Abstract
Background
The spontaneous perforation of gastric cancer is a rare fatal complication, occurring in 1% of patients with gastric cancer, and it has a wide hospital mortality range (0–82%). In addition, it has been reported that about 10–16% of all gastric perforations are caused by gastric carcinoma. The aim of this study is to evaluate the gastric perforations and improve an alternative pathway for the management of this disorder when a pathologist is not available.
Material and methods
We reviewed the medical records of 513 patients who had undergone surgical treatment for gastric perforation due to gastric ulcus or gastric carcinoma in two medical centers. Sixty-seven (13.06%) patients were treated for perforated gastric carcinoma. Perforations due to trauma and iatrogenic causes were excluded. The clinicopathologic features of all patients were analyzed on the basis of their medical records.
Results
According to the results of our analysis, we can suggest that if a patient with gastric perforation has an age more than 60 years, an ulcus diameter (with edema) more than 6 cm, a perforation diameter more than 0.5 cm, a symptom duration of more than 20 h, and a white blood cell count less than 15.103/μL, he might have a gastric carcinoma. This system has a specificity of 98.7%, a sensitivity of 53.7%, a negative predicted value of 93.4%, and positive predicted value of 85.7%.
Conclusion
The diagnosis of malignancy is often made only on postoperative or operative frozen pathologic examination. We suggest a new pathway for the gastric perforations, if a pathologist is not available during the operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although the incidence of gastric cancer is declining, it is still one of the commonest causes of cancer deaths worldwide [1]. Surgery is the only curative option for localized disease. Despite the many published studies on elective surgical treatment, there is insufficient information on complicated gastric cancer [1]. The spontaneous perforation of gastric cancer is a rare fatal complication, occurring in 1% of patients with gastric cancer [2, 3], and it has a wide hospital mortality range (0–82%) [1]. In addition, it has been reported that about 10–16% of all gastric perforations are caused by gastric carcinoma [4].
In most instances, gastric carcinoma is not suspected as the cause of perforation prior to emergency laparotomy, and the diagnoses of malignancy are often made only on postoperative pathologic examination. It is often difficult to recognize the kind of lesion that caused gastric perforation at the time of emergency surgery, particularly when pathologic evaluation of frozen sections is not available. The treatment should aim to manage both the emergency condition of peritonitis and the oncologic technical aspects of surgery: It may be hazardous to embark on a major procedure observing the principles of radical oncologic surgery; on the other hand, a limited procedure only may jeopardize long-term survival in a patient with potentially curable gastric malignancy. To further understand the optimal management of patients with gastric perforation, we reviewed the clinicopathologic features of our patients. It is aimed to evaluate the gastric perforations and improve an alternative pathway for the management of this disorder when a pathologist is not available.
Material and method
We reviewed the medical records of 513 patients who had undergone surgical treatment for gastric perforation due to gastric ulcus or gastric carcinoma in two medical centers between August 1995 and August 2007. Sixty-seven (13.06%) patients were treated for perforated gastric carcinoma. Perforations due to trauma and iatrogenic causes were excluded. The clinicopathologic features of all patients were analyzed on the basis of their medical records (Table 1). The final diagnosis of malignancy was made by histopathologic examination, which has been recorded.
Data analysis was performed by using SPSS for Windows, version 11.5. Descriptive statistics were shown as mean ± standard deviation for numerical data and percentages for categorical ones. Comparisons of median values between groups were analyzed by Mann–Whitney U test. Nominal variables were tested by Chi-square or Fisher’s exact test, where appropriate. To define independent predictors of outcome variable (ulcus/cancer), multiple logistic regression analysis was used. Statistically significant variables according to the univariate statistics were selected as candidate variables for multiple logistic regression analysis. Odds ratio (OR) and 95% confidence intervals (CI) for each independent predictors were calculated. A p value less than 0.05 were considered statistically significant.
Results
We found the ratio of having gastric perforation (due to peptic ulcus or gastric carcinoma) is higher at male gender. Furthermore, we figured out that female patients with gastric perforation are most likely to have gastric carcinoma than male ones. If a female patient has gastric perforation, she has a 2.03-time greater possibility of having gastric cancer (95% CI = 0.72–5.71) than a male one.
We suggested that (for our cohort) possibility of being gastric carcinoma increases 4.62 times for every 10 years of increase at age (95% CI = 3.11–6.73; p < 0.001). In addition, we found that the increasing of the diameter of the ulcus (with edema) for every 1 cm and the diameter of the perforation for every 1 mm increases the possibility of being gastric cancer 1.53 times (95% CI = 1.06–2.21; p < 0.001) and 1.33 times (95% CI = 1.07–1.65; p < 0.001), respectively.
