Abstract
Orthostatic testing, involving the transition from different body positions (e.g., from lying or sitting position to an upright or standing position), offers valuable insights into the autonomic nervous system (ANS) functioning and cardiovascular regulation reflected through complex adjustments in, e.g., measures of heart rate (HR) and heart rate variability (HRV). This narrative review explores the intricate physiological mechanisms underlying orthostatic stress responses and evaluates its significance for exercise science and sports practice. Into this matter, active orthostatic testing (e.g., active standing up) challenges the cardiovascular autonomic function in a different way than a passive tilt test. It is well documented that there is a transient reduction in blood pressure while standing up, leading to a reflex increase in HR and peripheral vasoconstriction. After that acute response systolic and diastolic blood pressures are usually slightly increased compared to supine lying body position. The ANS response to standing is initiated by instantaneous cardiac vagal withdrawal, followed by sympathetic activation and vagal reactivation over the first 25–30 heartbeats. Thus, HR increases immediately upon standing, peaking after 15–20 beats, and is less marked during passive tilting due to the lack of muscular activity. Standing also decreases vagally related HRV indices compared to the supine position. In overtrained endurance athletes, both parasympathetic and sympathetic activity are attenuated in supine and standing positions. Their response to standing is lower than in non-overtrained athletes, with a tendency for further decreased HRV as a sign of pronounced vagal withdrawal and, in some cases, decreased sympathetic excitability, indicating a potential overtraining state. However, as a significant main characteristic, it could be noted that additional pathophysiological conditions consist in a reduced responsiveness or counter-regulation of neural drive in ANS according to an excitatory stimulus, such as an orthostatic challenge. Hence, especially active orthostatic testing could provide additional information about HR(V) reactivity and recovery giving valuable insights into athletes' training status, fatigue levels, and adaptability to workload. Measuring while standing might also counteract the issue of parasympathetic saturation as a common phenomenon especially in well-trained endurance athletes. Data interpretation should be made within intra-individual data history in trend analysis accounting for inter-individual variations in acute responses during testing due to life and physical training stressors. Therefore, additional measures (e.g., psychometrical scales) are required to provide context for HR and HRV analysis interpretation. However, incidence of orthostatic intolerance should be evaluated on an individual level and must be taken into account when considering to implement orthostatic testing in specific subpopulations. Recommendations for standardized testing procedures and interpretation guidelines are developed with the overall aim of enhancing training and recovery strategies. Despite promising study findings in the above-mentioned applied fields, further research, thorough method comparison studies, and systematic reviews are needed to assess the overall perspective of orthostatic testing for training monitoring and fine-tuning of different populations in exercise science and training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In exercise science and practice monitoring of internal and external load markers (including resting physiology) is an essential part of the training process to address (the adaptation of) programming of specific load and recovery measures (Borresen and Lambert 2009). Successful approaches should lead to specific acute and chronic responses (adaptation), performance, and capacity improvements also ensuring a good state of overall health. While it is beyond the scope of this narrative review to provide a comprehensive overview of monitoring approaches, recently a fine-tuning approach has been proposed by Boullosa et al. (2023) to implement several valid and practical tools, instead of relying on a single parameter or tool, to improve the effectiveness of monitoring practices when added to the knowledge of an interdisciplinary expert group in sports research or practice. The choice of a combination of adequate tools and parameters for monitoring purposes within this fine-tuning approach in load and recovery management may support evidence-informed decision-making, by integrating both scientific evidence and methodological simplicity and practicality for the sport-specific conditions and underlying circumstances.
In this context, monitoring of cardiovascular autonomic nervous system (ANS) functioning and corresponding outcome measures of heart rate (HR) and HR variability (HRV) have a long tradition to complement such fine-tuning approaches (Aubert et al. 2003; Billman 2011; Buchheit 2014; Michael et al. 2017; Ernst 2017a; Lundstrom et al. 2023). HR is defined as the number of heartbeats per minute and HRV describes the beat-to-beat fluctuations of time intervals between adjacent heartbeats over a defined measurement period and reflects the dynamic end-organ response of the heart to physiologic and/or pathophysiologic perturbations, as well as environmental influences (Billman 2011; Gronwald et al. 2024). Further, HR and HRV are primarily generated by the non-linear interaction of the efferent positive chronotropic influence of the sympathetic, the negative chronotropic influence of the parasympathetic branch of the ANS, the intrinsic activity of the hearts pacemaker cells, and further internal and external non-neural factors that reflect the context-dependent psycho-neuro-endocrinological modulation of cardiovascular control according to the demands imposed on the organism (Benarroch 1993; Persson 1996; White and Raven 2014; Michael et al. 2017). This complex modulation integrates several feedforward and feedback mechanisms that act on different time scales depending on psycho-physiological demands and cardio-postural interaction (e.g., arterial and muscle-pump baroreflex, respiratory control, circulating catecholamines, muscle metabo-/mechanoreflex, and renin–angiotensin–aldosterone system) and is therefore related to the individual dynamic regulation of circulatory control of HR and arterial blood pressure.
In the subsequent sections, we will provide a short overview about HRV metrics and resting measurements including the application and potential of postural change maneuvers, known as orthostatic testing, as an autonomic challenge of interest. We will also provide an overview about the physiological background of postural change mechanisms and the application in exercise science and sports practice. The present narrative report aims to (1) describe the relevance of the cardiovascular autonomic mechanism behind orthostatic testing for sports medicine and exercise science, to (2) resume and discuss the available evidence on advantages and limitations implementing such an orthostatic testing approach for training monitoring and fine-tuning in exercise science and training applications, and to (3) give a short introduction how a standardized approach for the specific field could look like and might be further evaluated in future studies.
Heart rate variability metrics: a short overview
From a methodological point of view, the time series of successive R–R intervals (based on the detection of the R-waves in PQRST complexes of the electrocardiogram as gold standard assessment; Hurst 1998; Task Force 1996), the so-called “tachogram” builds the basis from which various metrics with different time intervals are derived (e.g., from ultra-short-term around 1 min, short-term around 5 min and longer up to long-term with 24 h depending on the context and field of application; Task Force 1996; Berntson et al. 1997; Achten and Jeukendrup 2003; Sassi et al. 2015; Sammito & Böckelmann 2015; Ernst 2017b; Shaffer and Ginsberg 2017). For that purpose, different HRV metrics of time-, frequency-, and/or non-linear domains are widely used as markers of human cardiovascular health and risk stratification (Thayer et al. 2010; Billman 2011; Shaffer et al. 2014; Billman et al. 2015; Ernst 2017a, b) or as measures of training load, exercise response and performance (Aubert et al. 2003; Hottenrott et al. 2006; Bellenger et al. 2016b; Hottenrott and Hoos 2018; Michael et al. 2017; Lundstrom et al. 2023). These metrics may capture quite different HRV components, and their interpretation is always context-sensitive and depends on the application setting (for recent reviews, e.g., Sassi et al 2015; Shaffer and Ginsberg 2017, 2020).
Time-domain indices may be expressed in original units or as the natural logarithm (Ln) of original units to provide a more normal distribution (Shaffer and Ginsberg 2017). Frequency-domain indices are calculated as original units, as percentages of total spectrum, or as normalized units and estimate the distribution of absolute or relative power into four frequency bands, that are the ultra-low frequency (ULF), very-low frequency (VLF), low-frequency (LF), and high-frequency (HF) bands with different representation of autonomic/physiologic mechanisms (Task Force 1996; Shaffer and Ginsberg 2017). Non-linear indices may allow to quantify non-linear dynamics, complexity, and rather qualitative characteristics of HR time series, since the mechanisms involved in ANS regulation probably interact in a non-linear way (Goldberger 1990; Goldberger et al. 2002; Huikuri et al. 2009; Nicolini et al. 2012; de Godoy 2016). When capturing autonomic status through monitoring purposes, most commonly applied linear time- and frequency-domain HRV metrics display rather general descriptive statistical features or the distribution of the frequency content of the signal. For example, the standard deviation of all normal-to-normal R–R intervals over a given time interval (SDNN) is a general estimate of the global variability of the time series. In addition, the amount of efferent vagal modulation can be estimated from several parasympathetic-dominated HRV metrics across different domains of analysis like root mean square of successive differences of normal-to-normal R–R intervals (RMSSD), HF power, or standard deviation 1 (SD1) from the Poincaré Plot, while metrics from different recording durations may not be used interchangeably and their physiological meaning may vary considerably (Task Force 1996; Shaffer and Ginsberg 2017; Laborde et al. 2017; Gronwald et al. 2024). Further, selected metrics can be directly related to each other (e.g., RMSSD and SD1, Ciccone et al. 2017).
Although 24-h-HRV-recordings are used to represent the “gold standard” for clinical HRV assessment (Shaffer et al. 2014), short-term and ultra-short-term measurements with pre-stabilization periods are increasingly employed and validated for specific applied settings (Shaffer et al. 2020). In addition, it should be noted that for several HRV metrics, there is still an ongoing debate about their practical relevance and optimal recording lengths as these strongly depend on the algorithm and the setting. However, for a valid use of HRV in the fields of sports medicine and exercise science, careful considerations on standardized assessment, preprocessing, analysis, and context-sensitive interpretation are mandatory (Laborde et al. 2017; Gronwald et al. 2024; see section “Practical recommendation for implementation”).
