Abstract
Background
Complete mesocolic excision (CME) with central ligation or D3 lymphadenectomy has been reported to provide increased lymph node retrieval with the prospect of superior oncological results in colon cancer. However, right hemicolectomy with CME or D3 lymphadenectomy by laparoscopy is considered to be a technically challenging and time-consuming procedure with a higher risk of causing intraoperative injuries. Here, we introduce a novel laparoscopic approach for the D3 right hemicolectomy and report its feasibility, safety, and efficacy in cancer clearance.
Methods
This purely medial to lateral approach of D3 hemicolectomy was characterized by the following two features: a series of repeated, unidirectional dissections along the superior mesentery vein (SMV) that were started below the ileocolic vein and ended at the pancreatic neck, followed by the exposure of the whole SMV and its colonic branches precisely before the ligation. From January 2012 to December 2015, 58 patients underwent this procedure. The short-term outcomes and long-term survival are reported.
Results
All 58 operations were finished with this procedure successfully, with one injury of the jejunal vein. The mean operation time was 164 ± 28.3 min, the mean blood loss was 64 ± 63.5 ml, and the mean number of retrieved lymph nodes was 28 ± 13.9. No mortality or major morbidity was observed. The 4-year overall survival was 78%, and the disease-free survival was 77%.
Conclusion
This novel, unidirectionally progressive, pancreas-oriented procedure for laparoscopic radical right hemicolectomy with D3 lymphadenectomy is safe and feasible, with the merit of providing an easier and safer way to tackle the variable tributaries of the SMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of the laparoscopic technique into colorectal surgery by Jacobs in the early 1990s [1], its superiority in short-term outcomes and non-inferiority in long-term survival for colorectal cancer have been successfully replicated by several randomized clinical trials [2,3,4]. Most of these trials did not give a clear definition of the extent of lymph node dissection. However, D3 lymphadenectomy, in both Japan and other east Asian countries, is a state-of-the-art surgery for advanced colon cancer, especially for T3–4/N+ tumors [5]. Similar to D3 lymphadenectomy [6], the complete mesocolic excision (CME), first suggested by Hohenberger in 2009 [7], offered a higher quality of surgery including longer central pedicle, increased lymph node harvest, and better disease-free survival [8], allowing the CME procedure to gain a wider acceptance as a new standard of care [9, 10].
Several reports have already reported the feasibility, safety, and effectiveness of the laparoscopic CME/D3 right hemicolectomy [10,11,12]. Due to its technical complexity and the frequent variation in vascular anatomy of superior mesentery vessels, however, there is still no consensus on the safety of routine applications of this extended lymphadenectomy and its real benefits. Issues about completeness of excision for transverse colon cancer and bleeding around the gastrocolic trunk of Henle (GTH) have also been raised [13]. With the risk posed by performing dissection near the superior mesenteric vein (SMV), here, we report a novel procedure, a unidirectionally progressive, pancreas-oriented procedure, to facilitate the laparoscopic right hemicolectomy with CME/D3 lymphadenectomy. The main feature of this procedure involves exposing the whole length of the SMV before ligating any named veins connected to the right-sided colon.
Methods
Surgical procedure
The patient was placed in a supine position with split-leg. The surgery was performed using five ports with the surgeon standing between the patient’s two legs (Fig. 1). The lymph node dissection and mobilization of the colon were done in five steps, as shown in Fig. 2a and the video.
