortality of this disease. A surgical model of myocardial infarction (MI) associated with left anterior descending (LAD) coronary artery ligation has been widely used in rodents to study post-ischemic ventricular remodeling [1, 10, 28, 34, 35, 43]. This surgery results in contractile dysfunction and contributes to the observed progressive thinning of the infarcted wall, ventricular enlargement, and heart failure [18]. Contractile dysfunction during ischemia is partly attributed to the effects of intracellular acidosis on the contractile apparatus [7, 30]. Intracellular acidosis and the resultant reduction in myofilament Ca2+ sensitivity may lead to the decreased contractility associated with myocardial ischemia [24]. This reduced Ca2+ sensitivity is ascribed to either a direct effect of pH on the binding of Ca2+ to cardiac troponin C (cTnC) [19, 26, 32] or an indirect effect via non-covalent modification of the C-terminal region of cardiac troponin I (cTnI) [6, 8, 23, 45, 46]. Our recent study showed that EGCg acts by modulating pH-induced changes in myofilament Ca2+ sensitivity in cardiac muscle by binding to the C-terminal sites of cTnC [25]. It was therefore of great interest to determine whether, in live animals, green tea polyphenols could improve cardiac functions by increasing myofilament Ca2+ sensitivity, thus overcoming the decreased contractility associated with myocardial injury.
In this study, we examined the myocardial protective effect of green tea extract (GTE) in terms of preventing progressive remodeling and improving myofilament Ca2+ sensitivity and thus contractile function in the ischemic myocardium utilizing the MI model in the rat with LAD ligation. Histopathological studies showed that GTE supplementation could prevent myocardial fibrosis and LV enlargement in the ischemic myocardium of post-MI animals. Echocardiography confirmed that the attenuation of the progressive remodeling of the myocardium was accompanied by improved cardiac performance in post-MI animals receiving GTE supplementation for 4, 18, or 46 days. To examine whether the myocardial protection provided by GTE supplementation involved enhancement of myofilament Ca2+ sensitivity, the actomyosin ATPase activity of cardiac myofibrils prepared from the hearts of 3- or 7-week post-MI rats was measured under controlled Ca2+ and pH conditions. This is the first study providing in vivo evidence for the myocardial protective effect of green tea polyphenols in preventing progressive remodeling in ischemic hearts and for the modulation of myofilament Ca2+ sensitivity in post-MI animals.
Materials and methods
Sunphenon 90DCF-T, decaffeinated green tea extract (GTE) powder was purchased from Taiyo Kagaku Co., Ltd. (Tokyo, Japan). According to the manufacturer’s information, the GTE powder contained >80% polyphenols, of which >80% were catechins and >45% EGCg, and <1% caffeine. Pure EGCg was purchased from Sigma and was prepared as a 10-mM stock solution in de-ionized water. All reagents used were ACS or MB grade.
Experimental animals
Male Wistar rats (300–350 g) aged 10–11 weeks were randomly divided into three different groups, control, LAD ligation without GTE supplementation, and LAD ligation with GTE supplementation for 4, 18, or 46 days. One hundred milligrams of GTE dissolved in 0.5 ml water (2%) was administered intra-gastrically to animals (400 mg/kg animal/day). This GTE concentration used is equivalent to 180 mg EGCg/kg animal/day. The animals were housed in small groups in a temperature- (24 ± 1°C), humidity- (55 ± 5%), and light- (12 h light:12 h dark) controlled room until the study. During this period, the rats had access to standard rat chow and distilled water ad libitum.
Left coronary artery ligation
The LAD ligation operation is described in supplementary data. After surgery, the animals remained in intensive care until fully conscious. The immediate post-operative mortality rate of this surgical procedure was 24%. All experimental procedures conformed to the “Guidelines for Proper Conduct of Animal Experiments” approved by the Animal Care and Use Committee of National Chung-Hsing University.
Histopathological analysis
Five to six rats for each of the three groups (control, GTE, and no GTE) at the time 3 weeks and 7 weeks post-LAD ligation were studied. Rats were anesthetized with ketamine (80 mg/kg IP) and the heart removed, washed with saline for 10 min, and fixed in neutral-buffered 4% formalin for 48 h. Paraffin-embedded samples were sectioned (4 μm thick) and stained with hematoxylin and eosin (H&E staining) to assess the overall morphology [11].