Postural change as an autonomic challenge of interest
In applied settings of sports and exercise science, HRV has been assessed under various conditions, at rest (e.g., Schmitt et al. 2006; Plews et al. 2013a, b), in supine and standing positions (e.g., Schmitt et al. 2013; da Silva et al. 2015) or seated (e.g., Plews et al. 2017), during sleep (e.g., Pichot et al. 2000; Garet et al. 2004; Nuuttila et al. 2022), during exercise (e.g., Sandercock and Brodie 2006; Michael et al. 2017; Gronwald and Hoos 2020), or during the post-exercise recovery period (e.g., Buchheit et al. 2007; Seiler et al. 2007; Stanley et al. 2013; Hug et al. 2014) using different recording lengths. Originally, resting HR and/or HRV was recorded and interpreted in a standardized relaxed situation (e.g., lying in supine position first thing in the morning after waking up or during the entire night) as part of an assessment of resting physiology less influenced by external factors (Aubert et al. 2003; Buchheit et al. 2005). For the comparability of measurement results, body position is very important, as different body positions, including supine, sitting, and standing positions have a significant influence on HR and HRV metrics (Task Force 1996; Holmes et al. 2020; Pomeranz et al. 1985; Hnatkova et al. 2019). For example, the highest global HRV is usually measured in a supine position, while HRV decreases in the sitting position, and further in the standing position. However, to assess the health and physiological state of the organism, it might be more useful to look at how the global system responds to a challenge or provocation approach (Task Force 1996) such as physiological perturbations like cough, respiration, the Valsava maneuver, physical exercise, or postural change (Lipsitz et al. 1990; Freeman 2006).
Active standing up as “active stand test” is the most practical postural maneuver to challenge the cardiovascular autonomic function and has a long tradition in clinical medicine with standardized assessment and analysis for clinical practice (Freeman 2006; Freeman et al. 2011; Finucane et al. 2019). In addition, recent evidence shows that HRV profiles in supine and standing positions are associated with health and lifestyle markers and may reveal abnormalities undetected at only one of the two positions (Goncalves et al. 2015; Grosicki et al. 2022). This potentially affects measurement sensitivity according to lifestyle factors (Hynynen et al. 2011), and long-term risk stratification in healthy individuals (Carnethon et al. 2002; McCrory et al. 2016), which may reflect dysregulation of the parasympathetic branch of the ANS aiding clinical decision-making. In addition, it has been hypothesized that measurements taken while sitting are more strongly associated with changes in fitness level than measurements taken while lying down (Rabbani et al. 2021). Thus, a sitting or standing body position possibly implies an autonomic stressor of interest, with enhanced sensitivity compared to a measurement in supine position. For example, in the case of highly trained endurance athletes (especially with high volume of low-intensity aerobic training), despite high parasympathetic activity, this cannot be meaningfully represented via supine HRV analysis. This so-called “parasympathetic saturation” is reflected in a rather unchanged or not detectable change in vagally related HRV metrics despite a decreased HR (and increased R–R intervals) (Kiviniemi et al. 2006; Plews et al. 2013b; Buchheit 2014). Here, vagally related HRV metrics reflect the magnitude of modulation in parasympathetic outflow as opposed to overall parasympathetic tone (Hedman et al. 1995).
In line with the active stand test in clinical medicine, that provides additional information, and overcomes some of the aforementioned limitations, a combination of HRV analysis during both supine and standing position or an active orthostatic test has been proposed for training monitoring purposes and a reliable testing procedure with the potential of additional information about HR(V) reactivity and recovery (Bosquet et al. 2008; Grant et al. 2012; Schmitt et al. 2013, 2015a, b; Hottenrott and Hoos 2018; Laborde et al. 2017, 2018; Manser et al. 2021). This test explores the reactivities of the parasympathetic branch (withdrawal) and the sympathetic branch (excitation) of ANS in response to the position change (Taylor 1994). In this context, Schmitt et al. (2015a) argued that HRV values obtained only from supine position do not provide information about the preserved or altered ability of dynamic adjustment of the ANS. They also suggested that especially HR(V) changes between supine and standing position as an orthostatic response assessment could better inform about cardiovascular autonomic functioning and fatigue state and may therefore potentially further enlighten non-functional overreaching and chronic overtraining response processes (Schmitt et al. 2013, 2015a, b; Meeusen et al. 2013a, b).
Physiological background of orthostatic testing
Besides dynamic physical exercise, upright posture represents a physical stressor that requires full capabilities of the reflexes for the regulation of cardiovascular function (Rowell 1993; Freeman 2006; Stewart 2012). Therefore, the detection of specific changes in HR and HRV during orthostatic testing could be a potential source of information about overload and overtraining associated with acute and chronic responses in the organism. Within the clinical setting, orthostatic challenges and corresponding testing scenarios typically include the standardized change in body position, either passive in a so-called tilt test with individuals lying down on a table that can be tilted or as an active change from a lying or sitting position to an upright or standing position (e.g., active standing up; Freeman 2006; Sutton et al. 2021; van Zanten et al. 2024). This change challenges acute regulation responses by means of stress due to gravity within the ANS in general and the cardiovascular system in particular. Therefore, a normal hemodynamic response to orthostatic challenge is driven by and highly dependent on a sequence of multiple interrelated mechanisms, including several redundant feedback loops to counteract inadequacy of any one subsystem involved (Freeman 2006; Stewart 2012; Soloveva et al. 2020; see Fig. 1).
Normal physiological and compensatory responses to active standing up modified from Soloveva et al. (2020) with information added from Rowell (1993) and Freeman (2006). CO cardiac output, BP blood pressure, HR heart rate, HRV heart rate variability, SV stroke volume, RAAS renin–angiotensin–aldosterone system
Gravitational forces due to orthostatic challenge shift approx. 400–800 ml of intra-vascular blood volume from the chest area into the splanchnic and pelvis compartment as well as into the buttock and leg vessels (Rowell 1993; Freeman 2006; Stewart et al. 2006; Stewart 2012; Soloveva et al. 2020). In addition, volume shifts out of the heart and the pulmonary vascular bed decrease the pressure of right ventricular filling with a subsequent reduction of cardiac output. In the follow-up due to decreased venous return (Sander-Jensen et al. 1986), blood pressure slightly decreases and HR increases. This cascade also triggers aortic (located in the walls of the large blood vessels, particularly in the aorta and the carotid sinus arteries) and carotid baroreceptor (located in the atria of the heart and in the blood vessels of the lungs) reflexes and stimulates brain ANS centers leading to sympathetic activation and vagal withdrawal accompanied by changes of plasma hormone concentrations (e.g., norepinephrine, epinephrine, vasopressin, angiotensin II; Sander-Jensen et al. 1986; Freeman 2006). This further stimulates compensatory vasoconstriction and HR increase (Ricci et al. 2015). Consequently, during an orthostatic challenge, autonomic regulatory processes strive to maintain blood pressure homeostasis (after short-term initial hypotension) and secure adequate hemodynamics to avoid cerebral hypoperfusion (Stewart 2012).
Beyond inter-individual variability, several pathophysiologic alterations in the cardiovascular responses to orthostatic challenges may occur, such as orthostatic hypotension, neurally mediated syncope and postural tachycardia syndrome (POTS) in all of which dysfunctional ANS-regulation, altered baroreflex sensitivity, and hypovolemia interact with other factors (e.g., genetics, aging, and postprandial status) and result in orthostatic intolerance (Freeman 2006; Barantke et al. 2008; Freeman et al. 2011; Stewart 2012; Swai et al. 2019; Soloveva et al. 2020). Furthermore, cardiovascular deconditioning and physical fitness were shown to interfere with normal orthostatic regulation, and the relationship with orthostatic intolerance was characterized as a shallow “U” shape, with a high incidence of orthostatic intolerance at both sides of the “fitness spectrum” (Levine 1993). This could be a potential limitation in the application of orthostatic testing, particularly for well-trained endurance athletes, and should be evaluated on an individual basis.
As a diagnostic tool for pathophysiologic alterations and the evaluation of physiologic reflex patterns, the application of position change from lying to standing was already introduced almost 50 years ago as a standardized test of autonomic function in diabetes (Ewing et al. 1978). As described before, the maneuver induces an integrated reflex response of the cardiovascular system, that trigger distinctive alterations in HR and blood pressure (Johnson and Spalding 1974; Ewing et al. 1978; Pomeranz et al. 1985). From a diagnostic perspective of the ANS, HR and HRV markers during passive tilt testing (e.g., Task Force 1996; Tulppo et al. 2001) typically indicate vagal withdrawal, and sympathetic excitation in a reciprocal fashion associated with an HR increase, a HF power decrease, and an increase in normalized LF power. In addition, increases in short-term correlation properties of HRV suggest reduced complexity in HR dynamics with the change to an upright body position, similar to low-intensity dynamic physical exercise (not detected by linear and other non-linear HRV metrics; Tulppo et al. 2001). However, passive tilt only entails a minimal engagement of central drive and muscular activity and is compatible with quite accurate stationary conditions (Malliani et al. 1991; Montano et al. 1994). These acute responses were reported in numerous studies and were mainly related to baroreflex activity and sensitivity (Tulppo et al. 2001; Montano et al. 1994; Nakamura et al. 1993; Butler et al. 1993, 1994). However, the exact responses (predominance of the cardiopulmonary or arterial baroreflex, predominance of norepinephrine or epinephrine, etc.) seem to be highly dependent on the specific protocol applied (e.g., mild vs. more pronounced tilt, velocity of inclination, and duration of the phases) and can differ substantially between different populations with direct effects on orthostatic tolerance (Mellingsæter et al. 2015; Sander-Jensen et al. 1986; Montano et al. 1994; Lipsitz et al. 1990; Butler et al. 1993; Levine 1993).
