Abstract
Purpose
This study compared the magnitude of excess post-exercise oxygen consumption (EPOC) between kettlebell complexes (KC) and high-intensity functional training (HIFT) and identified predictors of the EPOC response.
Methods
Active men (n = 11) and women (n = 10) (age 25 ± 6 yr) initially completed testing of resting energy expenditure and maximal oxygen uptake (VO2max), followed by lower and upper-body muscle endurance testing. On two subsequent days separated by ≥ 48 h, participants completed KC requiring 6 sets of kettlebell exercises (pushups, deadlifts, goblet squats, rows, and swings) with 60 s recovery between sets, and HIFT requiring 6 sets of bodyweight exercises (mountain climbers, jump squats, pushups, and air squats) with 60 s recovery. During exercise, gas exchange data and blood lactate concentration (BLa) were acquired and post-exercise, EPOC was assessed for 60 min.
Results
Results showed no difference in EPOC (10.7 ± 4.5 vs. 11.6 ± 2.7 L, p = 0.37), and VO2 and ventilation (VE) were significantly elevated for 30 and 60 min post-exercise in response to KC and HIFT. For KC and HIFT, HRmean and post-exercise BLa (R2 = 0.37) and post-exercise BLa and VE (R2 = 0.52) explained the greatest shared variance of EPOC.
Conclusion
KC and HIFT elicit similar EPOC and elevation in VO2 which is sustained for 30–60 min post-exercise, leading to 55 extra calories expended. Results show no association between aerobic fitness and EPOC, although significant associations were revealed for mean HR as well as post-exercise VE and BLa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical activity is one of the most promising nonpharmacological, noninvasive, and cost-effective methods of health promotion (Lachman et al. 2018). According to the 2018 Physical Activities Guidelines Advisory Committee Scientific Report, adults should perform 150 to 300 min a week of moderate-intensity, or 75 to 150 min a week of vigorous-intensity aerobic exercise, or an equivalent combination of moderate- and vigorous-intensity aerobic activity, to improve health status. In addition, they should perform resistance exercise (RE) on two or more days a week (Garber et al. 2011). However, approximately 80% of U.S. adults are insufficiently active (Piercy et al. 2018) which may reduce quality of life, physical function, and enhance risk of chronic disease.
The global prevalence of overweight and obesity has doubled since 1980, and approximately a third of the world’s population is now classified as overweight or obese (Chooi et al. 2019). Obesity represents a substantial health challenge because it dramatically increases risk of type 2 diabetes mellitus, fatty liver disease, hypertension, myocardial infarction, stroke, dementia, osteoarthritis, obstructive sleep apnoea, and several cancers, thereby contributing to a decline in both quality of life and life expectancy (Blüher 2019). Physical activity may significantly reduce fat mass as it enhances energy expenditure and in the case of RE, increases lean body mass (LBM) (Willis et al. 2012). This increase in LBM not only serves to enhance muscle strength and function, but it may also enhance basal metabolic rate (BMR). Therefore, regular RE is recommended for all adults including those with obesity to maintain LBM (Bennie et al. 2018).
Basal metabolic rate is the largest component of daily energy expenditure in Western societies (Johnstone et al. 2005). Prior data indicate a short-term elevation in BMR in response to acute exercise (generally termed the excess post-exercise O2 consumption; EPOC). EPOC appears to have two phases, one lasting < 2 h and a smaller much more prolonged response lasting up to 48 h (Speakman and Selman 2003). After moderate to vigorous exercise, temperature, heart rate (HR), and blood lactate concentration (BLa) are elevated, and concentrations of muscle glycogen and phosphocreatine are reduced (Hargreaves and Spriet 2020). To re-establish homeostasis which includes reversing exercise-induced increases in BLa, HR, temperature, and ventilation, repairing muscle damage, and restoring concentrations of muscle glycogen and phosphocreatine (PCr), energy expenditure (EE) is increased post-exercise resulting in EPOC. While some studies show that EPOC persists for several hours after RE (Schuenke et al. 2002), others suggest EPOC lasts < 1 h (Burleson et al. 1998). Nevertheless, a more prolonged and substantial EPOC occurs after high load versus moderate-load RE (Børsheim and Bahr 2003). Thus, the intensity of resistance exercise seems to be important for optimizing the magnitude of EPOC and potential changes in energy balance and in turn, body composition.