To delineate infarct size, hearts were sectioned from the apex to the base into five slices. Each slice (3 mm thick) was incubated for 10 min at 32°C in 1% triphenyltetrazolium chloride (TTC, Alfa Aesar, Ward Hill, MA, USA) in PBS, pH 7.4. The non-infarcted myocardium was stained red, while the infarcted myocardium appeared white. The slices were photographed using a digital camera and the images analyzed using the computerized Image-Pro Plus software (Media Cybernetics Inc, Silver Spring, MD, USA). Infarct size was expressed as a percentage of the mass of the whole myocardium [49].
Masson trichrome staining was performed to delineate the area of fibrosis in the myocardium [5]. Photographs were obtained using an Olympus SZX7 stereomicroscope and inverted microscope (Olympus Co., Tokyo, Japan). The slides were observed on a stereomicroscope with the whole field view (8×) and three non-contiguous slides per rat were randomly chosen to calculate the fibrotic area (blue) and viable area (red) normalized to the area of the whole heart using Image-Pro Plus computerized software (Media Cybernetics Inc). The largest endocardial circumference measured in the three LV sections was used as the index for the extent of LV enlargement.
Histoimmunocytochemistry was performed to detect osteopontin (OPN) expression in the myocardium. The paraffin-embedded hearts were sectioned (4 μm thick), the sections de-paraffinized, and endogenous peroxidase blocked with 0.3% hydrogen peroxide for 10 min. After washing with PBS, non-specific binding was minimized by blocking with 1% normal horse serum for 1 h and the sections washed with PBS and incubated overnight at 4°C with rabbit antiserum (1:200) against porcine OPN produced by a local biotechnology company (Ig Medica Biotechnology, Taiwan) using recombinant porcine OPN protein purified in our laboratory. The sections were then incubated with a biotin-conjugated goat anti-rabbit IgG secondary antibody (1:600, ABC kit, Vector Inc.), as recommended by the manufacturer. After the enzymatic reaction of horseradish peroxidase (HRP) with diaminobenzidine (DAB), OPN-containing tissues were stained brown [5].
Echocardiography
Rats were anesthetized with intraperitoneal ketamine (80 mg/kg) and echocardiographic images taken with a 7.5-MHz probe (Hewlett-Packard, MA, USA). The diastolic inter-ventricular septum thickness (IVSd), diastolic left ventricular inner diameter (LVIDd), end diastolic left ventricular thickness (EDLV), and systolic LV inner diameter (LVIDs) were measured by M-mode echocardiography in the control group and post-MI groups with or without GT supplementation for 4, 18, or 46 days. The LV fractional shortening (FS) and LV ejection fraction (EF) were determined using the equations:
ATPase activity assay
In this experiment, we have four groups of rats for study: control with and without GTE supplementation and MI animals with and without GTE supplementation. Myofibrils were prepared from the left ventricular muscle of four different groups of rats: (1) controls without GTE supplementation, (2) controls with GTE supplementation for 18 or 46 days, (3) 3 or 7 weeks post-LAD ligation without GTE supplementation, and (4) LAD ligation with GTE supplementation for 18 or 46 days, starting 3 days after LAD ligation, according to previously described procedures [27]. Myofibrillar ATPase activity was determined in the presence and absence of 0.1 mM EGCg by measuring inorganic phosphate release. The relationship between myofibrillar ATPase activity (nmol Pi/min/mg protein) and the pCa was determined using the Hill plot as described previously [25]. From the plot, the Hill coefficient (n), as a measure of cooperativity, and the Ca2+ concentration giving half-maximal activation (pCa1/2) were obtained for the Ca2+-activated myofibrillar ATPase activity.
Statistics
Quantitative values were expressed as the mean ± SEM. An unpaired two-tailed Student’s t test and one-way ANOVA were performed for between-group comparisons. Scheffe’s multiple range test was used following ANOVA to determine which groups differed from each other. For all tests, P values less than 0.05 were considered as significant.
Results
In this study, GTE was given daily to the test rats from day 3 after ligation, so the terms day 4, 18, and 46 of GTE treatment and weeks 1, 3, and 7 after ligation are equivalent. Figure 1 shows morphological changes in the heart associated with myocardial injury in the rats at 7 weeks post-MI. The hearts of the post-MI rats (“no GTE”) showed an increase in width (Fig. 1a, d), but not in length (Fig. 1a, c), and GTE supplementation for 46 days (“GTE”) restored the width back to control levels (Fig. 1a, c). Note that, both with and without GTE supplementation, the surface of the heart of the post-MI rats was covered with whitish fibrotic tissue (Fig. 1a). However, TTC staining showed an infarct size of 20.2 ± 1.7% of the whole heart mass in post-MI rats without GTE supplementation, but only 7.1 ± 1.3% in post-MI rats with GTE supplementation for 46 days (Fig. 1b, e). This shows that GTE administration attenuated myocardial infarction (↓65%) in post-MI animals.