The position of the trocar for the operation. A1, port mainly for assistant to retract transverse mesocolon; A2, port mainly for assistant to retract ileocolic mesocolon; camera: port for laparoscopy; S1, port for instrument of left hand of surgeon; Sr, port for right hand of surgeon. Green spot: umbilicus
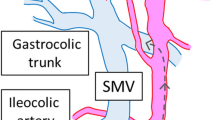
SMV: superior mesenteric vein, P: pancreas, MCA: middle colic artery, MCV: middle colic vein, ICV: ileocolic vein, ICA: ileocolic artery, GTH: gastrocolic trunk of Henle’s, RGEV: right gastroepiploic vein, RCV: right colic vein, AIPDVs: anterior inferior pancreaticoduodenal veins. a The main episode of the unidirectionally progressive, pancreas-oriented procedure for laparoscopic right hemicolectomy. b Locating the direction of dissection on transverse mesocolon between the Treitz ligament and the MCV. c Dissecting the terminal branch of SMV. Serving as the beginning of the dissection. d The path of dissection. e Exposing the artery across the SMV. f The whole course of SMV, the ICA run posterior to jejunal vein and anterior to the SMV. g Ligating the MCV, carefully distinguishing the jejunal vein, and the MCV is very close to the GTH. h Exposing the RGEV before ligating the RCV. i Relationship of GTH, RCV, RGEV, AIPDV. j After ligation of RCV, multiple AIPDVs, even some small vessels enter the GTH and RGEV, which may cause bleeding
supplementary video of the unidirectionally progressive, pancreas-oriented procedure for laparoscopic D3 right hemicolectomy (WMV 363693 kb)
After careful exploration for metastasis and resectability, the surgery started with entering the omental bursa either superior to the gastroepiploic vessels for transverse colon cancer or inferior to the gastroepiploic vessels for ascending colon cancer. The transverse mesocolon was then detached from the inferior border of the pancreatic neck. A gauze was left in the omental bursa as an indicator for the following dissection, and the omentum was reflected superiorly to explore the transverse mesocolon (Fig. 2b). Before the steps of lymph node dissection were performed, the pedicle of the middle colic vessel was identified and lifted to retract the transverse mesocolon superiorly and ventrally (Fig. 2b), and the pedicle of the ileocolic vessel was grasped and retracted ventrally (Fig. 2c). The second step commenced with creating a window left of the ileocolic vessels in the mesentery (Fig. 2c). The peritoneum over the superior mesenteric artery (SMA) was divided up to the left of the middle colic vessels until the omental bursa could be entered from below (Fig. 2b). This defined the course of the following steps (Fig. 2d).
Third, the SMV was identified below the ileocolic pedicle, and dissection was then carried out along the left side of the SMV. A very thin layer of fatty tissue overlying the SMV was taken into the jaws of the harmonic for dissection to avoid inadvertent injuries on vessels embedded in the thick adipose tissue. Two to three rounds of dissection in this manner, always following the pre-defined course, would expose the arteries to the colon, mainly the middle colic artery (MCA), or less commonly the ileocolic (ICA) and right colic arteries (RCA), which run ventrally to the SMV (Fig. 2e).
At the beginning of the fourth step, the whole length of the SMV had already been observed, and easy dissection in this areolar space in front of the SMV would render its tributaries from the colon into visualization (Fig. 2f). The ileocolic and middle colic veins were divided at their entry into the SMV (Fig. 2g).
Prior to manipulation of the GTH, the transverse mesocolon was separated from the duodenum, followed by tracing the GTH up to the right gastroepiploic veins (RGEVs). This allowed for the identification of the point where the right colic vein (RCV) connects with the GTH and where the RCV can be safely clipped and divided (Fig. 2h–j). If the tumor was located at the transverse colon or flexure, the RGEV would also be divided and then ligated while preserving the anterior inferior pancreaticoduodenal veins (AIPDVs).
Lastly, the transverse and ascending mesocolon was separated from its attachment to the posterior abdominal wall. The dissection was advanced laterally from the pre-pancreatic areolar space and inferiorly along the Toldt’s fascia. Finally, the peritoneum of the paracolic gutter was divided.
The transection and reconstruction of the bowel was completed through a mini-laparotomy around the umbilicus. After reinforcement of the transecting end of the colon, the bowel was returned into the abdomen. The mesenteric defect was closed by intracorporeal continuous sutures.
Patients
From January 2012 to December 2015, consecutive patients, who were diagnosed with right colon cancer by colonoscopy plus biopsy, a thoracic and abdominopelvic CT, and tumor markers, were included. Patients with an emergency situation were excluded. After recovery, patients were advised to visit the oncologist as the guideline suggested. Patients were then followed up every 6 months for 5 years. The short-term and long-term data were collected. This study was approved by the Ethics Committee of West China Hospital, and informed consent was obtained from all patients.
