Abstract
Purpose
Risk factors of ischemic gastropathy (IG) following distal pancreatectomy with en bloc celiac axis resection (DP-CAR) remain unclear.
Methods
Fifty consecutive patients with pancreatic cancer who underwent DP-CAR were retrospectively reviewed for possible risk factors for IG. This study was registered on the UMIN Clinical Trials Registry (UMIN 000028732).
Results
Complications higher than grade 3 were observed in 21 patients (42%) and mortality in 4 (8%). Left gastric artery (LGA) resection (P = 0.046) and a combination of left inferior phrenic artery (IPA) with LGA resection (P = 0.012) were risk factors of IG, and an elevated creatine kinase (CK) value ≥ 1005 IU/L (P = 0.025) was associated with IG. Among prognostic factors, IG (OR, 5.997; 95% CI, 1.543–23.309; P = 0.010), completion of adjuvant chemotherapy (OR, 0.282; 95% CI, 0.121–0.654; P = 0.003), longer operative time (OR, 2.261; 95% CI, 1.084–4.714; P = 0.030), and higher age (OR, 2.212; 95% CI, 1.081–4.524; P = 0.030) remained independent predictors of survival. Comparison at 2 and 3 months postoperatively showed nutritional values were higher in patients who underwent LGA-preserving DP-CAR than those with LGA-resecting DP-CAR: total protein (7.17 ± 0.56 vs 6.65 ± 0.66 g/dl, P = 0.007), albumin (4.04 ± 0.45 vs 3.43 ± 0.43 g/dl, P < 0.001), and total cholesterol (162.3 ± 34.7 vs 141.6 ± 27.2 mg/dl, P = 0.044).
Conclusions
The poorer prognosis in patients who undergo DP-CAR may be related to more advanced tumors. A combination of left IPA and LGA resection was a significant risk factor for IG. IG, completion of adjuvant chemotherapy, longer operative time, and higher age remain good independent predictors of survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is associated with poor prognosis and is predicted to be the second leading common cause of cancer-related mortality worldwide by 2030 [1]. Curative surgical resection with adjuvant therapy is considered the best option for longer survival [2]. Most pancreatic adenocarcinomas recur systemically, so tumors involving vascular structures recur frequently, even following aggressive surgery. As stronger chemotherapy is being introduced as a neoadjuvant therapy [3], combined arterial resection should be revisited [4], and a decision should be made regarding conversion surgery. The Appleby operation has been modified for safety and has re-emerged as a focus of pancreatic surgeons as a radical pancreatectomy for locally advanced/borderline resectable pancreatic body/tail carcinoma [5,6,7]. The modified Appleby operation, a synonym for distal pancreatectomy with en bloc celiac axis resection (DP-CAR), is a procedure enabling removal of T4 pancreatic body/tail carcinoma. Until now, in ordinary clinical settings, these have had unresectable status. More favorable prognosis is expected by increasing the R0 resection rate in T3 cases, taking a wider surgical margin. Moreover, cancer-related pain relief can be achieved by removal of tumors infiltrating plexuses [8, 9].
DP-CAR remains a controversial procedure because of unsolved issues, including ischemic complications of the stomach in the early postoperative period, and whether the procedure has genuine long-term survival benefits for patients with pancreatic cancer. In particular, the ischemic gastropathy (IG) after DP-CAR can sometimes become a severe and prolonged complication. In spite of this, the clinical impact of IG has not been sufficiently discussed. Several sources have reported the challenge in development of this procedure based on experience of severe complications in clinical studies [10, 11]. Incidences of morbidity and mortality remain high compared to other pancreatectomies [12].
In our institution, based on our initial experience of IG, we demonstrated that the LGA can be preserved in cases with LGA branches antecedently and those where the distance between the LGA and the tumor is more than 10 mm [9]. In patients who undergo DP-CAR, resection of LGA is an independent risk factor for delayed gastric emptying (DGE) [13].