GTE supplementation protects against myocardial injury in rats at 7 weeks post-MI. a Morphological changes in the heart associated with myocardial injury with and without GTE supplementation. The calibration bar is 1 cm. b TTC staining of tissue slices from controls or at 7 weeks post-MI with and without GTE supplementation. To determine infarct size, the heart was sectioned from the apex to the base into five slices which were stained with TTC. Non-infarcted myocardium is stained red, while infarcted myocardium is white. The calibration bar is 1 cm. c, d Quantitative analysis of the length (c) and width (d) normalized to body weight in controls (n = 5) and MI rats with (n = 9) and without (n = 8) GTE treatment. Each value is the mean ± SEM. *P < 0.1 between control and MI rats without GTE treatment. e Infarct size in the hearts of MI rats with (n = 7) and without GTE (n = 5) supplementation. Infarct size was determined as described in the Methods and expressed as a percentage of the mass of the whole heart. Each value is the mean ± SEM. *P < 0.05 between MI rats with and without GTE treatment
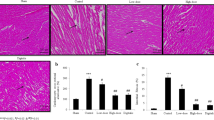
To further examine the effect of GTE supplementation on the progression of myocardial remodeling in post-MI rats, Masson trichrome staining for detection of fibrosis and histoimmunocytochemical detection of osteopontin (OPN) in the myocardium were performed. As shown in Fig. 2a and c, post-MI rats at 3 weeks showed myocardial fibrosis (24.1 ± 2.6%) associated with OPN expression in the myocardium and LV enlargement (LV circumference = control, 10.9 ± 1.9 mm; MI, 20.1 ± 1.1 mm; *P = 0.0008), and GTE supplementation for 18 days resulted in a significant 40% reduction in cardiac fibrosis (14.5 ± 2.6%) and a significant 17% reduction in LV enlargement (LV circumference = 16.6 ± 1.3 mm). The effects of GTE on myocardial fibrosis and LV enlargement were even more obvious at 7 weeks post-MI (Fig. 2b, d). Extensive interstitial fibrosis and LV enlargement were still seen in the myocardium of rats at 7 weeks post-MI (fibrosis = 19.0 ± 2.4%; LV circumference = 21.0 ± 1.0 mm), and GTE supplementation for 46 days resulted in a significant reduction in cardiac fibrosis (68%) and LV enlargement (30%) (fibrosis = 6.1 ± 1.0%; LV circumference = 14.6 ± 1.4 mm). Note that GTE supplementation for 18 days had a non-significant effect (P = 0.18) on the viable area of the myocardium in MI rats, whereas supplementation for 46 days resulted in a significant increase (↑8%, *P = 0.038) (Fig. 2c, d).
Histopathological analysis. a, b Masson trichrome staining and OPN histoimmunocytochemistry of control (a, d, g, j, m, p) and post-MI rats at 3 weeks (a) or 7 weeks (b) with (c, f, i, k, n, q) or without (b, e, h, l, o, r) GTE supplementation. Top row: photographs were obtained using a stereomicroscope (×8) for Masson trichrome staining of the myocardium. The calibration bar indicates 1 mm. The fibrotic area is stained blue and the viable area red. Center row: Photographs were taken using an inverted microscope (×200) for Masson trichrome staining of the myocardium. The calibration bar represents 50 µm. Bottom row: photographs of the histoimmunocytochemical detection of OPN in the myocardium. c, d Quantitative analysis of the effects of GTE on myocardial fibrosis and endocardial circumference in 3- (c) or 7 (d) week post-MI rats. Upper panels: the percentage of the total area that was fibrotic or viable was estimated in MI rats with or without GTE supplementation as described in the Methods. Lower panels: the LV circumference was estimated in control and post-MI rats with and without GTE supplementation as described in the Methods. Each value is the mean ± SEM for measurements taken from five to six animals. *Significant difference (P < 0.05) between control and MI rats with and without GTE treatment. #Significant difference (P < 0.05) between control and MI rats with or without GTE treatment. ##Significant difference (P < 0.05) between MI rats with and without GTE treatment