Contrary, active standing up produces different responses than a passive tilt test, adding information about changes occurring during actual tilting (Ewing et al. 1980). It is well documented that there is a transient fall in blood pressure while standing, with stimulation of the carotid baroreceptors and consequent reflex increase of HR and peripheral vasoconstriction in concert with cardio-postural interaction and skeletal muscle-pump activity (Johnson and Spalding 1974; Vinik et al. 2003; Xu et al. 2017). A reduction in blood pressure and initial hypotension occurs only during the initial transient mechanical disequilibrium. After that acute response systolic and diastolic blood pressures are usually slightly increased compared to supine lying body position (Stewart 2012). HR responses to standing have long been observed in healthy individuals (Hill 1895), while the immediate HR response has only been briefly documented at the beginning (Burke et al. 1977; Page and Watkins 1977). The ANS response to standing is reproducible and initiated by instantaneous cardiac vagal withdrawal followed soon after by sympathetic activation and vagal reactivation over the first 25–30 heartbeats. Thus, an increase of HR occurs immediately when standing up and HR will reach a peak after 15–20 beats and is less marked during passive tilting (Ewing et al. 1980; Vinik et al. 2003; Rooke and Sparks 2003), but the rebound only occurs with active orthostatic challenge, presumably due to the associated muscular activity of large muscle groups (Rowell 1993). Due to these observations, the 30:15 ratio was frequently used in clinical settings to assess physiological responses and cardiac vagal function during active standing up, which describes the ratio of HR increase at approx. 15 s after standing (the shortest R–R interval at or around the 15th beat) to the relative bradycardia that occurs at approx. 30 s after standing (the longest R–R interval at or around the 30th beat; Ewing et al. 1978, 1980). Due to variation in individual responses, an increase in sensitivity may be reached by enlarging the window of evaluation or measuring the ratio of the absolute maximum (peak HR) and minimum HR after standing up (Mitchell et al. 1983). Normal values for the 30:15 ratio were considered to range between 1.15 and 1.12 in 21–30-year-old individuals, 1.12 and 1.10 in 31–40-year-old individuals, 1.10 and 1.08 in 41–50-year-old individuals, and 1.08 and 1.07 in 51–60-year-old individuals (Ziegler et al. 1992; Freeman 2006).
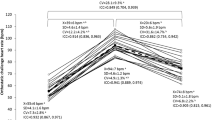
Hence, early on, it has been recognized that sitting and standing, rather than lying (alone), should be considered as additional resting physiology postures (Gauer and Tiron 1965; Burke et al. 1977). In addition, in sport and exercise science, an orthostatic test usually requires active standing up, which leads to a higher degree of change in HR and blood pressure, and seems to be more sensitive and feasible for monitoring purposes in different fields of sports application (Hottenrott and Gronwald 2014; Hottenrott and Hoos 2018). Figure 2 displays the temporal course of the HR and R–R intervals during an orthostatic test with an active postural change from supine lying to standing. In that regard, Hynynen et al. (2011) confirmed earlier results, that life stress induces autonomic perturbations that could be assessed via orthostatic testing and might be helpful to improve individual load and recovery management. Also, Lucini et al. (2002) showed higher incidence of stress symptoms associated with lower HRV during the orthostatic test after awakening. This association was found during both supine rest and standing positions. These findings are in line with Porges (1992), who suggested that the parasympathetic nervous system acts as the general modulator of stress vulnerability and reactivity, which could be directly investigated via orthostatic testing. In the next section, we will discuss further data and specific responses during orthostatic testing in the applied field of exercise science and sports practice.
Example of a heart rate (HR) and R–R interval profile of a 35-year-old healthy active individual without specific adaptation during an orthostatic test as part of a 3-min measurement while lying down in supine position, followed by a 3-min measurement while standing. The black vertical arrow indicates the change in body position due to active standing up and the compensatory transient reaction of the HR and R–R intervals (red shaded area). It is noticeable that the variability of the HR- / R–R-time series is much greater in the lying position compared to the standing position. Briefly, The compensatory reaction of the cardiovascular system leads to a peak HR of 103 beats per minute (Δ t: time to peak HR = 15 s) and an HR minimum of 72 beats per minute (Ratio = 1.43) before the time series stabilizes again—characterized by an increase in HR and both a reduction in the mean R–R interval and the RMSSD due to the change in body position. Green shaded areas display the 2-min steady-state windows of analysis during supine and standing position. From a qualitative perspective, the shape of the HR change after standing up shows a steep increase (in regard of the time to peak HR) and fast decrease which potentially stands for a good responsiveness and regulation quality
Orthostatic testing in exercise science and sports practice
In the last decades, several studies have shown the advantage of evaluating a standing position and the comparison between supine lying and standing (upright position) in response to active standing up (and tilting) in endurance and intermittent sports such as team sports (e.g., soccer). Here, prior studies reported additional sensitivity for the assessment of training status, performance level, and the dynamic adaptability of cardiac autonomic modulation (Gilder and Ramsbottom 2008; Rave and Fortrat 2016; Bellenger et al. 2016a; Maggioni et al. 2020), as well as for the reflection of fatigue states, parasympathetic hyperactivity, and overreaching (Uusitalo et al. 2000; Hedelin et al. 2000; Portier et al. 2001; Hynynen et al. 2008; Le Meur et al. 2013; Schmitt et al. 2013, 2015b; Bellenger et al. 2016a; Schneider et al. 2019; Barrero et al. 2020). In a conceptual framework for contextualizing HR measures for training and recovery prescription by Schneider et al. (2018), it is also summarized that the autonomic shift associated with an orthostatic challenge may be influenced by training-related phenomena, such as an overall vagal enhancement through low-intensity aerobic training, pre-competitive anxiety, overall stress (Hynynen et al. 2011), and fatigue phenomena from preceding exercise sessions or competition (Gratze et al. 2005; Schäfer et al. 2015), as well as overtraining (Hynynen et al. 2008). In a meta-analysis by Manresa-Rocamora et al. (2021), the authors concluded that resting vagal-related HRV indices measured in the standing position should be used to increase the sensitivity for parasympathetic hyperactivity in functionally overreached athletes. Measuring while standing might also counteract the above described issue of parasympathetic saturation as a common phenomenon among healthy individuals (Goldberger et al. 2001; Kiviniemi et al. 2004, 2006), especially associated with low-resting HR in well-trained endurance athletes (Janssen et al. 1993; Buchheit et al. 2004; Kiviniemi et al. 2007). Other studies emphasize the implementation and analysis of the combination of body positions (Flatt & Esco 2015). This supports the association that an orthostatic stimulus may be more favorable than supine lying or sitting measurements alone to obtain specific information on the status of the cardiac autonomic regulation (Kiviniemi et al. 2007).
In that regard, Le Meur et al. (2013) evaluated alterations in autonomic function associated with functional overreaching in triathletes, showing how standing measurements could be used to detect parasympathetic hyperactivity compared to supine measures. In addition, Bellenger et al. (2016a) found that RMSSD assessed in a supine position was less sensitive in detecting potential parasympathetic hyperactivity in overreached endurance athletes after heavy training phases, and also according to improvements in performance level, suggesting that standing measures of HRV are more appropriate for detecting autonomic disturbances in the present context. However, the results of Bellenger et al. (2016a) demonstrate that increases in RMSSD due to specific training stimuli may be indicative of both positive and negative physiological responses. Therefore, additional measures such as psychometrical data are required to add context information for HR and HRV analysis interpretation (Le Meur et al. 2013; Tian et al. 2013; Buchheit 2014). In this regard, Hottenrott et al. (2021) published a case study assessing a viral infection in an elite marathon runner. In line with the general notion that an increase in resting HR and a decrease in RMSSD suggest a suppression of vagal activity (Buchheit 2014; Laborde et al. 2018), the viral infection had a direct influence on HR and HRV during orthostatic testing. An increase in HR was accompanied by a decrease in RMSSD in the standing position. Further, the kinetics of HR during position change was significantly different in the HR increase (time to peak HR) from a typical course in a healthy condition with a slower increase due to infection. Although there are no further findings in the literature according to these observations, reactivity of HR(V) is supposed to bear the potential of additional information in HR time series analysis due to associations of a resilient system (Scheffer et al. 2018; Laborde et al. 2017, 2018; Manser et al. 2021). Bellenger et al. (2016c, 2017) also found a slower maximal rate of HR increase at the onset of physical exercise (e.g., assessed via submaximal testing scenarios) in athletes suffering from exercise-induced fatigue due to overload training. Therefore, this measure could also be sensitive for different kinds of stressors including internal or external factors (Nelson et al. 2014, 2017; Bellenger et al. 2016b, c, 2017). A second main finding of Hottenrott et al. (2021) is that HR and HRV values changed more substantially in the standing position compared to supine position during viral infection. This was particularly pronounced in the RMSSD values. The findings of this report are limited to one case but could have some implications for sports practitioners and coaches looking to both ensure the health of their athletes, and for using HRV as a tool to monitor training processes and the return to sport after a viral infection.