We can say that if a patient (any gender) has a perforation site at middle or upper third of the stomach, he has a 1.71 times greater risk of having gastric cancer (95% CI = 0.92–3.18). We also found that there is a statistically significant difference between peptic ulcus and gastric cancer for the duration of symptoms (p < 0.001). It seems that if the symptoms are resistible for the patient for at least 20 h, it might be a gastric carcinoma (OR = 1.08 and 95% CI = 1.03–1.13). Nevertheless, if a patient has a long duration of symptoms and his white blood cell count is unexpectedly less than 15.103/μL, then he has a high possibility of having gastric cancer (p < 0.001; Table 1). We did not find any statistically significant difference between two groups for steroid or nonsteroidal anti-inflammatory (NSAI) drugs usage and the presence of Helicobacter pylori.
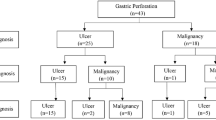
Statistically significant variables according to the univariate statistics were selected as candidate variables for multiple logistic regression analysis. According to the results of the analysis, we can suggest that if a gastric perforation patient has an age more than 60 years, an ulcus diameter (with edema) more than 6 cm, a perforation diameter more than 0.5 cm, a symptom duration of more than 20 h, and a white blood cell count less than 15.103/μL, he might have a gastric carcinoma. This system has a specificity of 98.7%, a sensitivity of 53.7%, a negative predicted value of 93.4%, and positive predicted value of 85.7% (Fig. 1).
Discussion
Preoperative diagnosis of malignancy is unusual, accounting for about 30% of cases [2, 3]; the other patients are usually accepted for acute abdomen at the emergency units where generic preoperative diagnosis of gastroduodenal perforation is made. The only preoperative feature that may guide the surgeon is the age of the patient: Perforated gastric carcinoma usually occurs in patients with a mean age of 65 years in contrast with the mean age of 51 years of the patients with perforated peptic ulcers [4, 5]. In our cohort, patients with perforated peptic ulcus were younger than these series. Even during surgery, the gastric ulcer is often difficult to be characterized as benign or malignant by the surgeon. Therefore, a biopsy and a frozen section should be performed in all gastric perforations when a pathologist is available. Histologic determination is fundamental for the surgeon to choose the type of operation and to perform it with oncologic criteria, for example considering adequate distance from the lesion and the resection margin. Malignant gastric perforation is more often a manifestation of advanced cancer with serosal invasion (55–82%) and lymph node metastasis (57–67%). Nevertheless, as confirmed by different observations [4, 6], gastric cancer can perforate at an early stage. Indeed, at the pathologic examination of specimens, the process of gastric wall perforation is sustained by infectious and ischemic factors due to tumoral neovascularization, which results in the shedding of the neoplastic tissue [4].
It is still debated whether positive peritoneal cytology has an independent prognostic impact in gastric cancer. Several studies have noted free gastric cancer cells in the peritoneum to be associated with poor prognosis [7, 8]. However, viable free cancer cells have not been demonstrated in the peritoneal cavity of patients with perforated gastric cancer, and the metastatic efficiency of gastric cancer cells possibly shedding during perforation is uncertain in the presence of the peritonitis; different studies report of long-term survivors [9]. When a curative operation can be performed, survival rates after gastric cancer perforation [2, 4] appear similar to survival rates observed in elective patients [10, 11].
The treatment of acute gastric perforation should aim to manage both the emergency condition of peritonitis and the oncologic technical aspects of surgery: It may be hazardous to embark on a major procedure observing the principles of radical oncologic surgery; on the other hand, a limited procedure only may jeopardize long-term survival in a patient with potentially curable gastric malignancy. Until now, the only preoperative feature that may guide the surgeon is the age of the patient. It is well known that if a patient has a gastric perforation, he might have a high possibility of having gastric cancer as we found in our cohort. We tried to take this a step higher. Gastric carcinoma, peptic ulcus, and peptic ulcus perforation are male-predominant disorders. However, we found that when a female has a gastric perforation, she has nearly two times greater possible risk of gastric carcinoma than a male one. It may be because of the small number of female patients. The count of white blood cell increases, in gastric perforation patients, until the perforation has been repaired or a septic process has settled. We figured out that if a patient has a long duration of symptoms and his white blood cell count is unexpectedly less than 15.103/μL, then he has a high possibility of having gastric cancer (p < 0.001).