Despite the fact that RE elicits sizable health and fitness-related benefits, it is not accessible to all adults. The requisite equipment (various machines and barbells) and exercise facilities such as gyms can be expensive and potentially prevent inactive adults from initiating this modality of physical activity (Vancini et al. 2019). Nevertheless, kettlebell (KB) exercise is an alternative to traditional RE which significantly increases muscular power and strength (Otto et al. 2012; Williams and Kraemer 2015). A kettlebell complex (KC) is characterized by repeated sets of KB exercises such as goblet squats, KB swings, and deadlift (Kang & Kim 2022). KB exercise including the swing leads to significant increases in blood lactate concentration (BLa) and heart rate (HR) to near-maximal values in resistance-trained men, reflecting its vigorous nature (Budnar et al. 2014). In young adults, 8 weeks of KB training significantly improved maximal oxygen uptake (VO2max), leg press, and core and grip strength (Beltz et al. 2013).
High intensity functional training (HIFT) incorporates numerous modalities including calisthenics and various body weight exercises targeting muscles of the entire body (McDougle et al. 2023). Feito et al. (2018) reported that repeated sets of bodyweight exercises including pushups, squats, and mountain climbers elicit significantly higher HR than high intensity interval training (HIIT). McRae et al. (2012) reported that 16 sessions of HIFT consisting of whole-body aerobic-resistance training (one set of 8 × 20 s of a single exercise, burpees, jumping jacks, mountain climbers, or squat thrusts) significantly improved VO2max and muscle endurance. In older adults, Bocalini et al. (2012) demonstrated significant reductions in body mass and body fat in response to 36 sessions of HIFT (circuit training).
The primary aim of this study was to compare the magnitude of EPOC between KC and HIFT. It is apparent that EPOC is substantial after RE (Greer et al. 2021), yet it is unknown if KC and HIFT also elicit a substantial EPOC. If these protocols exhibit prolonged EPOC, then they could be recommended as part of exercise regimens seeking not only to increase muscular fitness, but also to regulate body mass. The secondary aim was to identify predictors of the EPOC response which are unresolved. Compared to adults with low VO2max, Short and Sedlock (1997) identified a lower EPOC after moderate exercise at 70% VO2max in individuals with higher VO2max. More recently, Matsuo et al. (2012) showed an inverse association between VO2max and EPOC acquired after sprint interval exercise. It was hypothesized that KC would elicit higher EPOC than HIFT due to the increased load, which should augment the magnitude of metabolic stress (e.g., higher BLa and greater reductions in PCr) during exercise and in turn, evoke a more significant EPOC. It was also hypothesized that muscular fitness and VO2max will be significantly and inversely correlated with EPOC, as shown in a prior study between VO2max and EPOC from interval-based exercise (Matsuo et al. 2012).
Methods
Participants
Healthy, active, and non-obese men (n = 11) and women (n = 10) participated in this study. They resistance trained at least 2 days per week for the preceding 6 mo and were free of any condition which may preclude intense exercise, specifically joint pain and presence of hypertension, heart disease, or diabetes. Participants provided written informed consent, and the research protocol was approved by the University IRB. This study was registered with Open Science Framework on May 18th, 2023. The participants filled out a health history questionnaire and PARQ to confirm eligibility, and their physical characteristics are shown in Table 1.
Experimental design
This study used a randomized, within subjects crossover design. On day 1, resting VO2, ventilation (VE), heart rate (HR), and respiratory exchange ratio (RER) were determined, followed by VO2max assessment. Subsequently, participants underwent muscular fitness testing followed by familiarization to KC and HIFT. The subsequent two sessions were held at the same time of day within subjects and required completion of KC or HIFT followed by assessment of pulmonary gas exchange data to determine EPOC. Participants did not know which exercise session they would undergo until arriving to the laboratory.