Results
A total of 60 patients with right colon cancer were enrolled, and 58 completed this procedure successfully. The other two patients converted to open surgery due to adhesions from a previous major surgery. The basic characteristics are shown in Table 1. Of the excised right colon, all 58 cases achieved complete mesocolic excision. Mean harvested lymph nodes were 28 ± 13.9 (range 5–63), the number of harvested central lymph nodes was 9.7 ± 5.36 (range 1–20), and the length of distal colonic ending was 15.1 ± 5.39 cm. The pathologic features are shown in Table 2. Results of short-term outcomes are shown in Table 3. The average operation time was 164 ± 28.3 min, and mean blood loss was 64 ± 63.5 ml. One patient had a jejunal vein injury, which was sutured laparoscopically, and two patients had a postoperative chyle leakage. No postoperative mortality or anastomotic leaks occurred.
At a median follow-up of 48 months (range 6–60), two patients were lost. One patient had an adhesive obstruction, which was managed by conservative treatment. There were no other long-term complications. Of the 56 patients, 12 patients died, among whom two died from non-oncological causes. Twelve patients had distant metastasis, three had local relapse concurrent with distant metastasis, and one patient had local recurrence only. The 4-year overall survival was 78% and disease-free survival (DFS) was 77%. The Kaplan-Meier curve of these patients, which was stratified by stage, is shown in Fig. 3.
Discussion
Laparoscopic colorectal surgery has been reported to provide similar long-term outcomes and better short-term outcomes compared to open surgery in several large randomized controlled trials [2,3,4]. Hence, laparoscopic surgery is the procedure of choice by a majority of surgeons. Retrospective studies have also implied that an open CME or D3 lymphadenectomy might result in better long-term outcomes, while other reports have argued against its effectiveness. The Japanese prospective JCOG0404 trial failed to prove the non-inferiority of the laparoscopic D3 lymphadenectomy but found similarly unexpected advancements in safety and morbidity, justifying the application of this procedure in experienced hands [4].
CME or D3 lymphadenectomy for right colon cancer requires extended lymph node dissection along the thin-walled SMV and GTH, which is a challenging and risky procedure for surgeons, especially when performed laparoscopically or in obese patients. The notorious variations in the vascular anatomy of the right colon, especially around the GTH, further complicate this surgery. Zhao et al. [14] reported a bleeding rate of 43.6% in laparoscopic right hemicolectomy, and the main bleeding sites were at the head of the pancreas (16.4%) and the middle colic vessels (14.5%). To tackle this problem, some surgeons have turned to 3D reconstruction of mesenteric vessels, which certainly prepared the surgeon for the potential variations of vascular numbers and courses, increasing the safety of this procedure [15].
Our procedure provides an easier way for identifying tributaries of the SMV. The most distinguishing feature of our procedure is that, prior to seeking and dividing any colic veins, the whole course of the SMV is exposed, which is accomplished by several rounds of dissection along the long axis of the SMV and division of colic arteries. To avoid inadvertent injuries at the pancreatic neck, end of each round of dissection, which sometimes bears indistinguishable yellowish color as mesenteric fat, this approach is hence designed to detach in advance the transverse mesocolon from the pancreas, followed by insertion of a gauze between them. After visualization of the full course of the SMV, identifying its colic tributaries causes no further difficulties. Because, within the thin “sheath” covering the SMV, no thick adipose tissue wraps around the roots of the venous drainage to the SMV. Thus, each colonic vein is very likely to “come into view” with an easy dissection of areolar tissue or just gentle pushing, as shown in the video.
Another reason why this approach is easier and safer for manipulation of colic veins is that it takes advantage of little colonic veins draining into the left aspect of the SMV (Fig. 2a). This was also the difference between our technique and a similarly novel surgical technique undergone by Benz [16, 17]. The middle colic vein (MCV) usually joins to the middle-anterior aspect of the SMV or GTH, and the ICV, RCV, and/or GTH all go into its right aspect. Hence, nearly no colic veins will be encountered in the process of exposing the SMV and dividing colonic arteries, given that the dissection course is well held along the left side of the SMV. Comparing to Benz’s technique, our modified operation skeletonized the SMV and its tributaries more easily and sufficiently [16]. Even though identifying colic arteries in front of the SMV still poses challenges, the sheath of arteries is much thicker and thus less vulnerable to accidental injuries. In very rare cases (3.4%), MCV, as reported by Yamaguchi, drains into veins left to the SMV, such as the jejunal veins, splenic vein, or inferior mesenteric vein [18, 19]. Even in this situation, the MCV usually runs very superficially along the left aspect of the middle colic pedicle when it is stretched by the surgical assistant.
