Abstract
Purpose
The purpose of this study was to compare the feasibility and outcomes of two-stage hepatectomy in patients with or without accompanying digestive surgery.
Methods
We analyzed prospectively data from 56 patients with colorectal liver metastases undergoing two-stage hepatectomy between 1995 and 2009. Patients undergoing associated digestive resection (group I, n = 32) were compared with patients without associated digestive surgery (group II, n = 17).
Results
The feasibility rate was 87.5 % (49 patients). Neither the type and extent of hepatectomy nor the type of chemotherapy administered differed between the two groups. The median interval between hepatectomies was 1.79 and 2.07 months for groups I and II, respectively (not significant). One patient (group I) died of liver failure after the second hepatectomy. Postoperative morbidity rates were comparable: 37.5 % (group I) vs. 35.5 % (group II) after the first hepatectomy and 46.9 % (group I) vs. 52.9 % (group II) after the second hepatectomy. The median hospital stay after the first hepatectomy was longer in group I (13.5 days) than in group II (10 days) (P < 0.01). Median follow-up was 54 months. The median overall survival (OS) was 45.8 months, and 3- and 5-year OS were 58 and 31 %, respectively. Median OS was longer for group II (58 months) than for group I (34 months) (P = 0.048).
Conclusions
Digestive tract resection associated with two-stage hepatectomy does not increase postoperative mortality or morbidity nor does it lead to delay in chemotherapy or a reduction in cycles administered. The need for digestive tract surgery should not affect the surgical management of two-stage hepatectomy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resection is the only curative treatment for colorectal liver metastases: 5-year overall survival (OS) rates of up to 67 % have been reported for selected patients [1]. However, resection of liver metastases at the time of diagnosis is possible in only 15–25 % of patients [2]. The main reason for non-resectability is that the number, size and/or location of metastases means that—if all were to be resected in the same procedure—there would be insufficient future remnant liver (FRL). The technique of two-stage hepatectomy was first published in 2000 by Adam et al. to increase the proportion of patients presenting with bilobar and multiple liver metastases who were amenable to resection. The two-stage procedure results in morbidity and survival rates that are comparable with those of one-stage hepatectomy [3, 4]. This approach has been further developed in association with preoperative chemotherapy and portal vein embolization, achieving a 5-year overall survival rate of 42 % [1, 5].
The wisdom of performing a digestive procedure at the same time as hepatectomy is still debated [6]. This reluctance is explained by the increased risk of anastomotic leakage caused by intestinal oedema secondary to a Pringle manoeuvre [7]. Recently, combining resection of the primary tumour with first-stage hepatectomy has been shown to be feasible in a two-stage hepatectomy strategy for bilobar synchronous liver metastases [8]. However, the effect of undertaking a digestive procedure, defined by at least one anastomosis, during a two-stage hepatectomy has never been investigated in a comparative study. It is possible that performing a digestive procedure during a two-stage hepatectomy could modify patient management, limit the ability to administer chemotherapy and increase surgical morbidity. The aim of this study was to evaluate perioperative and postoperative outcomes when digestive surgery accompanies the first and/or second stage of a two-stage hepatectomy.
Methods
Patient selection
Between July 1995 and November 2009, 1,042 patients underwent liver resection for colorectal liver metastases at the Department of Surgery of the Léon Bérard Cancer Centre in Lyon, France. All patients with otherwise unresectable liver metastases identified as suitable for two-stage hepatectomy and having an Eastern Cooperative Oncology Group performance status of 0 to 1 were selected for this study. Initial unresectability was defined as the inability to treat all metastases in a single procedure while leaving sufficient FRL. The main causes of unresectability were vascular and biliary involvement, multinodular diseases and/or large tumour size.
Preoperative evaluation
All patients were evaluated preoperatively and after every 4 to 6 cycles of chemotherapy using computed tomography (CT) scans of the chest and abdomen. Magnetic resonance imaging was not routinely employed unless diagnostic doubts persisting after the CT scan required better characterization of the liver metastases. Positron emission tomography–CT has been used since 2005 to complete preoperative evaluation and to exclude extrahepatic disease not detected by CT scan.