Assessment of body composition, resting VO 2 , and VO 2 max
Initially, height and weight were assessed using a stadiometer and balance beam scale to determine body mass index (BMI in kg/m2). Percent body fat was calculated by measuring subcutaneous fat at 3 sites in rotational order, chest, abdominal, and thigh in men and, triceps, suprailiac, and thigh in women (Jackson & Pollock 1978; Jackson et al. 1980). The metabolic cart (ParvoMedics True One, Sandy, UT) was calibrated according to manufacturer guidelines, and gas exchange data (VO2, VE, and RER) were acquired every 30 s while participants sat quietly in a chair for 30 min to assess resting VO2. Resting VO2, VE, HR, and RER were calculated from the average of the last 10 min of data. Subsequently, participants performed a ramp-based test on an electronically braked cycle ergometer (Velotron Dynafit Pro, Quarq, Spearfish, SD) during which pulmonary gas exchange data were obtained every 15 s using the metabolic cart. Exercise began with a 2 min warm-up at 40–70 W and power output was increased by 20–35 W/min until volitional exhaustion. Peak power output (PPO) and HRmax were identified as the peak values attained, and VO2max was identified as the mean of the two highest 15 s values acquired in the last 30 s of the test (Astorino et al. 2009). Participants recovered for 2 min at 50 W followed by a 5 min passive recovery. BLa was measured 3 min after the VO2max test via a fingertip blood sample using a portable analyzer (Lactate Plus, Sports Research Group, New Rochelle, NY).
Assessment of muscular fitness and familiarization
Approximately 10 min after the VO2max test, participants completed a maximal pushup test to volitional fatigue, with men performing the standard push-up using proper form. Women were given the choice of performing push-ups on their knees. Participants completed a passive recovery for 5 min, and then performed a maximal goblet squat test with a kettlebell approximately 25% of their body mass. Subsequently, the participants were familiarized with KC and HIFT by completing one set of each exercise. Proper form was demonstrated to ensure that all participants followed standardized technique for all exercises.
Description of the KC and HIFT protocols
The order of exercise protocols was randomized for each participant using random.org’s sequence generator. These sessions were held at least 72 h after the initial session and separated by a minimum of 72 h to maximum of 7 d. All sessions were held at the same time of day within subjects (0800 am–1600 pm) and were preceded by 24 h abstention from intense exercise and a minimum 3 h fast. Participants recorded all food and drink consumed 24 h prior to the initial session on a written log, and were advised to replicate this intake before the last session.
Prior to exercise, a Hans Rudolph mask (Kansas City, MO) was placed over the participant’s head. VO2, VE, RER, and HR were assessed during and post-exercise and expressed every 30 s. Each protocol included a 3 min warm-up consisting of 2 min of low-intensity cycling and 1 min of jumping jacks. The HIFT session consisted of 10 push ups for men or modified push ups for women, 10 squat jumps, 10 mountain climbers (per leg), and 25 air squats, which was repeated six times with 1 min of walking in place between sets. The KC session consisted of six sets of 8 pushups, 8 deadlifts, 8 goblet squats, 8 rows, and 8 swings using a load equal to 25% body mass, separated by 1 min of walking in place. This load was selected to require greater muscle force generation, as Fung and Shore (2010) showed loads of approximately 13% body mass potentiate the VO2 and cardiovascular response to KC exercise. Participants performed each session at the highest degree of effort and velocity. Preliminary testing showed that each set required approximately 1 min of exercise to complete, with slightly greater durations needed across sets 1 to 6.
Assessment of EPOC
Immediately (~ 15 s) after the last repetition, participants were seated in a chair. Gas exchange data were assessed for 1 h post-exercise while participants remained seated alone in a quiet room. This duration was selected as preliminary data showed that VO2 returned to baseline within 60 min post-exercise. EPOC (in L) was determined as the difference between post-exercise VO2 and resting VO2 (Lamont et al. 2010; Tucker et al. 2016) which was determined on day 1. Mean VO2, RER, HR, and VE were determined as the average value from the entire 1 h post-exercise period; in addition, VO2, RER, and VE were estimated at 5, 15, 30, 45, and 60 min by taking an average of the 5 (0–5 min), 10 (5–15 min), and 15 min (15–30, 30–45, and 45–60 min) of data preceding each timepoint. Total VO2 (in L) was calculated by taking the sum of 24 (exercise) and 120 (recovery) VO2 values and dividing by two (the number of samples per minute). Blood lactate concentration was determined pre-exercise as well as at 5, 15, 30, 45, and 60 min post-exercise using a portable meter and lancet (Lactate Plus, Sports Research Group, New Rochelle, NY). Mean outcomes assessed from exercise were determined as the mean value from the entire session including recovery but excluding the warm-up.