Echocardiography was performed to assess LV dimensions and function. Figure 5 shows representative M-mode echocardiographic recordings (Fig. 3a) and average data for LV dimensions and function (Fig. 3b) in controls and post-MI rats with and without GTE supplementation for 4, 18, or 46 days. The diastolic inter-ventricular septum thickness (IVSd) was significantly greater (↑23%) in control rats (2.2 ± 0.1 mm, n = 5) than in post-MI rats (1.7 ± 0.1 mm, n = 12), while the diastolic LV inner diameter (LVIDd) was larger (↑25%) in post-MI rats (7.4 ± 0.1 mm, n = 12) than in controls (5.9 ± 0.1 mm, n = 5). Consistent with the histopathological data, the echocardiographic data indicated that the myocardial septum became thinner and the LV enlarged in post-MI rats. In addition, the contractile performance of the LV evaluated by the ejection fraction (EF % = control, 77.9 ± 2.6%; MI, 51.0 ± 5.3%) and fractional shortening (FS % = control, 53.3 ± 2.9%; MI, 30.1 ± 43%) was significantly reduced in post-MI rats (↓35% EF and↓43% FS). After GTE supplementation for 4, 18, or 46 days, the LVIDd was significantly reduced in post-MI rats (9% at day 4, n = 6; 19% at day 18, n = 9; 19% at day 46, n = 7) and the IVSd significantly increased (11% at day 4, n = 6; 20% at day 18, n = 9; 29% at day 46, n = 7). The calculated EF % (31% at day 4, 55% at day 18, 73% at day 46) and FS % (38% at day 4, 50% at day 18, 95% at day 46) for LV contractile performance were also increased by GTE supplementation. These results suggest that GTE supplementation prevents progressive remodeling of the myocardium and restores LV contractile performance.
Echocardiography. a Representative M-mode echocardiographic recordings and b average data for LV dimensions and function in controls and post-MI rats with and without GTE supplement for 4, 18, or 46 days. The diastolic inter-ventricular septum thickness (IVSd, left upper panel), diastolic left ventricular inner diameter (LVIDd, left lower panel), end diastolic left ventricular thickness (EDLV), and systolic LV inner diameter (LVIDs) were measured by M-mode echocardiography. LV fractional shortening (FS, right upper panel) and LV ejection fraction (EF, right lower panel) were determined as described in the Materials and methods. Each value is the mean ± SEM with measurements from six to seven animals. *Significant difference (P < 0.05) between control and MI rats with or without GTE treatment
To understand the mechanism of the GTE-induced improvement in myocardial performance, skinned cardiac myofibrils prepared from the hearts of control and post-MI rats with or without GTE supplementation for 18 or 46 days were tested for Ca2+-dependent actomyosin ATPase activity in the presence and absence of 0.1 mM EGCg (Fig. 4, Tables 1 and 2). As shown in Fig. 4a, when Ca2+-dependent myofibrillar ATPase activity was measured in the absence of EGCg, the Ca2+ sensitivity was not significantly different in control rats with (6.29 ± 0.09, n = 6) or without (6.28 ± 0.07, n = 6) GTE supplementation for 18 days. In contrast, rats at 3 weeks post-MI displayed depressed myofilament Ca2+ sensitivity, as shown by a right-shift of the plot of pCa vs. myofibrillar ATPase activity (pCa1/2 = 5.80 ± 0.07, n = 6), and GTE supplementation for 18 days restored myofilament Ca2+ sensitivity (pCa1/2 = 6.18 ± 0.13, n = 6) to controls levels (top panel). In contrast, when the assay was performed in the presence of 0.1 mM EGCg, the controls with GTE supplementation for 18 days (pCa1/2 = 6.33 ± 0.03, n = 6) showed a left-shift in Ca2+ sensitivity compared to those without GTE supplementation (pCa1/2 = 5.70 ± 0.03, n = 6) (bottom panel). This result confirmed our previous finding that 0.1 mM EGCg causes a decrease in myofilament Ca2+ sensitivity in the porcine cardiac myofibrillar ATPase assay [25]. In the presence of 0.1 mM EGCg, myofilament Ca2+ sensitivity was not significantly different in cardiac myofibrils isolated from the hearts of post-MI rats with (pCa1/2 = 6.21 ± 0.03, n = 6) or without GTE supplementation (pCa1/2 = 6.16 ± 0.03, n = 6). Similar results were seen in rats at 7 weeks post-MI (Fig. 4b).