One pitfall associated with dissection on the left side of the SMV is the risk of inadvertent injuries to jejunal veins, which occurred in one patient in this study. Jejunal veins cross the SMA in the D3 area anteriorly in 30.9% of patients (Fig. 2f), as reported by Nesgaard JM [20]. Awareness of this variation can be acquired easily by a routine preoperative CT, with no need of 3D reconstruction of vessels. In addition to this knowledge, careful dissection, picking up a thin layer of tissue in the jaws of harmonic each time, and avoiding any wandering to the left of the SMV are all useful preventions.
Another major challenge facing laparoscopic right CME is the manipulation of the GTH and its tributaries, which has been addressed by several reports with different approaches [13, 21,22,23]. Here, we provide a distinct resolution to this challenge: to dissect upwardly from the root of the GTH to gain visualization of the course of the right gastroepiploic vein, followed by dividing the RCV. The merit of this approach is that it has the least risk of tearing the AIPDV. Traditionally, the RCV is approached by dissecting in front of the pancreatic head, which calls for an upward retraction of the transverse mesocolon. Excessive traction applied on the mesocolon by a surgical assistant would likely pass the tension onto the fixed AIPDV, which can cause tearing of its small vessels and result in troublesome bleeding (Supplementary Fig. 1). Dissection along the pancreatic neck rightwards or along the RGEV puts no tension on AIPDVs. Dividing the GTH at its root should also be avoided, for tension would be transferred to the AIPDV in a similar manner. To avoid the excessive tension on the GTH and its branches, Matsuda et al. [22, 24] reported a cranial approach to address the GTH and MCV first and to dissect from cranial to caudal. Their approach was different from ours. However, the thought of exposing the inferior border of the pancreas and handling vessels first to reduce the risk of bleeding was similar.
Some surgeons doubted the real benefit of CME or D3 lymphadenectomy since the central lymph node positive rate was as low as 0–5.8% [9, 25]. However, after CME, the 5-year DFS of central lymph node positive patients was 36.4% [25], much higher than patients with distant metastasis. Furthermore, routine pathologic examination might miss cases with micrometastasis, and an immunohistochemical stain might increase the positive rate to 8.5% [26, 27]. Additionally, large-scale retrospective studies reported a better 5-year survival, which may be related to more lymph nodes dissected [8]. In our series, only one patient had positive central lymph nodes, which is similar to previous reports. The mean number of retrieved lymph nodes was 28 ± 13.9 (range 5–63) in the current study, which was higher than the Japanese society (mean 21, range 16–27), and lower than (mean 32, range 20–40) in Erlangen [28]. It might be related to the length of excised colon. Hohenberger suggested to excise all the right transverse colon [7], and our strategy was to divide the transverse colon at least 10 cm away from tumor. The mean distal length of colon was 15.1 ± 5.39 cm in our series, which was longer than that suggested in Japanese guidelines. For the long-term follow-up, the survival of this small cohort was similar to that of Danish [8] and Japanese data [29]. In the future, a comparable study will be performed.
One limitation of this small size study is the lack of comparison with open operation or other surgical approaches. However, we have compared our results with the published data and stressed the importance of certain indispensable technical steps in the procedure. It precluded us from drawing any definitive conclusions, however, our data, such as those obtained by other authors, indicate that our specific technique is safe and effective and that laparoscopic right colonic resection for carcinoma does not increase the risk of complications, morbidity, or mortality.
Conclusions
This novel, unidirectionally progressive, pancreas-oriented procedure for laparoscopic radical right hemicolectomy with D3 lymphadenectomy is safe and feasible. This procedure allows for identification of the whole length of SMV prior to handling venous branches, thus providing an easier way to locate branches of the SMV connected to the colon and facilitating lymph node dissection.