Chemotherapy
Chemotherapy was administered before, between and after the two hepatectomies. Regimens included folinic acid and fluorouracil (5FU) combined with oxaliplatin (FOLFOX), irinotecan (FOLFIRI) or both drugs (FOLFIRINOX). Bevacizumab and cetuximab have been used since 2006. Patients with rectal cancer received neoadjuvant chemo-radiotherapy with 45 Gy and 5FU or FOLFOX regimens.
Surgery and postoperative care
The type and extent of liver resections were classified according to the Brisbane 2000 system [9]. Operations were performed through a right J-shaped laparotomy or through a midline incision for left-sided resections and when there was a right-sided ostomy. Intraoperative liver ultrasonography was employed to detect occult liver tumours and to better evaluate the relationship between liver tumours and intrahepatic vascular structures. Extrahepatic vascular control and sectioning of the hepatic and portal veins and of the hepatic artery were performed whenever possible, and the hepatic bile duct was divided inside the liver during parenchyma transection. Transection of the liver parenchyma was performed using the crushing Kelly clamp technique, clips and water bipolar forceps. Intermittent clamping of the liver pedicle for 15–20 min, interrupted for 5 min (Pringle manoeuvre), was used to reduce blood loss. The drainage was standardized with three perihepatic suction drains for hemihepatectomy and one for wedge resection. Intraoperative cryosurgery ablation accompanied resection in patients with residual unresectable liver metastases. Unresectability was defined as proximity of the lesion to major vascular and biliary structures, which would have required resection of the whole FRL (thus making second-stage hepatectomy impossible), or by intraoperative detection of unresectable tumour deposits within the abdomen. Right portal vein embolization (PVE) was undertaken if the volume of the FRL (Segment II + III or Segment II + III + IV, ±Segment I) was less than 30 % of the whole liver volume. Right portal vein occlusion was performed intraoperatively during the first hepatectomy using ultrasound-guided n-butyl-2-cyanoacrylate glue injection (Histoacryl®, Braun Medical, Aesculap AG and Co., Tuttlingen, Germany) mixed with iodized oil (Lipiodol®, André Guerbet, Aulnay-sous-Bois, France). The right portal vein trunk was then stapled and sectioned. When the FRL consisted of the S I + II + III, the glissonian branches of the S IVb were ligated at their origin during first-stage hepatectomy. The digestive procedures were performed at the end of liver surgery. The liver was isolated in the best way possible from the rest of the abdomen using gauze swabs to minimize contamination of the cut liver surface by bowel contents. All anastomoses were performed manually except for low colorectal anastomoses, which were performed using a double stapling technique.
Follow-up
Postoperative morbidity and mortality were defined as events occurring within 90 days of surgery and were assessed according to the classification of Dindo et al. [10]. The first outpatient visit was scheduled 1 month after the operation. Follow-up consisted of a physical examination and measurement of serum carcinoembryonic antigen (CEA) and CA 19-9 levels every 3 months. CT scans of the chest and abdomen were performed at 3 months and then every 6 months. The duration of follow-up was defined as the time from diagnosis to the last outpatient visit or death.
Statistical analysis
The study population consisted of patients in whom two-stage hepatectomy was completed. These patients were divided into two groups: those who had undergone additional digestive tract surgery during the first or second hepatectomy (group I) and those who had not (group II). Demographic and tumour characteristics of the two groups and data concerning surgery were compared using Fisher's exact test for categorical variables and a non-parametric Wilcoxon test for continuous variables. OS was calculated from the date metastases were diagnosed to the date of death from any cause or date of the last follow-up (censored observation). Progression-free survival (PFS) was measured from the date metastases were diagnosed to the time of disease progression or death, or was censored at the last follow-up. Survival estimates were calculated using the Kaplan–Meier method. Differences in survival between groups were assessed by log-rank test. Median follow-up was calculated using a reverse Kaplan–Meier estimate. All analyses were performed with SAS® software version 9.1.
Results
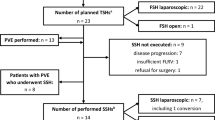
During the study period, 63 patients were candidates for two-stage hepatectomy. Of this number, seven were excluded because they did not in fact undergo a first hepatectomy, having only right PVE. Of 56 patients having the first hepatectomy, 7 did not undergo the planned second-stage hepatectomy because of progression of liver metastases (n = 5), the intraoperative discovery of diffuse peritoneal carcinomatosis during the second operation (n = 1) or development of portal vein thrombosis (n = 1). The 49 patients in whom two-stage hepatectomy was actually undertaken represent a feasibility rate of 87.5 %. Of these patients, 32 had digestive tract surgery during the first and/or second hepatectomy (group I) and 17 patients did not have such surgery (group II).