This study was approved by the Wakayama Medical University Hospital Institutional Review Board (No. 2066) and was registered on the UMIN Clinical Trials Registry (UMIN 000028732).
By analyzing the clinicopathological data of patients who have undergone DP-CAR, this study aims to clarify risk factors and clinical impact of IG.
Patients and methods
Patients
Enrolled in this study were 50 consecutive patients who underwent DP-CAR between October 2004 and May 2017 at Wakayama Medical University Hospital (Table 1). We investigated the risk factors for IG and performed analysis to identify prognostic indicators among these patients. No patients underwent preventive total gastrectomy to avoid gastric ischemic complications during DP-CAR. Pathologic stages were diagnosed according to the Union for International Cancer Control (UICC) seventh tumor-node-metastasis (TNM) classification [14]. Based on pathological diagnosis of the resected specimen, microscopic surgical margin status (R0 or R1) was examined. We defined R0 status as the absence of tumor cells within 1 mm of the resection margin and R1 status as the presence of tumor cells within 1 mm of the resection margin [15]. Until February 2010, upfront surgery was used but gave low R0 rates [9]. Since March 2010, our current treatment has included neoadjuvant therapy for all patients who are indicated for DP-CAR. In this study, borderline resectable pancreatic cancer (BRPC) was reviewed and defined according to National Comprehensive Cancer Network (NCCN) criteria version 2.2015 [16]. Routine preoperative endoscopic examination confirmed normal status of gastroduodenal mucosa in all patients. Patients underwent postoperative endoscopic examination if they presented gastrointestinal symptoms or required endoscopic intervention.
Preoperative arterial embolization
Preoperative arterial embolization was routinely performed for developing the blood flow via the pancreatic head arcade about a week prior to surgery to prevent ischemic complications of the liver and the stomach after DP-CAR. In patients who were to undergo LGA-resecting DP-CAR (conventional DP-CAR), arterial embolization was performed using interlocking detachable coils (IDCs) [17] in the celiac axis (CA), the common hepatic artery (CHA), and the left gastric artery (LGA) (Fig. 1a). In patients who were to undergo LGA-preserving DP-CAR (modified DP-CAR), arterial embolization was performed in only the CHA distal side of LGA branch (Fig. 1b).
Angiography examination showing arterial embolization performed using interlocking detachable coils in the celiac axis (CA), the common hepatic (CHA), and the left gastric arteries (LGA) in patients who were intended to undergo conventional DP-CAR (a), performed in only in the CHA distal side of LGA branch in patients who were intended to undergo modified DP-CAR (b). CA: celiac axis, CHA: common hepatic artery, PHA: proper hepatic artery, GDA: gastroduodenal artery, LGA: left gastric artery, SA: splenic artery
Neoadjuvant therapy
In our institution, patients with BRPC underwent neoadjuvant therapy as follows: between March 2010 and December 2011, patients with BRPC underwent neoadjuvant chemoradiation therapy involving external-beam radiation with 50 Gy plus concurrent alternate-day oral therapy with S-1 for 6 weeks [18]; between January 2012 and December 2013, neoadjuvant chemotherapy involving concurrent alternate-day oral therapy with S-1 and gemcitabine for 9 weeks [18]; between April 2014 and May 2015, modified FOLFIRINOX (without bolus 5-FU and LV, also decreased dose of irinotecan; FIRINOX) by 4 or 8 cycles repeated every 2 weeks [19]; and from July 2015, nab-paclitaxel plus gemcitabine for 8 weeks [20]. In cases with no disease progression, patients underwent DP-CAR within 4 weeks.
Surveillance and postoperative adjuvant therapy
Patients who had undergone surgery were expected to receive adjuvant therapy. Follow-up surveillance was performed as reported previously [18]. Postoperative adjuvant chemotherapy regimens also differed depending on the timing. Between October 2004 and August 2013, gemcitabine was taken intravenously based on the CONKO-001 study [21] with or without concurrent alternate-day oral therapy with S-1 [22]. From September 2013, oral therapy was undertaken with S-1 based on the JASPAC 01 study [23].