References
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1(3):144–150
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. https://doi.org/10.1002/bjs.8945
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol : Off J Am Soc Clin Oncol 25(21):3061–3068. https://doi.org/10.1200/jco.2006.09.7758
Kitano S, Inomata M, Mizusawa J, Katayama H, Watanabe M, Yamamoto S, Ito M, Saito S, Fujii S, Konishi F, Saida Y, Hasegawa H, Akagi T, Sugihara K, Yamaguchi T, Masaki T, Fukunaga Y, Murata K, Okajima M, Moriya Y, Shimada Y (2017) Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 2(4):261–268. https://doi.org/10.1016/s2468-1253(16)30207-2
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K, Japanese Society for Cancer of the C, Rectum (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20(2):207–239. https://doi.org/10.1007/s10147-015-0801-z
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol : Off J Am Soc Clin Oncol 30(15):1763–1769. https://doi.org/10.1200/JCO.2011.38.3992
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Color Dis : Off J Assoc Coloproctol G B Irel 11(4):354–364; discussion 364-355. https://doi.org/10.1111/j.1463-1318.2008.01735.x
Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gogenur I, Danish Colorectal Cancer G (2015) Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 16(2):161–168. https://doi.org/10.1016/S1470-2045(14)71168-4
Sondenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D'Hoore A, Kennedy RH, West NP, Kim SH, Heald R, Storli KE, Nesbakken A, Moran B (2014) The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery : proceedings of a consensus conference. Int J Color Dis 29(4):419–428. https://doi.org/10.1007/s00384-013-1818-2
Matsuda T, Iwasaki T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y (2016) Laparoscopic complete mesocolic excision for right-sided colon cancer using a cranial approach: anatomical and embryological consideration. Int J Color Dis 32:139–141. https://doi.org/10.1007/s00384-016-2673-8
Kontovounisios C, Kinross J, Tan E, Brown G, Rasheed S, Tekkis P (2015) Complete mesocolic excision in colorectal cancer: a systematic review. Color Dis : Off J Assoc Coloproctology G B Irel 17(1):7–16. https://doi.org/10.1111/codi.12793
Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2014) Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 21(7):2288–2294. https://doi.org/10.1245/s10434-014-3614-9
Li H, He Y, Lin Z, Xiong W, Diao D, Wang W, Wan J, Zou L (2016) Laparoscopic caudal-to-cranial approach for radical lymph node dissection in right hemicolectomy. Langenbeck's Arch Surg/ Dtsch Ges Chir 401(5):741–746. https://doi.org/10.1007/s00423-016-1465-5
Zhao LY, Li GX, Zhang C, Yu J, Deng HJ, Wang YN, Hu YF, Cheng X (2012) Vascular anatomy of the right colon and vascular complications during laparoscopic surgery. Zhonghua wei chang wai ke za zhi = Chinese journal of gastrointestinal surgery 15(4):336–341
Spasojevic M, Stimec BV, Fasel JF, Terraz S, Ignjatovic D (2011) 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surg Endosc 25(6):1883–1886. https://doi.org/10.1007/s00464-010-1480-5
Benz S (2016) The uncinate-first approach for laparoscopic complete mesocolic right hemicolectomy—a video vignette. Color Dis : Off J Assoc Coloproctology G B Irel 18(1):109. https://doi.org/10.1111/codi.13157
Benz S, Tam Y, Tannapfel A, Stricker I (2016) The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 30(5):1930–1937. https://doi.org/10.1007/s00464-015-4417-1
Yamaguchi S, Kuroyanagi H, Milsom JW, Sim R, Shimada H (2002) Venous anatomy of the right colon: precise structure of the major veins and gastrocolic trunk in 58 cadavers. Dis Colon Rectum 45(10):1337–1340. https://doi.org/10.1097/01.DCR.0000027284.76452.84
Ogino T, Takemasa I, Horitsugi G, Furuyashiki M, Ohta K, Uemura M, Nishimura J, Hata T, Mizushima T, Yamamoto H, Doki Y, Mori M (2014) Preoperative evaluation of venous anatomy in laparoscopic complete mesocolic excision for right colon cancer. Ann Surg Oncol 21(Suppl 3):S429–S435. https://doi.org/10.1245/s10434-014-3572-2