Based on previous findings (Le Meur et al. 2013; Buchheit 2014; Plews et al. 2012, 2013b) and experiences in coaching elite athletes, Hottenrott and Hoos (2018) displayed schematic examples of HR and RMSSD-based HRV alterations during orthostatic testing and with associated functional states (e.g., acute and chronic fatigue, parasympathetic saturation) following different training characteristics (e.g., high-intensity training, high-volume training) compared to baseline assessments. It is mentioned that the average HR in the standing position of healthy individuals is on average 10–25 beats higher than the HR at rest in supine position, depending on age (Dambrink and Wieling 1987; van Wijnen et al. 2017). Standing also induces a three- to four-fold decrease of vagally related HRV indices compared to the supine position (Aubert et al. 2003; Hottenrott and Hoos 2018). It is also stated that there are specific and inter-individual variations in accordance to acute and chronic responses of life and physical training stressors. Main characteristics could be that high-intensity exercise over several days can lead to sympathetic overreaching, which is reflected by increased HR and decreased vagal activity in both supine and standing position (Hottenrott and Hoos 2018). Furthermore, high-volume training combined with some intensity peak sessions induce similar changes of supine lying values, whereas there could be a lower counter-regulatory response in the standing position. Consequently, the HR difference between supine and standing position is reduced (Hottenrott and Hoos 2018; Hynynen et al. 2008). Further, an attenuation of both parasympathetic and sympathetic activity during both supine and standing positions could be found in overtrained endurance athletes (Hynynen et al. 2008). The response to the standing position seemed to be lower than in non-overtrained athletes with the tendency of further decreased HRV in the standing position as a sign of pronounced vagal withdrawal and in some cases decreased sympathetic excitability associated with a potential overtraining state (Hynynen et al. 2008). In further studies of active orthostatic testing, it could be shown that the variation in HR and HRV indices comparing supine and standing positions is strongly correlated with the fatigue status in endurance athletes (Barrero et al. 2019). Therefore, the difference between standing and supine HR and HRV after active standing up could be a simple analysis option, e.g., with lower HR change between supine and standing positions indicating a potential maladaptive training status (Barrero et al. 2019, 2020). In addition, extensive high-volume training with low exercise intensity may lead to parasympathetic saturation, which could be indicated by a low HR and high HRV in both supine and standing position. Due to the high vagal activity, there is almost no HR difference between both body positions. Potentially, physiological states of functional overreaching and some kind of overtraining can be distinguished by the alteration of these functional states through changes in training characteristics (e.g., intensity, volume, and frequency). For example, parasympathetic saturation can be acutely counteracted with a reduction in training volume, introducing more intensity peaks in the following micro-cycle (Hottenrott and Hoos 2018). However, as a significant main characteristic, it could be noted that additional pathophysiological conditions consist in a reduced responsiveness or counter-regulation of neural drive in ANS according to an excitatory stimulus, such as an orthostatic challenge (Montano et al. 2009).
Practical recommendation for implementation
Regular testing is mandatory for appropriate data interpretation from resting physiology that includes orthostatic testing. Regular assessment can enhance understanding of how the organism responds to training load and other lifestyle factors that may interfere with ANS activity (e.g., work-life stress, sleep quality and duration, alcohol, nutrition, infection, environmental conditions, and changes such as temperature or altitude; Sandercock et al. 2005; Buchheit 2014; Fatisson et al. 2016). Regular testing should comprise at least three-to-four times a week in standardized conditions (e.g., same days in the micro-cycle, first thing in the morning after waking up) and testing frequency may be increased in periods of accumulated workload or overall stress (e.g., altitude and training camps, travel) (Buchheit 2014). To obtain reliable results, standardized conditions and application of recommended standards (e.g., Task Force 1996; Quintana & Heathers 2014; Sassi et al. 2015; Laborde et al. 2017) are mandatory to derive appropriate interpretations. To minimize stimuli affecting ANS function, the test should be performed in a quiet, dimly lit room at a comfortable temperature of around 21–23 °C (Finucane et al. 2019). Reliability of specific HRV measures can be dependent on body position (Sandercock et al. 2005; da Cruz et al. 2019; Cassirame et al. 2019; Holmes et al. 2020). In addition, the speed of standing up and the magnitude of active muscle contraction can be influencing factors due to cardio-postural interaction and muscle-pump activity (Kamiya et al. 2009; Xu et al. 2017). Therefore, it is recommended to stand up with a steady and comparable speed for multiple testing. Specifically, HR and HRV from orthostatic testing were shown to be reproducible both prior and after postural change in healthy participants and in patients with a history of acute coronary disease (Dantas et al. 2010; Schäfer et al. 2015; Vescovi et al. 2019). Recording conditions can be well replicated for morning measurements, which seem to be less susceptible to daily stressors and more representative of upcoming daily readiness (Nuuttila et al. 2022). Morning HR(V) measurements can also be implemented using low-cost and easy-to-use wearable technologies (e.g., smartphone-based applications), whereas orthostatic testing needs to be further evaluated with photoplethysmography measurement principles (PPG; e.g., smartwatch at the wrist, ring at the index finger) due to motion artifact susceptibility and the ability to sensitively detect the physiological changes and thus beat-to-beat intervals occurring during and after postural changes (Esco et al. 2017; Charlton et al. 2022). In that regard, further technical improvements of PPG measurement principles and wearable applications are necessary. Hence, currently HR(V) monitors enabling electrophysiological measurement principles (e.g., electrodes embedded in chest strap devices, electrode patches) with internal memory, or Bluetooth/ANT + coupled with e.g., smartwatches or smartphones are recommended for orthostatic testing.
A short guide to orthostatic testing with a chest strap device:
-
Between waking up and recording, it is mandatory to use the bathroom (to empty bladder). In case you do not need to go to the bathroom, please simulate it.
-
Fit on the chest strap with the sensor device. Moisten the electrodes of the strap and fasten it comfortable but ensure that it does not slip.
-
There should be no disturbances around (e.g., talking people, television, music, and telephone noise).
-
Lie back and find a comfortable supine position, then start recording.
-
Be relaxed and calm. Keep your eyes open and try to breathe calmly and spontaneously. Avoid swallowing and yawning during the measurement.
-
Both when measuring while lying down and during standing position, try to move as little as possible.
-
After 3 min stand up with a steadily speed. Record another 3 min while standing. Remain in an upright position. The arms hang sideways next to the body, the knees are slightly bent, and the view is directed forward.
-
After that stop the recording.
-
Perform the test regularly in the same body positions and at the same time of day in the morning after awakening.
-
Compare your measurement values to your own historical data baseline.
After lying down, a stabilization period should be applied (at least 1 min of baseline leading to a steady signal and settlement of respiratory rhythm). The test continues with 2 min of steady-state analysis window recording in lying position followed by actively standing up for a minute of transient early response and 2 min of steady-state analysis in standing position (1 + 2 min in each position for 6 min in total; Bourdillon et al. 2017; Hottenrott and Hoos 2018). This time frame is recommended for time-domain HRV analysis (e.g., RMSSD). Beyond time-domain indices, the implementation of spectral HRV analysis for the potential clustering of fatigue-related processes was proposed (Schmitt et al. 2015a), although the presence of higher sensitivity to breathing pattern might complicate data interpretation (Saboul et al. 2013). However, due to influences of breathing pattern on vagal-related HRV indices (Laborde et al. 2022; Ritz 2024; You et al. 2024), spontaneous breathing is required and no paced breathing is recommended. If frequency-domain parameters are considered, the test procedure should be slightly extended in each position (1 + 4 min in each position for 10 min in total; Bourdillon et al. 2017). An example for test execution and the following suggestions for quantitative and qualitative analysis of an orthostatic test are shown in Fig. 2.
Quantitative outcomes for time-domain metrics:
-
HR and HRV (e.g., RMSSD) average for the last 2-min lying down
-
Peak HR and time to peak HR (Δ t) after active standing up
-
Ratio of peak HR and HR minimum after active standing up
-
HR and HRV average for the last 2 min in standing position
-
HR and HRV change between supine and standing positions (e.g., low HR change may reflect a maladaptive training status)
-
Absolute sum of the HR and HRV change in supine and standing positions compared to the previous test (e.g., HR is 2 bpm higher in supine position and 4 bpm higher in standing position compared to the previous day leading to an overall difference of 6 bpm)
As a qualitative outcome the shape of the HR change after standing up could be evaluated (e.g., a steep increase and fast decrease potentially stands for fast response and good regulation quality; a slow increase and decrease stands potentially for fatigue processes and disturbed regulation pattern).