Measurement of Ca2+-dependent myofibrillar ATPase activity. Cardiac myofibrils prepared from the hearts of control and post-MI rats with and without GTE supplementation for 18 (a) or 46 (b) days. Actomyosin ATPase activity was measured in the absence (upper panel) or presence (lower panel) of 0.1 mM EGCg as described in the Methods. The measured ATPase activity at pCa 8 was subtracted from that at the test pCa and the value normalized to that at pCa 4 and plotted against the pCa. Each value is the mean ± SEM for five measurements. * symbolizes the significant difference (P < 0.05) for one-way ANOVA among four different groups: (1) controls without GTE supplementation, (2) controls with GTE supplementation, (3) LAD ligation without GTE supplementation, and (4) LAD ligation with GTE supplementation
Intracellular acidosis is an important factor in the reduced contractility associated with myocardial ischemia. Consistent with our previous report [25], 0.1 mM EGCg caused a significant reduction in the acidic pH-induced decrease in Ca2+-dependent myofibrillar ATPase activity in control rats without GTE supplementation (top panel in Fig. 5a and b). In control rats supplemented with GTE for 18 or 46 days (second panel from top in Fig. 5a or b, respectively), 0.1 mM EGCg did not significantly affect the Ca2+ sensitivity at pH 7.0 or 6.5, but significantly increased the sensitivity at pH 6.0 (pCa1/2 ↑0.24 at 18 days; ↑0.34 at 46 days). In post-MI rats without GTE supplementation for 3 or 7 weeks (third panel from top in Fig. 5a or b, respectively), 0.1 mM EGCg caused a significant increase in Ca2+ sensitivity at pH 7 (pCa1/2 at 3 weeks = ↑0.36; 7 weeks = ↑0.43), pH 6.5 (3 weeks = ↑0.26; 7 weeks = ↑0.19), and pH 6.0 (3 weeks = ↑0.38; 7 weeks = ↑0.25). However, in post-MI rats with GTE supplementation for 18 or 46 days (bottom panel in Fig. 5a or b, respectively), the effects of EGCg on myofilament Ca2+ sensitivity were generally reduced over the pH range of 7 to 6. The summarized Ca2+ sensitivity (pCa1/2) and Hill coefficient (n) for the Ca2+-dependent myofibrillar ATPase activity measured in the presence and absence of 0.1 mM EGCg at pH 7.0, 6.5, and 6.0 for 3- and 7-week post-MI rats are listed in Tables 1 and 2, respectively. Clearly, green tea polyphenols modulated myofilament Ca2+ sensitivity in the myocardium in the animal model, while EGCg potentiated myofilament Ca2+ sensitivity for myocardial contractility under acidic conditions in vitro.
Quantitative analysis of the effects of EGCg on the pH-induced decrease in the Ca2+ sensitivity (pCa1/2) of myofibrillar ATPase activity. Control and post-MI rats with and without GTE supplementation for 18 (a) or 46 (b) days were compared. Actomyosin ATPase activity was measured in the presence (dotted lines) or absence (solid lines) of 0.1 mM EGCg. The normalized ATPase activity is plotted against the pCa. Each value is the mean ± SEM for five measurements. * symbolizes the significant difference (P < 0.05) of a Student’s t test between measurements of Ca2+-dependent actomyosin ATPase activity in the presence and absence of 0.1 mM EGCg
Discussion
In contrast to the well-known effects of green tea on vessels, little information is available regarding its myocardial effects. Using an experimental autoimmune myocarditis model in rats, Suzuki et al. showed that green tea catechins reduced inflammation and suppressed ventricular remodeling [40]. The same research teams utilizing another animal model of chronic myocardial ischemia in the rats with LAD ligation demonstrated that catechins attenuated chronic ventricular remodeling after myocardial ischemia due to the suppression of proinflammatory factors without systemic adverse effects [41]. A recent study with neonatal rat cardiomyocytes showed the cardioprotective effects of EGCg on H2O2-mediated oxidative stress via upregulation of antioxidative enzyme (e.g., heme oxygenase-1; HO-1) and activation of prosurvival signaling kinases (e.g., Akt, ERK1/2, p38 MAPK) [9]. Since inhibition of HO-1 and these signaling kinases does not abolish polyphenol-mediated protection, upregulation of antioxidative enzyme and activation of prosurvival signaling do not appear to play a major role in polyphenol-mediated cardioprotection.