Surgical procedures
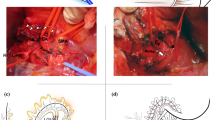
Indications and surgical procedures used during conventional DP-CAR were similar to those previously reported [9] (Fig. 2a). DP-CAR was applied in patients with tumors invading the plexus around the common hepatic artery, the root of the splenic artery, or the CA. Patients who had antecedent branching of the LGA and had a distance between the LGA and carcinoma greater than 10 mm underwent modified DP-CAR, where the artery was divided just below the branching of the LGA, as we previously reported [13, 24] (Fig. 2b). In all cases, nerve plexuses and lymph nodes in proximal portion along the SMA through the perineural spaces were also dissected. The left gastric veins and the posterior gastric vein were divided in all cases. In this series, the gastroduodenal arteries and the right gastric and right gastroepiploic arteries were identified and preserved in all cases. Based on the experience of patient-presented ischemic cholecystitis after DP-CAR, we have routinely performed cholecystectomy as a concomitant procedure since 2011.
Schema showing the relationship between the division site and the branching site of the left gastric artery (a) in conventional DP-CAR and (b) in distal pancreatectomy with resection of the common hepatic and splenic artery, with preservation of the left gastric artery (modified DP-CAR). Double-headed arrows indicate the site of the division. CA: celiac axis, SA: splenic artery, CHA: common hepatic artery, LGA: left gastric artery
We conducted a single center pilot study [10] and a multicenter randomized control trial (RCT) of pancreaticojejunostomy (PJ) with 17 patients [11] to evaluate whether pancreatic stump decreases the incidence after distal pancreatectomy (DP)/DP-CAR.
Investigation of the postoperative stomach blood flow
In the present study, elimination of the left inferior phrenic arteries (IPA) was investigated by postoperative computed tomography (CT) to compare/review preoperative images [25] and operative records as a risk factor of organ ischemia. The blood flow status postconventional DP-CAR with bilateral IPA combined resection is illustrated in Fig. 3.
Blood flow status after conventional DP-CAR with bilateral IPA combined resection. Solid lines represent arteries with blood flow and dotted lines represent arteries without blood flow after surgery. Ao: aorta, CA: celiac axis, SA: splenic artery, CHA: common hepatic artery, SMA: superior mesenteric artery, LGA: left gastric artery, RGA: right gastric artery, IPDA: inferior pancreatoduodenal artery, RGEA: right gastroepiploic artery, LGEA: left gastroepiploic artery, PGA: posterior gastric artery, SGA: short gastric artery, Rt IPA: right inferior phrenic artery, Lt IPA: left inferior phrenic artery
Investigation of the early phase nutritional status
To confirm the nutritional impact of single factors of LGA preservation, we investigated the preoperative/postoperative body weight/laboratory data including total protein (TP), albumin (Alb), and total cholesterol (TC) as nutrition-related factors. We compared them between the groups with conventional DP-CAR and modified DP-CAR. Preoperative data was defined as those obtained within 2 weeks before surgery, even in patients who underwent neoadjuvant therapy. Postoperative data was determined as those with highest values between postoperative months 2 and 3. We selected this postoperative period to remove the effect of potential cancer recurrence as much as possible without data loss.
Definition of postoperative complications
We defined DGE according to consensus and the clinical grading of postoperative DGE proposed by the International Study Group of Pancreatic Surgery (ISGPS) [26]. Definition of pancreatic fistula was set according to ISGPS guidelines [27]. Postpancreatic hemorrhage was also defined by the ISGPS [28]. IG was defined by gastroduodenal ulcer or perforation due to ischemic change of the gastric wall identified by endoscopy or surgery. Hepatic infarction (HI) was defined as lower density area of the liver on enhanced CT images within postoperative day 7 with abnormal liver function tests. Surgical site infections included surgical wounds or intra-abdominal abscesses with positive cultures. Intra-abdominal abscess including liver abscess was defined as intra-abdominal fluid collection with positive cultures identified by ultrasonography or computed tomography associated with persistent fever and elevation of the white blood cell count. Mortality was defined as in-hospital death from any cause.