Statistical analysis
All data are reported as mean ± SD and were analyzed using SPSS version 25 (IBM, Armonk, NY). The Shapiro–Wilk test was performed to assess normality, and all outcomes were normally distributed. Independent t-test was used to examine differences in demographic and physiological outcomes between men and women. Three-way repeated measures analysis of variance having two within-subjects factors of condition (KC vs. HIFT) and time (6 timepoints) and one between subjects factor (sex in an exploratory analysis) was used to identify differences in the primary outcomes. Two-way ANOVA with repeated measures was used to identify differences in EPOC and total VO2 between protocols (a within subjects factor), with sex as a between-subjects factor. If a significant F statistic was obtained, dependent or independent t-test was used to identify differences between means. The Greenhouse–Geisser correction was used if the sphericity assumption was violated. Partial eta-squared was used as an estimate of effect size for ANOVA, with η2 = 0.01 indicating a small effect, η2 = 0.06 indicating a medium effect, and η2 = 0.14 indicating a large effect. Cohen’s d was used as a measure of effect size for pairwise comparisons, with values between 0.15 and < 0.40 representing “small,” between 0.40 and < 0.75 representing “moderate,” and ≥ 0.75 representing “large.” Stepwise multiple regression was used to identify predictors of EPOC using two-predictor models to maintain a sample size to predictor ratio of > 10:1. Pearson’s product moment correlation was used to identify relationships between magnitude of EPOC, HR, VE, BLa, and indices of aerobic and muscular fitness. GPower was used to compute a likely sample size for identifying differences in EPOC between protocols with a one-tailed t-test with the following parameters: p value = 0.05; power = 0.80, and effect size = 0.80. This resulted in a minimum sample size of 12 participants, which is lower than our sample. Statistical significance was set at p < 0.05.
Results
Maximal exercise data
Table 2 shows data obtained from testing of VO2max and muscular endurance. Men revealed significantly higher relative and absolute VO2max, PPO, and VE than women, although muscular endurance and VO2max expressed according to FFM were similar. Their VO2max values are classified as average cardiorespiratory fitness according to age- and sex-specific norms for cycle ergometry (Kaminsky et al. 2017).
Changes in EPOC and total VO 2 in response to KC and HIFT
Data showed no significant difference in EPOC (10.7 ± 4.5 vs. 11.6 ± 2.7 L, p = 0.39, η2p = 0.04, d = 0.27) between KC and HIFT which was equivalent to an additional 55 kcal burned post-exercise. Resting VO2 was equal to 0.25 ± 0.04 and 0.23 ± 0.03 L/min in men and women (p = 0.09, d = 0.50). Total VO2 was significantly higher in response to HIFT versus KC (40.0 ± 5.7 vs. 36.9 ± 6.2 L, p = 0.002, η2p = 0.39, d = 0.51, a moderate effect).
Changes in VO 2 and ventilation in response to KC and HIFT
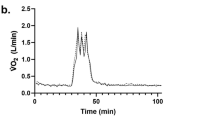
Mean VO2 increased almost six-fold in response to KC and HIFT from rest (Fig. 1a). For VO2, data showed a main effect of time (p < 0.001, η2p = 0.959), protocol (p = 0.012, η2p = 0.289), and protocol by time interaction (p < 0.001, η2p = 0.236), yet no other interactions were significant. Post hoc analyses showed that in response to KC, post-exercise VO2 at 5 (p < 0.001, d = 2.28) and 15 min (p < 0.001, d = 1.26) was higher versus rest yet at 30 min, VO2 was similar (p = 0.07, d = 0.20). For HIFT compared to rest, post exercise VO2 was significantly higher at 5 (p < 0.001, d = 2.28), 15 (p < 0.001, d = 1.81), 30 (p < 0.001, d = 0.95), 45 (p < 0.001, d = 0.94), and 60 min (p < 0.001, d = 0.96). VO2 was significantly greater in HIFT than KC at 15 min post-exercise (p = 0.035, d = 0.36, a small effect).
Data showed a main effect of time (p < 0.001, η2p = 0.917) and protocol (p = 0.021, η2p = 0.251) for VE, as well as a protocol by time (p < 0.001, η2p = 0.260) interaction (Fig. 1b). Compared to rest (8.4 ± 1.6 L/min), post-exercise VE was elevated at 5 min for KC (p < 0.001, d = 2.58) and HIFT (p < 0.001, d = 2.18). In addition, significant elevations in VE were shown at 15 (p < 0.001, d = 1.71 and p < 0.001, d = 1.69) and 30 min (p = 0.008, d = 0.69 and p = 0.002, d = 0.88) for KC and HIFT compared to rest. Post-hoc analyses showed a significantly lower exercise VE for KC compared to HIFT (p = 0.001, d = 0.47, a moderate effect).