As mentioned earlier, Schmitt et al. (2015a, b) also proposed the implementation of spectral HRV analysis such as LF and HF for more sensitivity in fatigue detection and monitoring of the adaptive training-recovery process as opposed to the sole usage of time-domain HRV indices. One argument in favor of this is that RMSSD does not provide isolated information on the sympathetic-related modulation. Here, a decrease in RMSSD may lead to a biased interpretation due to parasympathetic saturation (Kiviniemi et al. 2004); this could be the result of a sympathetic overactivity combined with a saturation phenomenon (Schmitt et al. 2015a). However, a reductionistic simplification and assignment to parasympathetic and/or sympathetic regulation pattern only is also not justified by specific frequency domains. Here, inconsistent results are often generated using the LF/HF-frequency-domain ratio, and therefore, it should be discouraged to be used as a simple metric for sympatho-vagal balance (e.g., Billman 2013; Michael et al. 2017). Moreover, non-linear analysis of HRV such as the evaluation of short-term correlation properties of detrended fluctuation analysis (DFAa1) of HR time series has been shown to be more sensitive compared to the classical spectral and time-domain indices during postural change. Alterations in DFAa1 suggest reduced complexity in HR dynamics with an upright body position depending on inter-individual data history and may therefore be significant for specific organismic responses (de Souza et al. 2014; Tulppo et al. 2001). Further studies are necessary to evaluate the recommended outcomes in relation to other domains of analysis to ensure an integrated evaluation of the orthostatic response in the future.
Interpretation:There are specific and inter-individual differences in accordance with acute and chronic responses to orthostatic stress. Even if normative values for vagally related HRV metrics for resting conditions can be helpful on a statistical level to distinguish certain individuals on a population level (e.g., age, disease vs. no disease; sedentary vs. aerobically-trained), it is noticeable that there is still a large overlay of the actual data of compared populations (Huikuri et al. 2000; Aubert et al. 2003; Acharya et al. 2004; Zhang et al. 2020). Hence, an intra-individual regulation pattern as an individual profile should be primarily addressed and applied as the base of an interpretation together with further contextual data. Additional information from easily accessible psychometrical scales (e.g., Hooper-Index, Hooper et al. 1995) and other contextual information (e.g., training load analysis, performance test results) may overcome the lack of specificity in the interpretation of HR and HRV values, as these are strongly influenced by multiple factors (Sandercock et al. 2005; Buchheit 2014; Fatisson et al. 2016; Plews et al. 2012). As indicated by Schneider et al. (2018, 2019), it is recommended first to gain experience at an individual level through observation of data history to avoid inappropriate adjustments in exercise and training prescription due to overly simplistic training-response models. In addition, multiple studies and practical applications of HR and HRV trend analysis recommend the utilization of weekly HRV and rolling averages of a minimum of three-to-seven measurements as a baseline to be more sensitive compared to single-day values and to assess physiological alterations in vagal modulation and adaptation to training (Le Meur et al. 2013; Plews et al. 2013a, b, 2014; Flatt and Esco 2015; Medeiros et al. 2021). In addition, the use and interpretation of the coefficient of variation (CV) as the ratio of the standard deviation (SD) and the mean × 100 (%) of a data series and some kind of smallest worthwhile change (SWC) approach as a factor of ± CV or ± SD (implemented as a normal range) could be helpful for decision-support in load and recovery management (Plews et al. 2013a, b; Buchheit 2014; Gronwald et al. 2024). As an alternative, a fixed reference (average over multiple measurements) for the baseline of comparable phases of periodization or lifestyle could be used. This could be also applied to the different outcome measures of an orthostatic test (Polar Research and Technology 2024). All these information could be useful and relevant on an individual level, but their sensitivity should be evaluated in the applied field. In addition, these simple statistical implementations may help to increase the signal-to-noise ratio and the reproducibility of the applied measures improving quality and robustness of the monitoring process (Buchheit 2014; Schmitt et al. 2015a). Further, computational approaches with mathematical modeling are promising for quantifying ANS functionality reducing mathematical complexity and offering an easy-to-implement tool for clinical assessment and monitoring through innovative wearable technology (Wang et al. 2024).
Perspective and conclusion
In monitoring processes, physiological parameter interpretation is often difficult in isolation and/or with contrasting information involved. Therefore, a fine-tuned approach is recommended to implement several valid and practical tools, and to improve the effectiveness of monitoring practices when added to experts’ knowledge (Boullosa et al. 2023). The ANS response to active standing up in orthostatic testing is initiated by instantaneous cardiac vagal withdrawal, followed by sympathetic activation and vagal reactivation. Generally, standing decreases vagally related HRV indices compared to the supine position. In overtrained endurance athletes, both parasympathetic and sympathetic activity are attenuated in supine and standing positions. The response to standing is lower than in non-overtrained athletes, with a tendency for further decreased HRV as a sign of pronounced vagal withdrawal and, in some cases, decreased sympathetic excitability. Therefore, the comparison of both body positions in a regular monitoring procedure could provide additional information and sensitivity about HR(V) reactivity and recovery due to health issues, fatigue processes, and overtraining phenomena. Measuring while standing might also counteract the issue of parasympathetic saturation as a common phenomenon especially in well-trained endurance athletes with low-resting HR (< 50 bpm). However, a likely increased incidence of orthostatic intolerance at both sides of the “fitness spectrum” should be evaluated on an individual level and must be taken into account when considering to implement orthostatic testing in specific subpopulations. Given the presented compilation of study findings, HR(V) analysis during orthostatic testing bears the potential to provide sensitive outcome measures about altered physiological conditions which should be interpreted within intra-individual data history in trend analysis and in regards of additional measures (e.g., psychometrical scales) to provide context for data interpretation. The effort involved should be considered in relation to the benefits for the specific field of application (e.g., sport-specific circumstances, individual situation after awakening). Thorough method comparison studies and systematic analysis of the literature must be conducted to further evaluate whether the use of regular orthostatic testing is able to offer an innovative, non-invasive, ecological valid, and time-efficient application to assess the health status and performance alterations of different populations, performance level, sport-specific demand profiles (modality and type of physical exercise), and could enhance decision-support for load and recovery management in exercise science and practice.
Abbreviations
- ANS:
-
Autonomic nervous system
- BP:
-
Blood pressure
- CO:
-
Cardiac output
- CV:
-
Coefficient of variation
- DFAa1:
-
Short-term scaling exponent of detrended fluctuation analysis
- HF:
-
High frequency
- HR:
-
Heart rate
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- POTS:
-
Postural tachycardia syndrome
- PPG:
-
Photoplethysmography
- RAAS :
-
Renin–angiotensin–aldosterone system
- RMSSD:
-
Root mean square of successive differences of normal-to-normal R–R intervals
- SD:
-
Standard deviation
- SD1:
-
Standard deviation 1 from the Poincaré Plot
- SDNN:
-
Standard deviation of normal-to-normal R–R intervals
- SV:
-
Stroke volume
- SWC :
-
Smallest worthwhile change
- ULF:
-
Ultra-low frequency
- VLF:
-
Very-low frequency
- Δ t:
-
Time to peak HR
References
Acharya UR, Kannathal N, Sing OW, Ping LY, Chua T (2004) Heart rate analysis in normal subjects of various age groups. Biomed Eng Online 3(1):24
Achten J, Jeukendrup AE (2003) Heart rate monitoring: applications and limitations. Sports Med (Auckland NZ) 33(7):517–538
Aubert AE, Seps B, Beckers F (2003) Heart rate variability in athletes. Sports Med (Auckland NZ) 33(12):889–919
Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H (2008) Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19(12):1296–1303
Barrero A, Schnell F, Carrault G, Kervio G, Matelot D, Carré F, Le Douairon Lahaye S (2019) Daily fatigue-recovery balance monitoring with heart rate variability in well-trained female cyclists on the Tour de France circuit. PLoS ONE 14(3):e0213472
Barrero A, Le Cunuder A, Carrault G, Carré F, Schnell F, Le Douairon Lahaye S (2020) Modeling stress-recovery status through heart rate changes along a cycling grand tour. Front Neurosci 14:576308
Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD (2016a) Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med 46:1461–1486
Bellenger CR, Karavirta L, Thomson RL, Robertson EY, Davison K, Buckley JD (2016b) Contextualizing parasympathetic hyperactivity in functionally overreached athletes with perceptions of training tolerance. Int J Sport Physiol Perform 11:685–692
Bellenger CR, Thomson RL, Howe PR, Karavirta L, Buckley JD (2016c) Monitoring athletic training status using the maximal rate of heart rate increase. J Sci Med Sport 19:590–595
Bellenger CR, Thomson RL, Robertson EY, Davison K, Nelson MJ, Karavirta L, Buckley JD (2017) The effect of functional overreaching on parameters of autonomic heart rate regulation. Eur J Appl Physiol 117(3):541–550
Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68(10):988–1001
Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW (1997) Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34(6):623–648
Billman GE (2011) Heart rate variability - a historical perspective. Front Physiol 2:86
Billman GE (2013) The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4:26
Billman GE, Huikuri HV, Sacha J, Trimmel K (2015) An introduction to heart rate variability: methodological considerations and clinical applications. Front Physiol 6:55
Borresen J, Lambert MI (2009) The quantification of training load, the training response and the effect on performance. Sports Med (Auckland NZ) 39(9):779–795
Bosquet L, Merkari S, Arvisais D, Aubert AE (2008) Is heart rate a convenient tool to monitor over-reaching? A systematic review of the literature. Br J Sports Med 42(9):709–714
Boullosa D, Claudino JG, Fernandez-Fernandez J, Bok D, Loturco I, Stults-Kolehmainen M, García-López J, Foster C (2023) The fine-tuning approach for training monitoring. Int J Sports Physiol Perform 18(12):1374–1379
Bourdillon N, Schmitt L, Yazdani S, Vesin JM, Millet GP (2017) Minimal window duration for accurate HRV recording in athletes. Front Neurosci 11:456
Buchheit M (2014) Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol 5:73
Buchheit M, Simon C, Piquard F, Ehrhart J, Brandenberger G (2004) Effects of increased training load on vagal- related indexes of heart rate variability: a novel sleep approach. Am J Physiol Heart Circ Physiol 287:H2813–H2818
Buchheit M, Simon C, Charloux A, Doutreleau S, Piquard F, Brandenberger G (2005) Heart rate variability and intensity of habitual physical activity in middle-aged persons. Med Sci Sports Exerc 37(9):1530–1534
Buchheit M, Papelier Y, Laursen PB, Ahmaidi S (2007) Noninvasive assessment of cardiac parasympathetic function: postexercise heart rate recovery or heart rate variability? Am J Physiol Heart Circul Physiol 293(1):H8–H10
Burke D, Sundlöf G, Wallin G (1977) Postural effects on muscle nerve sympathetic activity in man. J Physiol 272(2):399–414
Butler GC, Yamamoto Y, Xing HC, Northey DR, Hughson RL (1993) Heart rate variability and fractal dimension during orthostatic challenges. J Appl Physiol (Beth Md 1985) 75(6):2602–2612
Butler GC, Yamamoto Y, Hughson RL (1994) Heart rate variability to monitor autonomic nervous system activity during orthostatic stress. J Clin Pharmacol 34(6):558–562
Carnethon MR, Liao D, Evans GW, Cascio WE, Chambless LE, Rosamond WD, Heiss G (2002) Does the cardiac autonomic response to postural change predict incident coronary heart disease and mortality? The Atherosclerosis Risk in Communities Study. Am J Epidemiol 155(1):48–56
Cassirame J, Chevrolat S, Mourot L (2019) Effects of RR time series accuracy on heart rate variability indexes. Move Sport Sci Sci Motr 4:27–35
Charlton PH, Kyriacou PA, Mant J, Marozas V, Chowienczyk P, Alastruey J (2022) Wearable photoplethysmography for cardiovascular monitoring. Proceedings of the IEEE. Inst Elect Electr Eng 110(3):355–381. https://doi.org/10.1109/JPROC.2022.3149785
Ciccone AB, Siedlik JA, Wecht JM, Deckert JA, Nguyen ND, Weir JP (2017) Reminder: RMSSD and SD1 are identical heart rate variability metrics. Muscle Nerve 56(4):674–678
da Cruz CJG, Porto LGG, da Silva Rolim P, de Souza Pires D, Garcia GL, Molina GE (2019) Impact of heart rate on reproducibility of heart rate variability analysis in the supine and standing positions in healthy men. Clinics (Sao Paulo, Brazil) 74:e806
Da Silva VP, De Oliveira NA, Silveira H, Mello RG, Deslandes AC (2015) Heart rate variability indexes as a marker of chronic adaptation in athletes: a systematic review. Ann Noninv Electrocardiol off J Int Soc Holter Noninv Electrocardiol Inc 20(2):108–118
Dambrink JH, Wieling W (1987) Circulatory response to postural change in healthy male subjects in relation to age. Clinical Science (Lond Eng 1979) 72(3):335–341
Dantas EM, Gonçalves CP, Silva AB, Rodrigues SL, Ramos MS, Andreão RV, Pimentel EB, Lunz W, Mill JG (2010) Reproducibility of heart rate variability parameters measured in healthy subjects at rest and after a postural change maneuver. Braz J Med Biol Res 43(10):982–988
de Godoy MF (2016) Nonlinear analysis of heart rate variability: a comprehensive review. J Cardiol Therapy 3(3):528–533
de Souza AC, Cisternas JR, de Abreu LC, Roque AL, Monteiro CB, Adami F, Vanderlei LC, Sousa FH, Ferreira LL, Valenti VE (2014) Fractal correlation property of heart rate variability in response to the postural change maneuver in healthy women. Int Arch Med 7:25
Ernst G (2017a) Heart-rate variability-more than heart beats? Front Public Health 5:240
Ernst G (2017b) Hidden signals-the history and methods of heart rate variability. Front Public Health 5:265
Esco MR, Flatt AA, Nakamura FY (2017) Agreement between a smartphone pulse sensor application and electrocardiography for determining lnRMSSD. J Strength Cond Res 31(2):380–385
Ewing DJ, Campbell IW, Murray A, Neilson JM, Clarke BF (1978) Immediate heart-rate response to standing: simple test for autonomic neuropathy in diabetes. BMJ 1(6106):145–147
Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF (1980) Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Env Exer Physiol 49(5):809–814
Fatisson J, Oswald V, Lalonde F (2016) Influence diagram of physiological and environmental factors affecting heart rate variability: an extended literature overview. Heart Int 11(1):e32–e40
Finucane C, van Wijnen VK, Fan CW, Soraghan C, Byrne L, Westerhof BE, Freeman R, Fedorowski A, Harms MPM, Wieling W, Kenny R (2019) A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res off J Clin Auton Res Soc 29(4):427–441
Flatt AA, Esco MR (2015) Smartphone-derived heart-rate variability and training load in a women’s soccer team. Int J Sports Physiol Perform 10(8):994–1000
Freeman R (2006) Assessment of cardiovascular autonomic function. Clin Neurophysiol off J Int Federat Clin Neurophysiol 117(4):716–730
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res off J Clin Auton Res Soc 21(2):69–72
Garet M, Tournaire N, Roche F, Laurent R, Lacour JR, Barthélémy JC, Pichot V (2004) Individual Interdependence between nocturnal ANS activity and performance in swimmers. Med Sci Sports Exerc 36(12):2112–2118
Gauer OH, Tiron HL (1965) Postural changes in the circulation. In: Hamilton WF, Dow P (eds) Handbook of physiology. American physiological society, Washington, D.C., pp 2409–2439
Gilder M, Ramsbottom R (2008) Change in heart rate variability following orthostasis relates to volume of exercise in healthy women. Auton Neurosci Basic Clin 143(1–2):73–76
Goldberger AL (1990) Nonlinear dynamics, fractals and chaos: applications to cardiac electrophysiology. Ann Biomed Eng 18(2):195–198
Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH (2001) Relationship of heart rate variability to parasympathetic effect. Circulation 103(15):1977–1983
Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PCH, Peng CK, Stanley HE (2002) Fractal dynamics in physiology: alterations with disease and aging. Proc Nat Acad Sci US Am 99(1):2466–2472
Gonçalves TR, de Farinatti PT, Gurgel JL, da Silva Soares PP (2015) Correlation between cardiac autonomic modulation in response to orthostatic stress and indicators of quality of life, physical capacity, and physical activity in healthy individuals. J Stren Condit Res 29(5):1415–1421
Grant CC, Viljoen M, Janse van Rensburg DC, Wood PS (2012) Heart rate variability assessment of the effect of physical training on autonomic cardiac control. Ann Noninv Electrocardiol off J Int Soc Holter Noninv Electrocardiol 17(3):219–229
Gratze G, Rudnicki R, Urban W, Mayer H, Schlögl A, Skrabal F (2005) Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. J Appl Physiol (Bethesda Md 1985) 99(5):1728–1735
Gronwald T, Hoos O (2020) Correlation properties of heart rate variability during endurance exercise: a systematic review. Ann Noninv Electrocardiol off J Int Soc Holter Noninv Electrocardiol 25(1):e12697
Gronwald T, Schaffarczyk M, Reinsberger C, Hoos O (2024) Heart rate variability: methods and analysis in sports medicine and exercise science. Germ J Sports Med 75(3):113–118
Grosicki GJ, Culver MN, McMillan NK, Cross BL, Montoye AHK, Riemann BL, Flatt AA (2022) Self-recorded heart rate variability profiles are associated with health and lifestyle markers in young adults. Clin Auton Res off J Clin Auton Res Soc 32(6):507–518
Hedelin R, Wiklund U, Bjerle P, Henriksson-Larsén K (2000) Cardiac autonomic imbalance in an overtrained athlete. Med Sci Sports Exerc 32(9):1531–1533
Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumäki MO (1995) The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic “tone.” Acta Physiol Scand 155(3):267–273
Hill L (1895) The influence of the force of gravity on the circulation of the blood. J Physiol 18(1–2):15–53
Hnatkova K, Šišáková M, Smetana P, Toman O, Huster KM, Novotný T, Schmidt G, Malik M (2019) Sex differences in heart rate responses to postural provocations. Int J Cardiol 297:126–134
Holmes CJ, Fedewa MV, Dobbs WC, Liu Y, Flatt AA, Nakamura FY, Esco MR (2020) The effects of different body positions on the accuracy of ultra-short-term heart rate variability indexes. J High Technol Managem Res 31(1):100375
Hooper SL, Mackinnon LT, Howard A, Gordon RD, Bachmann AW (1995) Markers for monitoring overtraining and recovery. Med Sci Sports Exerc 27(1):106–112
Hottenrott K, Gronwald T (2014) Bedeutung der Herzfrequenzvariabilität für die Re-generationssteuerung. Leistungssport 44(5):9–13
Hottenrott K, Hoos O (2017) Heart rate variability analysis in exercise physiology. In: Jelinek HF, Cornforth DJ, Khandoker AH (eds) ECG time series variability analysis: engineering and medicine. CRC Press, Boca Raton, FL, pp 249–279
Hottenrott K, Hoos O, Esperer HD (2006) Herzfrequenzvariabilität und Sport Heart rate variability and physical exercise. Current status. Herz 31(6):544–552
Hottenrott L, Gronwald T, Hottenrott K, Wiewelhove T, Ferrauti A (2021) Utilizing heart rate variability for coaching athletes during and after viral infection: a case report in an elite endurance athlete. Front Sports Act Liv 3:612782
Hug B, Heyer L, Naef N, Buchheit M, Wehrlin JP, Millet GP (2014) Tapering for marathon and cardiac autonomic function. Int J Sports Med 35(8):676–683
Huikuri HV, Mäkikallio TH, Peng CK, Goldberger AL, Hintze U, Møller M (2000) Fractal correlation properties of R–R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 101(1):47–53
Huikuri HV, Perkiömäki JS, Maestri R, Pinna GD (2009) Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philosophical transactions. Ser A Math Phys Eng Sci 367(1892):1223–1238
Hurst JW (1998) Naming of the waves in the ECG, with a brief account of their genesis. Circulation 98(18):1937–1942
Hynynen E, Uusitalo A, Konttinen N, Rusko H (2008) Cardiac autonomic responses to standing up and cognitive task in overtrained athletes. Int J Sports Med 29(7):552–558
Hynynen E, Konttinen N, Kinnunen U, Kyröläinen H, Rusko H (2011) The incidence of stress symptoms and heart rate variability during sleep and orthostatic test. Eur J Appl Physiol 111(5):733–741
Janssen MJ, de Bie J, Swenne CA, Oudhof J (1993) Supine and standing sympathovagal balance in athletes and controls. Eur J Appl Physiol 67(2):164–167
Johnson RH, Spalding JMK (1974) Disorders of the autonomic nervous system. Blackwell, Oxford
Kamiya A, Kawada T, Shimizu S, Iwase S, Sugimachi M, Mano T (2009) Slow head-up tilt causes lower activation of muscle sympathetic nerve activity: loading speed dependence of orthostatic sympathetic activation in humans. Am J Physiol Heart Circ Physiol 297(1):H53–H58
Kiviniemi AM, Hautala AJ, Seppänen T, Mäkikallio TH, Huikuri HV, Tulppo MP (2004) Saturation of high-frequency oscillations of R–R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Am J Physiol Heart Circ Physiol 287(5):H1921–H1927
Kiviniemi AM, Hautala AJ, Mäkikallio TH, Seppänen T, Huikuri HV, Tulppo MP (2006) Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R–R interval. Eur J Appl Physiol 96(6):686–692
Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP (2007) Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol 101(6):743–751
Laborde S, Mosley E, Thayer JF (2017) Heart rate variability and cardiac vagal tone in psychophysiological research: recommendations for experiment planning, data analysis, and data reporting. Front Psychol 8:213
Laborde S, Mosley E, Mertgen A (2018) Vagal tank theory: the three Rs of cardiac vagal control functioning–resting, reactivity, and recovery. Front Neurosci 12:458
Laborde S, Allen MS, Borges U, Dosseville F, Hosang TJ, Iskra M, Mosley E, Salvotti C, Spolverato L, Zammit N, Javelle F (2022) Effects of voluntary slow breathing on heart rate and heart rate variability: a systematic review and a meta-analysis. Neurosci Biobehav Rev 138:104711
Le Meur Y, Pichon A, Schaal K, Schmitt L, Louis J, Gueneron J et al (2013) Evidence of parasympathetic hyperactivity in functionally overreached athletes. Med Sci Sports Exerc 45:2061–2071
Levine BD (1993) Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc 25(6):727–732
Lipsitz LA, Mietus J, Moody GB, Goldberger AL (1990) Spectral characteristics of heart rate variability before and during postural tilt Relations to aging and risk of syncope. Circulation 81(6):1803–1810
Lucini D, Norbiato G, Clerici M, Pagani M (2002) Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension (Dallas Tex 1979) 39(1):184–188
Lundstrom CJ, Foreman NA, Biltz G (2023) Practices and applications of heart rate variability monitoring in endurance athletes. Int J Sports Med 44(1):9–19
Maggioni MA, Rundfeldt LC, Gunga HC, Joerres M, Merati G, Steinach M (2020) The advantage of supine and standing heart rate variability analysis to assess training status and performance in a walking ultramarathon. Front Physiol 11:731
Malliani A, Pagani M, Lombardi F, Cerutti S (1991) Cardiovascular neural regulation explored in the frequency domain. Circulation 84(2):482–492
Manresa-Rocamora A, Flatt AA, Casanova-Lizón A, Ballester-Ferrer JA, Sarabia JM, Vera-Garcia FJ, Moya-Ramón M (2021) Heart rate-based indices to detect parasympathetic hyperactivity in functionally overreached athletes. A meta-analysis. Scand J Med Sci Sports 31(6):1164–1182
Manser P, Thalmann M, Adcock M, Knols RH, de Bruin ED (2021) Can reactivity of heart rate variability be a potential biomarker and monitoring tool to promote healthy aging? A systematic review with meta-analyses. Front Physiol 12:686129
McCrory C, Berkman LF, Nolan H, O’Leary N, Foley M, Kenny RA (2016) Speed of heart rate recovery in response to orthostatic challenge. Circ Res 119(5):666–675
Medeiros AR, Leicht AS, Michael S, Boullosa D (2021) Weekly vagal modulations and their associations with physical fitness and physical activity. Eur J Sport Sci 21(9):1326–1336
Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D et al (2013a) Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc 45:186–205
Meeusen R, Duclos M, Foster C, Fry A, Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen A, European College of Sport Science and American College of Sports Medicine (2013b) Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc 45(1):186–205
Mellingsæter MR, Wyller TB, Ranhoff AH, Wyller VB (2015) Fit elderly men can also stand: orthostatic tolerance and autonomic cardiovascular control in elderly endurance athletes. Aging Clin Exp Res 27(4):499–505
Michael S, Graham KS, Davis GM, Oam (2017) Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals-a review. Front Physiol 8:301
Mitchell EA, Wealthall SR, Elliott RB (1983) Diabetic autonomic neuropathy in children: immediate heart-rate response to standing. Aust Paediatr J 19(3):175–177
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A (1994) Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90(4):1826–1831
Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, Iellamo F (2009) Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev 33(2):71–80
Nakamura Y, Yamamoto Y, Muraoka I (1993) Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. Jappl Physiol (Bethesda Md 1985) 74(2):875–881
Nelson MJ, Thomson RL, Rogers DK, Howe PR, Buckley JD (2014) Maximal rate of increase in heart rate during the rest-exercise transition tracks reductions in exercise performance when training load is increased. J Sci Med Sport 17(1):129–133
Nelson MJ, Bellenger CR, Thomson RL, Robertson EY, Davison K, Olstad DS, Buckley JD (2017) Maximal rate of heart rate increase correlates with fatigue/recovery status in female cyclists. Eur J Appl Physiol 117(12):2425–2431
Nicolini P, Ciulla MM, De Asmundis C, Magrini F, Brugada P (2012) The prognostic value of heart rate variability in the elderly, changing the perspective: from sympathovagal balance to chaos theory. Pac Clin Electrophysiol PACE 35(5):622–638
Nuuttila OP, Seipäjärvi S, Kyröläinen H, Nummela A (2022) Reliability and sensitivity of nocturnal heart rate and heart-rate variability in monitoring individual responses to training load. Int J Sports Physiol Perform 17(8):1296–1303
Page MM, Watkins PJ (1977) The heart in diabetes: autonomic neuropathy and cardiomyopathy. Clin Endocrinol Metab 6(2):377–388
Persson PB (1996) Modulation of cardiovascular control mechanisms and their interaction. Physiol Rev 76(1):193–244
Pichot V, Roche F, Gaspoz JM, Enjolras F, Antoniadis A, Minini P, Costes F, Busso T, Lacour JR, Barthélémy JC (2000) Relation between heart rate variability and training load in middle-distance runners. Med Sci Sports Exerc 32(10):1729–1736
Plews DJ, Laursen PB, Kilding AE, Buchheit M (2012) Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Eur J Appl Physiol 112(11):3729–3741
Plews DJ, Laursen PB, Kilding AE, Buchheit M (2013a) Evaluating training adaptation with heart-rate measures: a methodological comparison. Int J Sports Physiol Perform 8(6):688–691
Plews DJ, Laursen PB, Stanley J, Kilding AE, Buchheit M (2013b) Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med 43:773–781