Statistical analysis
Statistical comparison between the two groups was made using the chi-square statistic, Fisher’s exact test, or the Mann-Whitney U test, where appropriate. Baseline characteristics, operative outcomes, and postoperative complications were compared between the patients with and without IG by means of the chi-square test for continuous and categorical variables. Univariate analyses (chi-square test or the Kaplan–Meier method) were primarily used for selecting variables on the basis of a p value < 0.05. Significant variables and clinically effective factors were subjected to forward logistic regression analysis to determine net effect for each predictor while controlling the effects of the other factors. Odds ratios (ORs) and their 95% confidential intervals (CIs) were used to assess independent contributions of significant factors. Data were expressed as median. The cutoff value for the identified parameter (creatine kinase [CK], lactate dehydrogenase [LDH]) was determined to maximize the difference between the cases with and without IG by receiver operating characteristic (ROC) curve. The relationship between the two groups divided by the cutoff value was analyzed by chi-square test for categorical variables. Cumulative overall survival was calculated by Kaplan–Meier method, and a comparison of the survival curves was analyzed using the log-rank test. All survival times were evaluated from the operative day. Statistically significant difference was considered to be p < 0.05. All analyses were performed using the statistical software package SPSS II (version 20.0; SPSS, Inc., Chicago, IL).
Results
Patient characteristics
Table 1 shows the characteristics of the 50 consecutive patients with pancreatic body/tail carcinoma. Combined resection of the portal venous system was performed for 13 patients (26%). Of the 50 patients, 26 received preoperative therapy (14 with chemotherapy, 11 with chemoradiotherapy) and the remaining 24 patients underwent upfront surgery. Regional lymph node metastases developed in 31 patients (62%). For one patient, a minute peritoneal nodule at the mesenterium of the small intestine found in closing the abdomen proved to be an M1 lesion and was diagnosed as stage 4 disease. R0 resection was achieved in 31 patients (62%), whereas R1/2 resection was found in 19 patients (38%); at the pancreatic cut end margin in one patient, retroperitoneal dissected tissue in 18 patients. The positive margin resection rate declined from 58% in the upfront strategy period to 19% in the period of neoadjuvant therapy strategy (P = 0.005).
Surgical outcome
Surgical outcomes including the incidence of operative morbidity, mortality, and the completion of postoperative adjuvant chemotherapy are shown in Table 2. Major complications of higher than grade 3 were observed in 21 patients (42%). Mortality rate was 8%. Three (75%) reoperation cases (n = 4) were associated with ischemia-related complications (total gastrectomy, n = 2; cholecystectomy, n = 1), and the other one (25%) was performed due to the impossibility of hemostasis with interventional radiology (IVR), each reflecting DP-CAR-specific issue complications. Twenty-eight (56%) patients completed the planned adjuvant chemotherapy of more than 60% dose intensity (Table 2).
Operative mortality
Regarding operative mortality, the first patient died of rapidly progressed peritoneal dissemination from residual cancer on day 51. The second patient died of uncontrollable hemorrhage from the portal vein without pancreatic fistula on day 28. The third patient died of multiple organ failure from clinically relevant pancreatic fistula which leads to post-pancreatectomy hemorrhage from the pseudoaneurysm located on the CHA stump on day 50. The last patient died of severe cardiac failure following acute myocardial infarction the previous day, 11 days postoperatively.