Patient characteristics
Patient characteristics, details of the location of the primary tumour and the nature of their metastatic disease are shown in Table 1. Three patients presented with extrahepatic disease: one with localized peritoneal carcinomatosis (group II), one with left iliac nodal metastases (group II) and one with left adrenal metastases (group I). In all cases, this extrahepatic disease was resected during first-stage hepatectomy.
Chemotherapy
Details of chemotherapy regimens and cycles administered before the first hepatectomy, between procedures and after the second hepatectomy are shown in Table 2. All but two patients from group I, both entered during the early period of the study, received preoperative chemotherapy. A median number of 9 cycles (range 2–27) were administered, with no difference between the groups. The median number of cycles given between the two hepatectomies was also similar.
Perioperative results
The median interval between the two hepatectomies was 1.79 months (range 0.92–8.97) and 2.07 months (range 1.05–10.58) for groups I and II, respectively. The rate of major hepatectomy during the second stage of the procedure was 91.8 % (45/49 patients) overall, with no difference between the two groups (Table 3). The rate of cryosurgery was higher during first-stage hepatectomy (75.5 %, 37/49 patients) than during the second procedure, but there was again no difference between groups I and II. During the first stage, the median duration of the operation was longer in group I, who had associated digestive surgery, than in group II, who did not have such surgery (330 (160–440) vs. 253 (175–420) min; P = 0.019). Total pedicle occlusion was more often used in group II (10/17 (52.4 %) vs. 8/32 (25.0 %); P = 0.030). Fourteen patients underwent additional surgical procedures, their frequency being similar in the two groups: seven patients had diaphragmatic resections, three had adrenalectomies, two had inferior vena cava partial resection, one had resection of isolated peritoneal metastases and one had unilateral pelvic lymph node dissection. The other parameters evaluated did not differ between the two groups (Table 3).
Digestive surgery procedures
The digestive procedures undertaken in the 32 patients in group I are listed in Table 4. Five had digestive surgery at both hepatectomies, while three had digestive surgery only during the second stage. In 8 of 32 patients, (4 during first-stage hepatectomy), digestive surgery did not consist of primary tumour removal.
Postoperative outcomes
Overall morbidity rates were 36.7 and 49 % for the first- and the second-stage hepatectomy, respectively, and did not differ significantly between groups (Table 3). Postoperative complications are listed in Table 5. One patient in group I (3 %) died of postoperative liver failure 27 days after the second-stage hepatectomy. One patient in group II (2 %) required reoperation for control of haemorrhage from the cut surface of the liver on the day of operation. Other complications were managed non-surgically. Overall median hospital stay was 12 days (range 6–28) after the first-stage hepatectomy and again 12 days (range 7–42) after the second stage. The median hospital stay after the first hepatectomy was longer in group I (13.5 days) than in group II (10 days) (P < 0.01).
Survival and recurrence
Median follow-up was 54 months (range 12–98). OS rates at 3 and 5 years for patients who completed the two-stage hepatectomy (n = 49) were 58 and 31 %, respectively. Median OS was 45.8 months (95 % confidence interval (CI) 28.4–58.2). Median OS was longer for group II patients (58 months) than for those in group I (34 months) (P = 0.048; Fig. 1a). Three- and five-year OS rates were 54 and 23 % in group I and 71 and 44 % in group II, respectively. Median PFS for all patients included in the study was 17.8 months (95 % CI 16.3–20.1; Fig. 1b). One- and three-year PFS rates were 84 and 6.5 % in group I and 94 and 12 % in group II, respectively, with no significant differences between the two groups.
Discussion
We compared patients who had digestive surgery in the course of a two-stage hepatectomy strategy with those who did not have such additional surgery. The endpoints of the study were postoperative morbidity and survival and patient management in terms of chemotherapy administration and the interval between the two hepatectomies.