Heart rate and RER responses to KC and HIFT
For HR, data showed a main effect of time (p < 0.001, η2p = 0.948) and protocol by time interaction (p = 0.031, η2p = 0.113), yet no main effect of protocol (p = 0.094, η2p = 0.141) (Fig. 1c). Mean HR increased twofold in response to exercise, and post-exercise HR was significantly elevated at 5 min for KC (p < 0.001, d = 2.17) and HIFT (p < 0.001, d = 2.34). Significant increases were also shown at 15 (p < 0.001, d = 2.01 and p < 0.001, d = 2.30), 30 (p < 0.001, d = 1.45 and p < 0.001, d = 1.85), 45 (p < 0.001, d = 0.97 and p < 0.001, d = 1.33), and 60 min (p < 0.001, d = 0.83 and p < 0.001, d = 0.88) post-exercise for both modalities compared to resting (72 ± 10 b/min). In addition, post-hoc analyses showed significantly greater post-exercise HR (a “small” effect) at 15 (p = 0.005, d = 0.32), 30 (p = 0.004, d = 0.37) and 45 min (p = 0.017, d = 0.29) in HIFT versus KC.
Data showed a main effect of time (p = 0.001, η2p = 0.866) for RER as well as a protocol by time interaction (p = 0.013, η2p = 0.130) (Fig. 1d). Post-Hoc analyses showed that RER was significantly greater at 5 min post exercise for KC and HIFT (p < 0.001, d = 3.63 and p < 0.001, d = 3.53) and 15 min post-exercise (p < 0.001, d = 1.57 and p = 0.003, d = 1.05) compared to rest. RER was significantly lower at 30 (p = 0.032, d = 0.72) 45 (p = 0.007. d = 0.80), and 60 min (p = 0.049, d = 0.56) post-exercise for HIFT compared to rest. RER was significantly greater in KC than in HIFT at 15 (p = 0.037, d = 0.59) and 45 (p = 0.034, d = 0.42) minutes post-exercise, showing a moderate effect.
Change in BLa in response to KC and HIFT
Data showed a main effect for BLa across time (p < 0.001, η2p = 0.796), but there was no effect of protocol (p = 0.092, η2p = 0.149) (Fig. 2). Data showed no significant protocol by time (p = 0.333, η2p = 0.061) interaction. Post-hoc analyses showed KC BLa values were significantly higher compared to rest (1.47 ± 0.66 mM) at 5 (p < 0.001, d = 2.40), 15 (p < 0.001, d = 2.09) 30 (p < 0.001, d = 1.29), and 45 (p = 0.018, d = 0.84) minutes post-exercise. For HIFT, BLa values were significantly higher compared to rest (1.40 ± 0.53 mM) at 5 (p < 0.001, d = 2.88), 15 (p < 0.001, d = 2.22), 30 (p < 0.001, d = 2.0), 45 (p < 0.001, d = 1.47) and 60 min (p < 0.001, d = 1.19) post-exercise.
Exploratory analysis of sex differences in responses to KC and HIFT
Data showed no sex X protocol interaction for EPOC (p = 0.24) between men and women for KC (9.2 ± 4.4 vs. 12.5 ± 4.2 L) or HIFT (11.0 ± 2.7 vs. 12.2 ± 2.8 L). There was also no sex X protocol interaction for total VO2 (37.2 ± 7.3 vs. 36.5 ± 5.2 L and 41.5 ± 6.2 vs. 38.3 ± 5.0 L for men and women, respectively, in response to KC and HIFT, p = 0.16, η2p = 0.10). Data for our outcomes separated by sex are shown in Figs. 3–5. Results showed a significant time X sex (p < 0.001, η2p = 0.451) interaction for VO2 as exercise VO2 was higher in men compared to women for KC (p = 0.04) and HIFT (p < 0.001). In addition, there was a significant time by sex interaction for VE (p < 0.001, η2p = 0.213). Data showed no significant difference in exercise VE for men versus women in KC (52.8 ± 15.3 vs. 46.2 ± 8.9 L/min, p = 0.248, d = 0.59) or HIFT (61.0 ± 16.6 vs. 50.3 ± 9.7 L/min, p = 0.089, d = 0.88). Nevertheless, there was a significantly greater post-exercise VE (a “large” effect) in response to KC at 5 (p = 0.024, d = 1.13), 15 (p = 0.025, d = 1.12), 30 (p = 0.001, d = 1.77), 45 (p = 0.011, d = 1.3), and 60 min (p = 0.25, d = 1.12) in women versus men. For HIFT, there was a significantly greater post-exercise VE at 60 min (p = 0.034, d = 1.06, a large effect) in women versus men.