Ischemic gastropathy
Refractory gastroduodenal ulcer was seen in two patients and necrotic change of the gastric wall in three patients. Univariate analysis was also performed for the risk factors of IG (Table 3). To calculate the cutoff value of postoperative peak CK/LDH for prediction of discrimination between patients with and without IG, we employed ROC analysis in this study. The areas under the curve (AUC) for cutoff value were 0.893 (95% confidential interval = 0.779–1.000) for CK and 0.899 (95% confidential interval = 0.724–1.000) for LDH, respectively. The determined cutoff values to discriminate between patients with and without IG were 1005 IU/L for CK and 494 IU/L for LDH, respectively. Although LGA resection (P = 0.046), combined left IPA and LGA resection (P = 0.012), and CK ≥ cutoff value of IG (P = 0.025) were significant risk factors for IG, these variables were not in the equation logistic regression in the multivariate analysis because all these factors were positive in patients who presented IG. Therefore, multivariate logistic regression analysis could not be demonstrated in the study.
Survival
Median follow-up time was 16 (0–125) months. The estimated 1-, 2-, and 5-year survival rates were 66, 36, and 8%, respectively, and estimated median survival time (MST) was 16 months in all patients, including four cases of mortality. Estimated recurrence-free survival time (RFS) was 8 (2–122) months. Regarding comparison of estimated overall survival (OS) and RFS of conventional DP-CAR and modified DP-CAR, there was no significant difference between the two groups (P = 0.101 and P = 0.827, respectively, log-rank test). According to the local recurrence rate excluding the mortality cases, there was no significant difference between the two groups (P = 0.182). Table 4 shows the univariate and multivariate logistic regression analysis of prognostic factors. Among these clinicopathologic prognostic factors, independent predictors of prognosis even after control for the other variables were IG (OR, 5.997; 95% CI, 1.543–23.309; P = 0.010), the completion of planned adjuvant chemotherapy (OR, 0.282; 95% CI, 0.121–0.654; P = 0.003), operative time (OR, 2.261; 95% CI, 1.084–4.714; P = 0.030), and age (OR, 2.212; 95% CI, 1.081–4.524; P = 0.030). All patients with IG had tumor invasion to both of LGA and left IPA, required longer median operative time of 471 min (233–846), and failed to complete adjuvant chemotherapy. In addition, the estimated overall survival rate in patients with IG (n = 5) was lower than that of the other patients (n = 45) (P < 0.001, log-rank test) (Fig. 4).
Postoperative nutritional status
There were no differences in preoperative nutritional data between patients who underwent conventional DP-CAR and modified DP-CAR, including the values of TP (7.20 ± 0.54 vs 7.18 ± 0.64 g/dl, P = 0.899), Alb (4.24 ± 0.25 vs 4.21 ± 0.41 g/dl, P = 0.791), and TC (177.3 ± 23.9 vs 187.5 ± 37.0 mg/dl, P = 0.319). Postoperative values were significantly higher in patients who underwent modified DP-CAR than those with conventional DP-CAR in TP (7.17 ± 0.56 vs 6.65 ± 0.66 g/dl, P = 0.007), Alb (4.04 ± 0.45 vs 3.43 ± 0.43 g/dl, P < 0.001), and TC (162.3 ± 34.7 vs 141.6 ± 27.2 mg/dl, P = 0.044). There were no differences, however, in body weight loss between the two groups (− 7.03 ± 3.8 vs − 5.40 ± 3.0 kg, P = 0.127).
Discussion
In the present study, we investigated the risk factors for IG and the prognostic factors for survival by analyzing clinicopathological data including ischemic complications in patients who underwent DP-CAR. Both left IPA and LGA resection and CK ≥ cutoff value of IG were significant risk factors for IG, and IG remained an independent prognostic predictor of the mortality or early recurrence. This result might be interpreted as the significance of LGA reconstruction especially in the cases with resection of combined left IPA and LGA after DP-CAR [29, 30], and an elevated CK value ≥ 1005 IU/L was associated with IG. CK is an abundantly present enzyme in skeletal, cardiac, and smooth muscles. If muscle necrosis occurs in an organ, CK appears in the bloodstream and serum concentration level is elevated. The predictive ability of CK is controversial for intestinal ischemia [31, 32], but the significance of peak CK level for organ necrosis depends on the type of organ [33] or the muscle mass volume of each organ. CK was revealed as an independent risk factor for IG in the present study, possibly reflecting more muscle mass in the stomach than in the intestine.