We defined a digestive procedure as any surgery of the digestive tract involving an anastomosis. All but four digestive procedures (listed in Table 4) during first-stage hepatectomy were primary colorectal tumour resection, and several studies have reported the outcome of resecting the primary tumour and liver metastases at the same time [6]. However, everyday surgical practice may require procedures other than primary colorectal tumour resection. Examples include bowel resections, Hartmann reversal and ostomy closure. In the two-stage hepatectomy strategy, the need to associate a digestive procedure with liver resection can arise for several reasons: patients referred after being operated on for their primary tumour at other institutions may present with an ileostomy or colostomy and patients seen in an emergency setting for bowel occlusion or perforation. Furthermore, the intraoperative finding of localized peritoneal carcinomatosis or the presence of tight adhesions may require a bowel resection at the same time as that of the liver, and transverse colectomy can be required because of major adhesions to a previous site of focal liver destruction, as reported in our series. The potential impact of these digestive procedures on outcome is as relevant as that of primary tumour resection: postoperative morbidity of 48.5 % and mortality of 1.7 % have been reported following Hartmann's reversal [11], and studies have reported an overall morbidity rate between 11 and 37 %, with mortality of up to 3.3 %, following closure of a defunctioning stoma [12, 13].
Two-stage hepatectomy remains a challenging procedure with feasibility rates of 70–87 % reported [8, 14]. In our study, the feasibility rate was relatively high, with 87.5 % of patients completing the two stages. The major reason for ineligibility for the second procedure was disease progression. A possible bias is that patients who were initially candidates for two-stage hepatectomy but who did not complete the second stage may have been missed when searching the database. Two-stage hepatectomy usually requires bilateral, complex liver procedures involving numerous liver segments, with 90-day mortality rates of up to 6 % reported [15]. We hypothesized that combining it with an additional digestive procedure might increase postoperative morbidity and potentially delay second hepatectomy and compromise chemotherapy. Even though the best strategy for dealing with synchronous colorectal liver metastases is debated [6], the safety of simultaneous liver and colorectal resection has been demonstrated [16, 17]. This approach avoids a second laparotomy and reduces overall complication rate without affecting operative mortality. It permits faster patient recovery and the possibility of earlier postoperative chemotherapy [17]. Recently, Karoui et al. suggested combining first-stage hepatectomy with resection of the primary colorectal tumour. In the two-stage hepatectomy setting, this association might reduce the number of procedures and optimize chemotherapy administration [8]. Even if it has been demonstrated that hepatectomies can be performed at the same time as colorectal surgery with a total morbidity lower than that in staged resection, the morbidity in simultaneous resection is higher than that after a single procedure and can interfere with the sequencing of two-stage hepatectomy. A successful outcome after the first stage is essential if the patient is to proceed to the second stage and/or chemotherapy. Another factor to consider is that difficulties related to abdominal and perihepatic adhesions arising from the initial procedure may complicate the second hepatectomy.
In our study, an associated digestive procedure did not worsen overall morbidity and mortality rates. The mortality of 2 % was due to liver failure after a right hepatectomy, worsened by persistent sepsis. Postoperative morbidity rates following the first and the second hepatectomy were not influenced by adding a digestive procedure to the liver resection. The mean interval between the two stages was similar in the two groups, as was the number of cycles of chemotherapy given between the two stages.
Synchronous liver and colorectal resections are usually performed in cases of right-sided primary tumour associated with small and limited liver metastases. Digestive surgery can be performed at the same time as major hepatectomy [7]. During a two-stage hepatectomy, the first stage often consists of a minor hepatectomy in association with a digestive procedure. We found that a digestive procedure can safely be performed at the second stage even in conjunction with a major hepatectomy. Postoperative morbidity could be determined by the complexity of the digestive procedure, rather than of the hepatectomy. Surgery of the lower rectum, for example, is a major digestive procedure with a reported morbidity of up to 40 % [18].
Management of metastatic rectal cancer could also be integrated into two-stage hepatectomy. In our study, rectal surgery with preoperative chemo-radiotherapy was used in approaching a third of group I patients. When a defunctioning stoma is required, closure can safely be performed during the second-stage hepatectomy. The mean interval between the two stages was 2 months, which is the same as the interval for stoma closure in rectal surgery.