One of the novel features of the present study was the use of a surgical model of MI in rats with LAD ligation to evaluate the myocardial protective effect of GTE. In this animal model, severe myocardial injury, including altered cardiac performance and the appearance of extensive fibrosis and LV enlargement, was seen. Histopathological studies showed that GTE supplementation for 18 or 46 days attenuated progressive remodeling after myocardial injury (Figs. 1 and 2). Transthoracic echocardiography confirmed that GTE supplementation prevented LV enlargement and improved LV performance in post-MI rats (Fig. 3). The data reported here validate the novel action of green tea polyphenols in protecting post-MI rats against myocardial damage and enhancing cardiac contractility.
Troponin (Tn) is a myofilament switch which directly controls the Ca2+-dependent activation of striated muscle contraction [12]. The Tn complex contains three subunits, troponin C (TnC), the Ca2+-binding inhibitory subunit troponin I (TnI), and troponin T (TnT), which binds the complex to tropomyosin. Phosphorylation of cTnI and cTnT mediated by protein kinases A and C can modulate myofilament Ca2+ sensitivity and cross-bridge cycling in the myocardium [7, 30]. Covalent modification of cTnI and cTnT is thought to play an essential role in tuning myocardium performance in both normal and diseased hearts [7, 19, 24, 30]. Accordingly, our study showing that post-MI rats displayed depressed myofilament Ca2+ sensitivity of cardiac myofibrils could be attributed to the modification of these myofilament proteins (Fig. 4).
A recent study showed that mutation of Gly159 of cTnC to Asp reduces the myofilament desensitization induced by phosphorylation of Ser23 and Ser24 of cTnI by altering Ca2+ binding to cTnC and that a site made up of the N-terminal region of cTnI, the C-lobe of cTnC, and the C-terminus of cTnT is important in modulating myofilament Ca2+ sensitivity in cardiac muscle [3]. More recently, studies with NMR spectroscopy [42] and biochemical approaches [25] have shown that EGCg binds to the C-lobe of cTnC near the region involved in the interaction with the N-terminal helix of cTnI and that this might alter Ca2+ binding to the C-terminal Ca2+-binding sites. By disrupting and weakening the interaction of cTnI34–71 with the cTnC C-lobe in myofilaments, EGCg has compensatory effects on increasing Ca2+ sensitivity of cardiac myofibrils in post-MI rats (Fig. 4).
Non-covalent modification of cardiac Tn also occurs, e.g., the C-terminal region of cTnI (residues 153–164) is proton-sensitive and non-covalent modification occurs on intracellular acidification [6, 8, 23, 26, 32, 45, 46]. Recently, we showed that EGCg binding to the C-lobe of cTnC reduces the acidic pH-induced decrease in Ca2+ sensitivity [25]. Based on circular dichroism (CD) and intrinsic fluorescence spectroscopy measurements, the K d for the binding of EGCg to cTnC was estimated as 3–4 μM. The binding of EGCg to cTnC was found to be Ca2+ independent, but dependent on the proton concentration. The interaction between EGCg and cTnC was twice as strong at pH 7 as at pH 6.5 [25]. In the present study, we confirmed further that green tea polyphenols potentiate myofilament Ca2+ sensitivity of cardiac myofibrils prepared from the hearts of both control and post-MI animals under acidic conditions (Fig. 5).
There are some studies indicating that a patient needs to drink at least 1 l of tea (about four cups)/day to gain some degree of benefit after a myocardial infarction [14, 33]. A cup of green tea contains 20–100 mg EGCg. According to the process of extraction of green tea reported by Yang et al., green tea leaves were first decaffeinated using supercritical carbon dioxide, followed by extraction with boiling water and lyophilization [48]. This decaffeinated GTE powder, containing 7.3% EGCg and 11.5% other catechins (e.g., ECG, EGC, EC), was used to study bioavailability of green tea polyphenols in human [48]. Their measurements indicated that the maximal plasma concentration of EGCg (0.3–1.1 μg/ml) was observed at 1.4–2.4 h after GTE ingestion. The half-life of EGCg and other catechins was 5.0–5.5 h and 2.5–3.4 h, respectively. A similar study with bioavailability of green tea polyphenols in rats was also conducted by Chen et al. [4] to investigate the absorption, distribution, and elimination of EGCg, and catechins after administration of GTE (200 mg or 15 mg EGCg equivalent/kg animal) or pure EGCg (75 mg/kg animal). Their data showed that the maximal plasma concentration of EGCg (16.3 ng/ml) appeared at 1.24 h after GTE ingestion. The elimination half-life of EGCg was 3.5 h after GTE ingestion. However, EGCg displayed different pharmacokinetic behavior when EGCg was given in the GTE or pure EGCg. In comparison with GTE supplementation, pure EGCg given to animals showed a 3.6-fold lower absorption rate constant, a 6.9-fold smaller distribution, and a 1.6-fold faster elimination of EGCg, respectively. This different pharmacokinetic behavior might be due to complex formation between EGCg and other components in GTE [4]. In our study, we showed that GTE supplementation (400 mg/kg animal/day, equivalent to180 mg EGCg/kg animal/day) for 18 or 46 days attenuated progressive remodeling after myocardial injury, prevented LV enlargement, and improved LV performance in post-MI rats. Apparently, the GTE concentration used in this study is relatively high. We do not rule out the possibility that a high dose of GTE will trigger additional protective signal transduction pathways in cardiomyocytes. It should be useful to determine the concentration-dependent effects of GTE or pure EGCg on myocardial protection/recovery from MI.