Nesgaard JM, Stimec BV, Bakka AO, Edwin B, Ignjatovic D, group RCCs (2015) Navigating the mesentery: a comparative pre- and per-operative visualization of the vascular anatomy. Color Dis :Off J Assoc Coloproctology G B Irel 17(9):810–818. https://doi.org/10.1111/codi.13003
Mori S, Baba K, Yanagi M, Kita Y, Yanagita S, Uchikado Y, Arigami T, Uenosono Y, Okumura H, Nakajo A, Maemuras K, Ishigami S, Natsugoe S (2015) Laparoscopic complete mesocolic excision with radical lymph node dissection along the surgical trunk for right colon cancer. Surg Endosc 29(1):34–40. https://doi.org/10.1007/s00464-014-3650-3
Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Maekawa Y, Tanaka T, Shimada E, Kakeji Y (2015) Cranial-to-caudal approach for radical lymph node dissection along the surgical trunk in laparoscopic right hemicolectomy. Surg Endosc 29(4):1001. https://doi.org/10.1007/s00464-014-3761-x
Zou L, Xiong W, Mo D, He Y, Li H, Tan P, Wang W, Wan J (2016) Laparoscopic radical extended right hemicolectomy using a caudal-to-cranial approach. Ann Surg Oncol 23(8):2562–2563. https://doi.org/10.1245/s10434-016-5215-2
Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Maekawa Y, Tsugawa D, Sugita Y, Sumi Y, Shimada E, Kakeji Y (2015) Cranially approached radical lymph node dissection around the middle colic vessels in laparoscopic colon cancer surgery. Langenbeck's Arch Surg/ Dtsch Ges Chir 400(1):113–117. https://doi.org/10.1007/s00423-014-1250-2
Kanemitsu Y, Komori K, Kimura K, Kato T (2013) D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 56(7):815–824. https://doi.org/10.1097/DCR.0b013e3182919093
Merrie AE, Phillips LV, Yun K, McCall JL (2001) Skip metastases in colon cancer: assessment by lymph node mapping using molecular detection. Surgery 129(6):684–691. https://doi.org/10.1067/msy.2001.113887
Chen G, McIver CM, Texler M, Lloyd JM, Rieger N, Hewett PJ, Sen Wan D, Hardingham JE (2004) Detection of occult metastasis in lymph nodes from colorectal cancer patients: a multiple-marker reverse transcriptase-polymerase chain reaction study. Dis Colon Rectum 47(5):679–686. https://doi.org/10.1007/s10350-003-0118-2
Kobayashi H, West NP, Takahashi K, Perrakis A, Weber K, Hohenberger W, Quirke P, Sugihara K (2014) Quality of surgery for stage III colon cancer: comparison between England, Germany, and Japan. Ann Surg Oncol 21(Suppl 3):S398–S404. https://doi.org/10.1245/s10434-014-3578-9
Moritani K, Hasegawa H, Okabayashi K, Ishii Y, Endo T, Kitagawa Y (2014) Difference in the recurrence rate between right- and left-sided colon cancer: a 17-year experience at a single institution. Surg Today 44(9):1685–1691. https://doi.org/10.1007/s00595-013-0748-5
Acknowledgements
We also thank a doctor of our team, Yazhou He (MD, Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China; and Institute of Genetics and Molecular Medicine, Western General Hospital, Edinburgh University, Edinburgh, UK), who revised this manuscript.
Funding
This study was funded by the Scientific and Technology department of Sichuan Province (grant no. 2016SZ0043).
Author information
Authors and Affiliations
Contributions
Xiangbing Deng and Tao Hu analyzed the data and drafted of manuscript; Tao Hu, Mingtian Wei, Qingbin Wu, and Tinghan Yang contributed for the acquisition and interpretation of the data; Wenjian Meng and Ziqiang Wang revised this manuscript; Wenjian Meng and Ziqiang Wang contributed for the study conception, design, and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, this study was approved by the Ethics Committee of West China Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Fig 1
the enlarged and vulnerable AIPDVs. Red arrows: AIPDVs; black arrows: RGEV; if the operation ligating the GTH is shown as a white arrow, the AIPDVs may cause troublesome bleeding, and haemostatic procedure may injure the pancreas. (PNG 16020 kb)
Rights and permissions
About this article
Cite this article
Deng, X., Hu, T., Wei, M. et al. Feasibility of a unidirectionally progressive, pancreas-oriented procedure for laparoscopic D3 right hemicolectomy. Langenbecks Arch Surg 403, 761–768 (2018). https://doi.org/10.1007/s00423-018-1703-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1703-0