The survival outcomes in our study were consistent with those reported by other authors [1, 5]. Such results may be greatly influenced by selection of patients on the basis of tumour response to chemotherapy. Furthermore, the definition of unresectability differs between surgical teams. Recently, very favourable survival outcomes (64 % 5-year OS and 20 % 5-year PFS) have been reported in patients who responded to preoperative chemotherapy and completed two-stage hepatectomy [15]. Patients in our group II showed better median OS when compared with patients in group I, even though associated digestive surgery did not modify the interval between the two stages of hepatectomy or delay chemotherapy.
Patient characteristics in the two groups were generally similar. We cannot be sure why this difference in survival occurred. However, the poorer median OS in group I might be explained by the presence of a higher proportion of patients with node-positive primary tumours, synchronous liver metastases, a high level of preoperative CEA and larger metastases. Even though their effect may have been weak in our study, these factors have been reported as predictors of adverse outcome following liver resection for colorectal liver metastases [19]. We also considered the possible role of selection bias. Early primary tumour resection may have led to selection of better prognosis patients as candidates for two-stage hepatectomy. This has plausibility since the majority of these patients (in group II) were initially treated at another institution and only those with a good response are referred to our centre.
In conclusion, within the limits inherent in the small size of our population, it seems that combining a digestive procedure with two-stage hepatectomy did not worsen patients' perioperative mortality or morbidity when compared with patients with similar preoperative characteristics who underwent two-stage hepatectomy without associated digestive surgery. The slightly worse median OS in patients having digestive surgery could derive at least in part from the characteristics of their disease, rather than from the additional surgery. In patients with unresectable liver metastases who are candidates for two-stage hepatectomy, performing a digestive procedure during the first and/or second hepatectomy is safe and may optimize patient management.
References
Wicherts DA, Miller R, de Haas RJ et al (2008) Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 248:994–1005
Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M (2006) Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 94:982–999
Chun YS, Vauthey JN, Ribero D et al (2007) Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg 11:1498–1504, discussion 504-5
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H (2000) Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 232:777–785
Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P (2004) A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 240:1037–1049, discussion 49-51
Hillingso JG, Wille-Jorgensen P (2009) Staged or simultaneous resection of synchronous liver metastases from colorectal cancer—a systematic review. Color Dis 11:3–10
Capussotti L, Ferrero A, Vigano L, Ribero D, Lo Tesoriere R, Polastri R (2007) Major liver resections synchronous with colorectal surgery. Ann Surg Oncol 14:195–201
Karoui M, Vigano L, Goyer P et al (2010) Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg 97:1354–1362
Pang YY (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2:333–339, HPB (Oxford) 2002; 4:99
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Aydin HN, Remzi FH, Tekkis PP, Fazio VW (2005) Hartmann's reversal is associated with high postoperative adverse events. Dis Colon Rectum 48:2117–2126
Kaiser AM, Israelit S, Klaristenfeld D, Selvindoss P, Vukasin P, Ault G, Beart RW (2008) Morbidity of ostomy takedown. J Gastrointest Surg 12:437–441
Akiyoshi T, Fujimoto Y, Konishi T, Kuroyanagi H, Ueno M, Oya M, Yamaguchi T (2010) Complications of loop ileostomy closure in patients with rectal tumor. World J Surg 34:1937–1942
Tsim N, Healey AJ, Frampton AE et al (2011) Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol 18:1939–1946
Brouquet A, Abdalla EK, Kopetz S et al (2011) High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 29:1083–1090
Martin RC 2nd, Augenstein V, Reuter NP, Scoggins CR, McMasters KM (2009) Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg 208:842–850, discussion 50-2
Martin R, Paty P, Fong Y et al (2003) Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 197:233–241, discussion 41-2
Breukink S, Pierie J, Wiggers T (2006) Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 4:CD005200
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318, discussion 18-21
Acknowledgments
We thank Thérèse Gargi for data management and Rob Stepney (medical writer, Charlbury, UK) for editing the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stella, M., Dupre, A., Chabaud, S. et al. A comparative study of patients with and without associated digestive surgery in a two-stage hepatectomy setting. Langenbecks Arch Surg 397, 1289–1296 (2012). https://doi.org/10.1007/s00423-012-1002-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-1002-0