Next, we investigated the nutritional data in two DP-CAR groups. There were no differences in loss of body weight between the two groups, but they have better nutrition in patients who underwent modified DP-CAR than those who underwent conventional DP-CAR. Taken together, modified DP-CAR could have indirectly improved the completion rate of adjuvant therapy by decreasing the incidence of IG and preventing postoperative nutritional deterioration. Currently, we perform LGA reconstruction by middle colic artery-left gastric artery (MCA-LGA) bypass after conventional DP-CAR [30], and a direct bypass from the aorta to the LGA could be another reconstruction option.
On investigation of prognostic risk factors, completion of planned adjuvant chemotherapy remained a significant predictor of favorable prognosis. This might be a universal result as similarly demonstrated in large scale prospective studies [2]. In this context, we had expected that preserving the LGA in modified DP-CAR directly improved survival time by decreasing the incidence of IG. There was no significant survival benefit in modified DP-CAR, however, compared with conventional DP-CAR. It could be due to the oncologic characteristic of tumor-involving arteries tending to recur systemically and rapidly even after radical resection.
In contrast, the incidence of morbidity and mortality was high in patients who underwent DP-CAR compared with several other series [12, 34] and impacting on the overall survival rate. All morbidity requiring reoperation was directly caused by DP-CAR-specific complications, and most mortality indirectly associated with intra-abdominal hemorrhage from the DP-CAR-specific arterial stump, which is extremely difficult to rescue by IVR. Thus, DP-CAR is still a developing surgery, which should be modified for safely against ischemia-related complications, and risk factors for post-pancreatectomy hemorrhages such as pancreatic fistula should be reduced. Contrary to previous reports, indications for DP-CAR remain controversial with regard to the curability, survival benefit, and high mortality rate. In this study, pathologically positive invasion of CA was only 12%. It remains debatable whether DP-CAR was really beneficial for all tumors involving CA. However, the microscopically positive margins were detected more frequently in patients with tumors situated near the origin of SA in previous study [9]. We therefore believe that DP-CAR is one surgical option to obtain R0 resection for the clinical T4 pancreatic body tumors. In the landmark era of a safer Appleby operation and introduction of stronger regimens of chemotherapy for borderline resectable or locally advanced pancreatic carcinoma [3, 35], pancreatic surgeons will return to this procedure again as a radical pancreatectomy. The procedure may be justified in strictly selected patients owing to the potential survival benefits as there will be increased chances to evaluate indication of DP-CAR.
Our results may be limited by being from a single institution and the retrospective nature of the study. More importantly, the outcome occurs over a very long period of time, and the patients and their treatments, both surgical and chemotherapeutic, evolve over time. The high degree of well-moderate differentiated cancer is typically reported in Asia but comparatively less common in other populations. This may have an effect on the reported response of chemotherapy [36, 37].
In conclusion, the poorer prognosis in patients who undergo DP-CAR may be related to more advanced tumors. Combined left IPA and LGA resection was a significant risk factor for IG. Among clinicopathologic prognostic factors, IG remains an independent predictor of prognosis in patients who undergo DP-CAR.