In summary, our results show that green tea polyphenols protect against myocardial damage and enhance cardiac contractility by modulating myofilament Ca2+ sensitivity in post-MI rats.
References
Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D'Agati V, Liu R, Homma S, Schmidt AM, Yan SF, Ramasamy R (2008) RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol 294:H1823–H1832
Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B (2004) Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 10:55–62
Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP (2007) The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res 100:1486–1493
Chen L, Lee MJ, Li H, Yang CS (1997) Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos 25:1045–1050
Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA (2004) Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol 43:1698–1705
Dargis R, Pearlstone JR, Barrette-Ng I, Edwards H, Smillie LB (2002) Single mutation (A162H) in human cardiac troponin I corrects acid pH sensitivity of Ca2+-regulated actomyosin S1 ATPase. J Biol Chem 277:34662–34665
Day SM, Westfall MV, Metzger JM (2007) Tuning cardiac performance in ischemic heart disease and failure by modulating myofilament function. J Mol Med 85:911–921
Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D'Alecy LG, Ingwall JS, Metzger JM (2006) Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med 12:181–189
Dreger H, Lorenz M, Kehrer A, Baumann G, Stangl K, Stangl V (2008) Characteristics of catechin- and theaflavin-mediated cardioprotection. Exp Biol Med (Maywood) 233:427–433
Fishbein MC, Maclean D, Maroko PR (1978) Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol 90:57–70
Gao XM, Dart AM, Dewar E, Jennings G, Du XJ (2000) Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res 45:330–338
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Hao J, Kim CH, Ha TS, Ahn HY (2007) Epigallocatechin-3 gallate prevents cardiac hypertrophy induced by pressure overload in rats. J Vet Sci 8:121–129
Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, Nakano A, Chiba T, Kawamura N (2007) Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci 80:1020–1032
Hirano R, Momiyama Y, Takahashi R, Taniguchi H, Kondo K, Nakamura H, Ohusuzu F (2002) Comparison of green tea intake in Japanese patients with and without angiographic coronary artery disease. Am J Cardio 90:1150–1153
Hotta Y, Huang L, Muto T, Yajima M, Miyazeki K, Ishikawa N, Fukuzawa Y, Wakida Y, Tushima H, Ando H, Nonogaki T (2006) Positive inotropic effect of purified green tea catechin derivative in guinea pig hearts: the measurements of cellular Ca2+ and nitric oxide release. Eur J Pharmacol 552:123–130
Jochmann N, Baumann G, Stangl V (2008) Green tea and cardiovascular disease: from molecular targets towards human health. Curr Opin Clin Nutr Metab Care 11:758–765
Klocke R, Tian W, Kuhlmann MT, Nikol S (2007) Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 74:29–38
Kobayashi T, Solaro RJ (2005) Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67:39–67
Kuriyama S (2008) The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr 138:1548S–1553S
Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296:1255–1265
Li D, Yang C, Chen Y (2008) Identification of a PKCε-dependent regulation of myocardial contraction by epicatechin-3-gallate. Am J Physiol 294:345–353
Li G, Martin AF, Solaro RJ (2001) Localization of regions of troponin I important in deactivation of cardiac myofilaments by acidic pH. J Mol Cell Cardiol 33:1309–1320
Li P, Hofmann PA, Li B, Malhotra A, Cheng W, Sonnenblick EH, Meggs LG, Anversa P (1997) Myocardial infarction alters myofilament calcium sensitivity and mechanical behavior of myocytes. Am J Physiol 272:H360–H370
Liou YM, Kuo SC, Hsieh SR (2008) Differential effects of a green tea-derived polyphenol (−)-epigallocatechin-3-gallate on the acidosis-induced decrease in the Ca2+ sensitivity of cardiac and skeletal muscle. Pflugers Arch 456:787–800
Liou YM, Chang JCH (2004) Differential pH effect on calcium-induced conformational changes of cardiac troponin C complexed with cardiac and fast skeletal isoforms of troponin I and troponin T. J Biochem 136:683–692