According to the results of our analysis, we can suggest that if a patient with gastric perforation has an age more than 60 years, an ulcus diameter (with edema) more than 6 cm, a perforation diameter more than 0.5 cm, a symptom duration of more than 20 h, and a white blood cell count less than 15.103/μL, he might have a gastric carcinoma (Fig. 1). This system has a specificity of 98.7%, a sensitivity of 53.7%, a negative predicted value of 93.4%, and positive predicted value of 85.7%. However, the absolute numbers and potential bias with data that seem to be selected make you think that this algorithm is uncertain. Thus, we suggest prospective studies about this subject. Then, we think that this manuscript will be a footstep at this point.
In the last decade, laparoscopic closure of the perforation has been the first-choice treatment if the patient condition allows it. Many authors like Kirshtein et al. [12] reported that laparoscopic repair of gastroduodenal perforations is a safe alternative treatment offering certain significant advantages over open surgery. In such case, we can make the definite diagnosis by endoscopic biopsy and perform oncologic treatment for cancer patients. However, none of our patients were operated laparoscopically because of technical limitations. Thus, our study includes the ones who were treated by the conventional open surgery technique.
Another limitation of our study is the low number of gastric cancer perforation cases. In addition, we think that the reason why we found the presence of H. pylori between the two groups not statistically significant is the unavailability of H. pylori tests for all the patients. A new trial with a large number of patients may be needed to confirm the effectiveness of the decisional flowchart that we suggested. We did not come cross any study in which emergency gastric perforations were studied in this way.
Conclusion
It has been reported that about 10–16% of all gastric perforations are caused by gastric carcinoma, and because of that, treatment should aim to manage both the emergency condition of peritonitis and the oncologic technical aspects of surgery. The diagnosis of malignancy is often made only on postoperative or operative frozen pathologic examination. If a pathologist is not available during the operation, what can we do? We suggest a new pathway for gastric perforations (Fig. 1). This system has a specificity of 98.7%, a sensitivity of 53.7%, a negative predicted value of 93.4%, and positive predicted value of 85.7%. Anyway, if a pathologist is available, we prefer to take a biopsy.
References
Ozmen MM, Zulfikaroglu B, Kece C, Aslar AK, Ozalp N, Koc M (2002) Factors influencing mortality in spontaneous gastric tumour perforations. J Int Med Res 30:180–184
Adachi Y, Mori M, Maehara Y, Matsumata T, Okudaira Y, Sugimachi K (1997) Surgical results of perforated gastric carcinoma: an analysis of 155 Japanese patients. Am J Gastroenterol 92(3):516–518
Kasakura Y, Ajani JA, Fujii M, Mochizuki F, Takayama T (2002) Management of perforated gastric carcinoma: a report of 16 cases and review of world literature. Am Surg 68:434–440
Roviello F, Rossi S, Marrelli D, De Manzoni D, Pedrazzani C, Morgagni P, Corso G, Pinto E (2006) Perforated gastric carcinoma: a report of 10 cases and review of the literature. World J Surg Oncol 4:19
Lehnert T, Buhl K, Dueck M, Hinz U, Herfarth C (2000) Two-stage radical gastrectomy for perforated gastric cancer. Eur J Surg Oncol 26:780–784
Kitakado Y, Tanigawa N, Muraoka R (1997) A case report of perforated early gastric cancer. Nippon Geka Hokan 66:86–90
Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ (1996) Prognostic value of positive cytology findings from abdominal washing in patients with gastric cancer. Br J Surg 83:672–674
Wu CC, Chen JT, Chang MC, Ho WL, Chen CY, Yeh DC, Liu TJ, P’eng FK (1997) Optimal surgical strategy for potentially curable serosa-involved gastric carcinoma with intraperitoneal cancer cells. J Am Coll Surg 184:611–617
Adachi Y, Aramaki M, Shiraishi N, Shimoda K, Yasuda K, Kitano S (1998) Long-term survival after perforation of advanced gastric cancer: case report and review of the literature. Gastric Cancer 1:80–83
Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P (1999) Patient survival after D1 and D2 resections for gastric cancer: long term results of the UK MRC randomised surgical trial. Br J Cancer 79:1522–1530
Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ (1999) Extended lymph-node dissection for gastric cancer. N Engl J Med 340:908–914
Kirshtein B, Bayme M, Mayer T, Lantsberg L, Avinoach E, Mizrahi S (2005) Laparoscopic treatment of gastroduodenal perforations: comparison with conventional surgery. Surg Endosc 19:1487–1490
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ergul, E., Gozetlik, E.O. Emergency spontaneous gastric perforations: ulcus versus cancer. Langenbecks Arch Surg 394, 643–646 (2009). https://doi.org/10.1007/s00423-008-0331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0331-5