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian L (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921
Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, Rawcliffe CL, Bassi C, Stocken DD, Cunningham D, O'Reilly D, Goldstein D, Robinson BA, Karapetis C, Scarfe A, Lacaine F, Sand J, Izbicki JR, Mayerle J, Dervenis C, Oláh A, Butturini G, Lind PA, Middleton MR, Anthoney A, Sumpter K, Carter R, Büchler MW (2014) Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 32:504–512
Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, Truty M, Kindler H, Lowy AM, Bekaii-Saab T, Philip P, Talamonti M, Cardin D, LoConte N, Shen P, Hoffman JP, Venook AP (2016) Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg 151:e161137
Fortner JG, Kim DK, Cubilla A, Turnbull A, Pahnke LD, Shils ME (1977) Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg 186:42–50
Appleby LH (1953) The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer 6:704–707
Kondo S, Katoh H, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T (2003) Results of radical distal pancreatectomy with en bloc resection of the celiac artery for locally advanced cancer of the pancreatic body. Langenbeck's Arch Surg 388:101–106
Hishinuma S, Ogata Y, Tomikawa M, Ozawa I (2007) Stomach-preserving distal pancreatectomy with combined resection of the celiac artery: radical procedure for locally advanced cancer of the pancreatic body. J Gastrointest Surg 11:743–749
Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K (2007) Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 246:46–51
Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H (2013) Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection? Surgery 153:365–372
Kawai M, Hirono S, Okada K, Sho M, Nakajima Y, Eguchi H, Nagano H, Ikoma H, Morimura R, Takeda Y, Nakahira S, Suzumura K, Fujimoto J, Yamaue H (2016) Randomized controlled trial of pancreaticojejunostomy versus stapler closure of the pancreatic stump during distal pancreatectomy to reduce pancreatic fistula. Ann Surg 264:180–187
Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H (2014) Isolated Roux-en-Y anastomosis of the pancreatic stump in a duct-to-mucosa fashion in patients with distal pancreatectomy with en-bloc celiac axis resection. J Hepatobiliary Pancreat Sci 21:193–198
Klompmaker S, de Rooij T, Korteweg JJ, van Dieren S, van Lienden KP, van Gulik TM, Busch OR, Besselink MG (2016) Systematic review of outcomes after distal pancreatectomy with coeliac axis resection for locally advanced pancreatic cancer. Br J Surg 103:941–949
Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H (2014) Preservation of the left gastric artery on the basis of anatomical features in patients undergoing distal pancreatectomy with celiac axis en-bloc resection (DP-CAR). World J Surg 38:2980–2985
Sobin LH, Gospodarowicz MK, Witterkind C (2009) TNM classification of malignant tumors, 7th edn. Wiley, New York
Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS (2009) Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 11:18–24
National Comprehensive Cancer Network. NCCN practice guidelines for pancreatic cancer, version 2. 2015 Available at: http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Accessed 15 Jan 2016
Abo D, Hasegawa Y, Sakuhara Y, Terae S, Shimizu T, Tha KK, Tanaka E, Hirano S, Kondo S, Shirato H (2012) Feasibility of a dual microcatheter-dual interlocking detachable coil technique in preoperative embolization in preparation for distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer. J Hepatobiliary Pancreat Sci 19:431–437
Hirono S, Kawai M, Okada KI, Miyazawa M, Shimizu A, Kitahata Y, Ueno M, Yamaue H (2016) Treatment strategy for borderline resectable pancreatic cancer with radiographic artery involvement. Pancreas 45:1438–1446
Okada K, Kawai M, Hirono S, Satoi S, Yanagimoto H, Ioka T, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H (2016) Impact of treatment duration of neoadjuvant FIRINOX in patients with borderline resectable pancreatic cancer: a pilot trial. Cancer Chemother Pharmacol 78:719–726
Okada KI, Hirono S, Kawai M, Miyazawa M, Shimizu A, Kitahata Y, Ueno M, Hayami S, Yamaue H (2017) Phase I study of Nab-paclitaxel plus gemcitabine as neoadjuvant therapy for borderline resectable pancreatic cancer. Anticancer Res 37:853–858