Liou YM, Jiang MJ, Wu MC (2000) Altered expression of cardiac myosin isozymes associated with the malignant hyperthermia genotype in swine. Anesthesiology 93:1312–1319
Liu YH, Yang XP, Nass O, Sabbah HN, Peterson E, Carretero OA (1997) Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am J Physiol 272:H722–H727
Lorenz M, Hellige N, Rieder P (2008) Positive inotropic effects of epigallocatechin-3-gallate (EGCG) involve activation of Na+/H+ and Na+/Ca2+ exchangers. Eur J Heart Fail 10:439–445
Metzger JM, Westfall MV (2004) Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res 94:146–158
Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA (2002) Tea consumption and mortality after acute myocardial infarction. Circulation 105:2476–2481
Solaro RJ, Kumar P, Blanchard EM, Martin AF (1986) Differential effects of pH on calcium activation of myofilaments of adult and perinatal dog hearts. Evidence for developmental differences in thin filament regulation. Circ Res 58:721–729
Peters U, Poole C, Arab L (2001) Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol 154:495–503
Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA (1979) Myocardial infarct size and ventricular function in rats. Circ Res 44:503–512
Shioura KM, Geenen DL, Goldspink PH (2007) Assessment of cardiac function with the pressure–volume conductance system following myocardial infarction in mice. Am J Physiol 293:H2870–H2877
Stangl V, Dreger H, Stangl K, Lorenz M (2007) Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc Res 73:348–358
Stangl V, Lorenz M, Stangl K (2006) The role of tea and tea flavonoids in cardiovascular health. Mol Nutr Food Res 50:218–228
Stephanou A (2004) Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med 8:519–525
Sumpio BE, Cordova AC, Berke-Schlessel DW, Qin F, Chen QH (2006) Green tea, the “Asian paradox,” and cardiovascular disease. J Am Coll Surg 202:813–825
Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M (2007) Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail 9:152–159
Suzuki J, Ogawa M, Maejima Y, Isobe K, Tanaka H, Sagesaka YM, Isobe M (2007) Tea catechins attenuate chronic ventricular remodeling after myocardial ischemia in rats. J Mol Cell Cardiol 42:432–440
Tadano N, Yumoto F, Morimoto S, Nagata K, Tanokura M, Ohtsuki I. Epigallocatechin gallate, a major polyphenol in green tea, binds to cardiac troponin C and desensitizes cardiac muscle contraction to Ca2+. International Symposium Celebrating 40th Anniversary of Troponin Discovery, the 33rd NIPS Conference, Okazaki, Japan, October 25–28, 2005 (Abstract)
Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML (2007) Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102:2104–2111
Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A (2004) Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J 18:1621–1623
Westfall MV, Metzger JM (2007) Single amino acid substitutions define isoform-specific effects of troponin I on myofilament Ca2+ and pH sensitivity. J Mol Cell Cardiol 43:107–118
Westfall MV, Borton AR, Albayya FP, Metzger JM (2002) Myofilament calcium sensitivity and cardiac disease: insights from troponin I isoforms and mutants. Circ Res 91:525–531
Wolfram S (2007) Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr 26:373S–388S
Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP (1998) Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev 7:351–354
Ytrehus K, Liu Y, Tsuchida A, Miura T, Liu GS, Yang XM, Herbert D, Cohen MV, Downey JM (1994) Rat and rabbit heart infarction: effects of anesthesia, perfusate, risk zone, and method of infarct sizing. Am J Physiol 267:H2383–H2390
Acknowledgments
This work was supported in part by the National Science Council of Taiwan (NSC 95-2320-B-005-005) and cooperative projects between the Taichung Veterans General Hospital and the NCHU (TCVGH-NCHU 967602).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsieh, SR., Tsai, DC., Chen, JY. et al. Green tea extract protects rats against myocardial infarction associated with left anterior descending coronary artery ligation. Pflugers Arch - Eur J Physiol 458, 631–642 (2009). https://doi.org/10.1007/s00424-009-0655-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0655-1