Plews DJ, Laursen PB, Le Meur Y, Hausswirth C, Kilding AE, Buchheit M (2014) Monitoring training with heart rate-variability: how much compliance is needed for valid assessment? Int J Sports Physiol Perform 9(5):783–790
Plews DJ, Scott B, Altini M, Wood M, Kilding AE, Laursen PB (2017) Comparison of heart-rate-variability recording with smartphone photoplethysmography, polar H7 chest strap, and electrocardiography. Int J Sports Physiol Perform 12(10):1324–1328
Polar research and technology (2024). Polar orthostatic test. White paper downloaded on January 18th 2024: https://www.polar.com/en/img/static/whitepapers/pdf/polar-orthostatic-test-white-paper.pdf
Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ (1985) Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248(1 Pt 2):H151–H153
Porges SW (1992) Vagal tone: a physiologic marker of stress vulnerability. Pediatrics 90(3 Pt 2):498–504
Portier H, Louisy F, Laude D, Berthelot M, Guézennec CY (2001) Intense endurance training on heart rate and blood pressure variability in runners. Med Sci Sports Exerc 33(7):1120–1125
Quintana DS, Heathers JA (2014) Considerations in the assessment of heart rate variability in biobehavioral research. Front Psychol 5:805
Rabbani M, Agha-Alinejad H, Gharakhanlou R, Rabbani A, Flatt AA (2021) Monitoring training in women’s volleyball: supine or seated heart rate variability? Physiol Behav 240:113537
Ravé G, Fortrat JO (2016) Heart rate variability in the standing position reflects training adaptation in professional soccer players. Eur J Appl Physiol 116(8):1575–1582
Ricci F, De Caterina R, Fedorowski A (2015) Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol 66(7):848–860
Ritz T (2024) How to account for respiration in respiratory sinus arrhythmia: Publication standards for heart rate variability studies in Biological Psychology. Biol Psychol 190:108806
Rooke TW, Sparks HV (2003) Control mechanisms in circulatory function. In RA Rhoades and GA Tanner (Eds.), Medical physiology (pp. 290–308), Philadelphia, PA: Lippincott Williams and Wilkins)
Rowell LB (1993) Human cardiovascular control. Oxford University Press, USA
Saboul D, Pialoux V, Hautier C (2013) The impact of breathing on HRV measurements: implications for the longitudinal follow-up of athletes. Eur J Sport Sci 13(5):534–542
Sammito S, Böckelmann I (2015) Analyse der Herzfrequenzvariabilität. Math Basis Prak Anwend Herz 40(Suppl 1):76–84
Sandercock GR, Brodie DA (2006) The use of heart rate variability measures to assess autonomic control during exercise. Scand J Med Sci Sports 16(5):302–313
Sandercock GR, Bromley PD, Brodie DA (2005) The reliability of short-term measurements of heart rate variability. Int J Cardiol 103(3):238–247
Sander-Jensen K, Secher NH, Astrup A, Christensen NJ, Giese J, Schwartz TW, Warberg J, Bie P (1986) Hypotension induced by passive head-up tilt: endocrine and circulatory mechanisms. Am J Physiol Regulat Integr Comp Physiol 251(4):R742–R748
Sassi R, Cerutti S, Lombardi F, Malik M, Huikuri HV, Peng CK, Schmidt G, Yamamoto Y (2015) Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europ Euro Pac Arrhyth Card Electrophysiol J Work Groups Card Pac Arrhyth Card Cell Electrophysiol Europ Soc Cardiol 17(9):1341–1353
Schäfer D, Nil M, Herzig D, Eser P, Saner H, Wilhelm M (2015) Good reproducibility of heart rate variability after orthostatic challenge in patients with a history of acute coronary syndrome. J Electrocardiol 48(4):696–702
Scheffer M, Bolhuis JE, Borsboom D, Buchman TG, Gijzel SM, Goulson D, Olde Rikkert MG (2018) Quantifying resilience of humans and other animals. Proc Nat Acad Sci 115(47):11883–11890
Schmitt L, Hellard P, Millet GP, Roels B, Richalet JP, Fouillot JP (2006) Heart rate variability and performance at two different altitudes in well-trained swimmers. Int J Sports Med 27(3):226–231
Schmitt L, Regnard J, Desmarets M, Mauny F, Mourot L, Fouillot JP, Coulmy N, Millet G (2013) Fatigue shifts and scatters heart rate variability in elite endurance athletes. PLoS ONE 8(8):e71588
Schmitt L, Regnard J, Millet GP (2015a) Monitoring fatigue status with HRV measures in elite athletes: an avenue beyond RMSSD? Front Physiol 6:343
Schmitt L, Regnard J, Parmentier AL, Mauny F, Mourot L, Coulmy N, Millet GP (2015b) Typology of “Fatigue” by heart rate variability analysis in elite nordic-skiers. Int J Sports Med 36(12):999–1007
Schneider C, Hanakam F, Wiewelhove T, Döweling A, Kellmann M, Meyer T, Pfeiffer M, Ferrauti A (2018) Heart rate monitoring in team sports-a conceptual framework for contextualizing heart rate measures for training and recovery prescription. Front Physiol 9:639
Schneider C, Wiewelhove T, Raeder C, Flatt AA, Hoos O, Hottenrott L, Schumbera O, Kellmann M, Meyer T, Pfeiffer M, Ferrauti A (2019) Heart rate variability monitoring during strength and high-intensity interval training overload microcycles. Front Physiol 10:582
Seiler S, Haugen O, Kuffel E (2007) Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc 39(8):1366–1373
Shaffer F, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Health 5:258
Shaffer F, McCraty R, Zerr CL (2014) A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 5:1040
Shaffer F, Meehan ZM, Zerr CL (2020) A critical review of ultra-short-term heart rate variability norms research. Front Neurosci 14:594880
Soloveva A, Fedorova D, Villevalde S, Zvartau N, Mareev Y, Sitnikova M, Shlyakhto E, Fudim M (2020) Addressing orthostatic hypotension in heart failure: pathophysiology, clinical implications and perspectives. J Cardiovasc Transl Res 13(4):549–569
Stanley J, Peake JM, Buchheit M (2013) Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med (Auckland NZ) 43(12):1259–1277
Stewart JM (2012) Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol (Bethesda Md 1985) 113(10):1659–1668
Stewart JM, Medow MS, Glover JL, Montgomery LD (2006) Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circul Physiol 290(2):H665–H673
Swai J, Hu Z, Zhao X, Rugambwa T, Ming G (2019) Heart rate and heart rate variability comparison between postural orthostatic tachycardia syndrome versus healthy participants; a systematic review and meta-analysis. BMC Cardiovasc Disord 19(1):320
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93(5):1043–1065
Taylor AA (1994) Autonomic control of cardiovascular function: clinical evaluation in health and disease. J Clin Pharmacol 34(5):363–374
Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141(2):122–131
Tian Y, He ZH, Zhao JX, Tao DL, Xu KY, Earnest CP, Mc Naughton LR (2013) Heart rate variability threshold values for early-warning nonfunctional overreaching in elite female wrestlers. J Strength Cond Res 27(6):1511–1519
Tulppo MP, Hughson RL, Mäkikallio TH, Airaksinen KE, Seppänen T, Huikuri HV (2001) Effects of exercise and passive head-up tilt on fractal and complexity properties of heart rate dynamics. Am J Physiol Heart Circ Physiol 280(3):H1081–H1087
Uusitalo AL, Uusitalo AJ, Rusko HK (2000) Heart rate and blood pressure variability during heavy training and overtraining in the female athlete. Int J Sports Med 21(1):45–53
van Wijnen VK, Finucane C, Harms MPM, Nolan H, Freeman RL, Westerhof BE, Kenny RA, Ter Maaten JC, Wieling W (2017) Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 282(6):468–483
van Zanten S, Sutton R, Hamrefors V, Fedorowski A, de Lange FJ (2024) Tilt table testing, methodology and practical insights for the clinic. Clin Physiol Funct Imaging 44(2):119–130
Vescovi JD (2019) Intra-individual variation of HRV during orthostatic challenge in elite male field hockey players. J Med Syst 43(12):328
Vinik AI, Erbas T (2013) Diabetic autonomic neuropathy. Handb Clin Neurol 117:279–294
Wang T, Wu J, Qin F, Jiang H, Xiao X, Huang Z (2024) Computational modeling for the quantitative assessment of cardiac autonomic response to orthostatic stress. Physiol Meas 45:7. https://doi.org/10.1088/1361-6579/ad63ee
White DW, Raven PB (2014) Autonomic neural control of heart rate during dynamic exercise: revisited. J Physiol 592(12):2491–2500
Xu D, Verma AK, Garg A, Bruner M, Fazel-Rezai R, Blaber AP, Tavakolian K (2017) Significant role of the cardiopostural interaction in blood pressure regulation during standing. Am J Physiol Heart Circ Physiol 313(3):H568–H577
You M, Laborde S, Ackermann S, Borges U, Dosseville F, Mosley E (2024) Influence of respiratory frequency of slow-paced breathing on vagally-mediated heart rate variability. Appl Psychophysiol Biofeedback 49(1):133–143. https://doi.org/10.1007/s10484-023-09605-2
Zhang L, Wu H, Zhang X, Wei X, Hou F, Ma Y (2020) Sleep heart rate variability assists the automatic prediction of long-term cardiovascular outcomes. Sleep Med 67:217–224
Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H, Mayer P, Gries FA (1992) Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med J Br Diabet Assoc 9(2):166–175
Funding
None of the authors received funding for this work from any organization, other than salary support for the authors from their respective institutions.
Author information
Authors and Affiliations
Contributions
TG conducted the literature search and drafted the raw manuscript. All authors provided critical comments on the manuscript, read, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gronwald, T., Schaffarczyk, M. & Hoos, O. Orthostatic testing for heart rate and heart rate variability monitoring in exercise science and practice. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05601-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05601-4