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Yamaue H, Satoi S, Kanbe T, Miyazawa M, Tani M, Kawai M, Hirono S, Okada K, Yanagimoto H, Kwon AH, Mukouyama T, Tsunoda H, Chijiiwa K, Ohuchida J, Kato J, Ueda K, Yamaguchi T, Egawa S, Hayashi K, Shirasaka T (2014) Phase II clinical study of alternate-day oral therapy with S-1 as first-line chemotherapy for locally advanced and metastatic pancreatic cancer. Cancer Chemother Pharmacol 73:97–102
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y, JASPAC 01 Study Group (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Marco-Clement I, Martinez-Barco A, Ahumada N, Simon C, Valderrama JM, Sanudo J, Arrazola J (2016) Anatomical variations of the celiac trunk: cadaveric and radiological study. Surg Radiol Anat 38:501–510
Aslaner R, Pekcevik Y, Sahin H, Toka O (2017) Variations in the origin of inferior phrenic arteries and their relationship to celiac axis variations on CT angiography. Korean J Radiol 18:336–344
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic Surgery (ISGPS) (2017) The 2016 update of the International Dtudy Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25
Sato T, Saiura A, Inoue Y, Takahashi Y, Arita J, Takemura N (2016) Distal pancreatectomy with en bloc resection of the celiac axis with preservation or reconstruction of the left gastric artery in patients with pancreatic body cancer. World J Surg 40:2245–2253
Okada KI, Hirono S, Kawai M, Hayami S, Asamura S, Wada Y, Ueno M, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H (2017) Left gastric artery reconstruction after distal pancreatectomy with celiac axis en-bloc resection: how we do it. Gastrointest Tumors 4:28–35
van der Voort PH, Westra B, Wester JP, Bosman RJ, van Stijn I, Haagen IA, Loupatty FJ, Rijkenberg S (2014) Can serum L-lactate, D-lactate, creatine kinase and I-FABP be used as diagnostic markers in critically ill patients suspected for bowel ischemia. BMC Anesthesiol 14:111
Matsumoto S, Sekine K, Funaoka H, Yamazaki M, Shimizu M, Hayashida K, Kitano M (2014) Diagnostic performance of plasma biomarkers in patients with acute intestinal ischaemia. Br J Surg 101:232–238
Palmerini T, Mehran R, Dangas G, Nikolsky E, Witzenbichler B, Guagliumi G, Dudek D, Genereux P, Caixeta A, Rabbani L, Weisz G, Parise H, Fahy M, Xu K, Brodie B, Lansky A, Stone GW (2011) Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the Harmonizing Outcome with Revascularization and Stent in Acute Myocardial Infarction trial. Circulation 123:2829–2837
Nakamura T, Hirano S, Noji T, Asano T, Okamura K, Tsuchikawa T, Murakami S, Kurashima Y, Ebihara Y, Nakanishi Y, Tanaka K, Shichinohe T (2016) Distal pancreatectomy with en bloc celiac Axis resection (modified Appleby procedure) for locally advanced pancreatic body cancer: a single-center review of 80 consecutive patients. Ann Surg Oncol 23(Suppl 5):969–975
Sadot E, Doussot A, O'Reilly EM, Lowery MA, Goodman KA, Do RK, Tang LH, Gönen M, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Allen PJ (2015) FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol 22:3512–3521
Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M, Bassi C, Pederzoli P, Falconi M (2012) Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery 152:S112–S119
Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F, Werner J, Büchler MW (2015) Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 261:961–969
Acknowledgements
We would like to thank the Clinical Research Center, Wakayama Medical University, for proofreading and editing the manuscript.
Author information
Authors and Affiliations
Contributions
KO drafted the manuscript. MK and SH helped to draft the manuscript. MM, YK, MU, and SH supported acquisition of data. HY helped in the revision of the article. TS supervised statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Okada, Ki., Kawai, M., Hirono, S. et al. Ischemic gastropathy after distal pancreatectomy with en bloc celiac axis resection for pancreatic body cancer. Langenbecks Arch Surg 403, 561–571 (2018). https://doi.org/10.1007/s00423-018-1692-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1692-z