Our results showed a time by sex interaction for HR (p < 0.001, η2p = 0.422), and mean exercise HR was higher (a “large” effect) in women versus men in response to KC (160 ± 10 b/min vs. 132 ± 17, p < 0.001, d = 2.08) and HIFT (154 ± 14 b/min vs. 130 ± 18, p = 0.001, d = 1.55). Post-exercise, there was a significantly higher HR (a “large” effect) for KC at 5 (p = 0.002, d = 1.61), 15 (p = 0.010, d = 1.33), and 30 min (p = 0.024, d = 1.13) in women versus men. Similar large effects were shown in response to HIFT, as there was a significant difference in HR between men and women at 5 (p = 0.003, d = 1.55), 15 (p = 0.006, d = 1.43), and 30 min (p = 0.023, d = 1.14). RER results showed a significant time by sex interaction (p = 0.007, η2p = 0.141). Post-hoc analyses also showed that post-exercise RER (a “large” effect) was significantly lower in women versus men for KC at 30 (p = 0.016, d = 1.15), 45 (p = 0.004, d = 1.56), and 60 min (p = 0.008, d = 1.33). In regards to the change in BLa, no sex X time or sex X protocol interactions occurred (p > 0.05).
Predictors of EPOC in response to KC and HIFT
Results showed no association between VO2max (r = −0.13, p = 0.29) or PU (r = −0.21, p = 0.18) and EPOC acquired from KC. However, data showed significant associations between EPOC and HRpeak (r = 0.42, p = 0.025), HRmean (r = 0.52, p = 0.008), VE (r = 0.52, p = 0.008), BLa at 5 min post-exercise (r = 0.52, p = 0.008), and GS (r = −0.43, p = 0.03). Multiple regression revealed several two-predictor models which significantly predicted EPOC, as shown in Table 3. A model including HRmean and BLa at 5 min post-exercise led to the highest R2 value (0.37) for EPOC from KC. In the case of HIFT, significant associations were revealed between magnitude of EPOC and PU (r = −0.52, p = 0.008), VE at 5 min post-exercise (r = 0.70, p < 0.001), and BLa at 5 min post-exercise (r = 0.60, p = 0.002), yet no association was shown for VO2max (r = −0.34, p = 0.07), HRmean (r = 0.29, p = 0.10) or GS (r = −0.29, p = 0.10). Ventilation and BLa acquired at 5 min post-exercise comprised the model with the highest R2 value (0.52) for HIFT.
Discussion
This study compared the magnitude of post-exercise metabolism between two highly accessible, low-volume RE protocols, KC and HIFT, and identified predictors of the EPOC response. Results showed no difference in EPOC between protocols, although the magnitude of EPOC is equivalent to an additional 55 kcal expended after exercise, which may be significant if these protocols are performed long-term. Significant predictors of EPOC included mean exercise HR and BLa for KC and post-exercise BLa and VE, which explained 37 and 52% of the variance in EPOC for KC and HIFT, respectively. HIFT elicited a significantly higher exercise VO2, HR, and VE than KC as well as a higher total VO2, suggesting a greater stress on the cardiorespiratory system. This result is likely due to the “all-out” nature of HIFT. In addition, our results show a more pronounced post-exercise ventilatory and HR response to exercise in women compared to men, suggesting that sex may impact recovery from these protocols.
Our results show no difference in EPOC between protocols, which is likely related to the similar BLa response between protocols (Fig. 2) since BLa is a primary contributor to EPOC (Brooks et al. 1973). Fourteen participants exhibited higher (0.34–9.57 L) EPOC in response to HIFT; whereas, seven participants with prior KC experience exhibited higher EPOC (0.91–6.67 L) in response to this modality. In addition, participants with higher EPOC in response to HIFT typically performed the exercises faster and in turn, demonstrated higher %HRmax and BLa than in KC. It is possible that our practice session was inadequate to familiarize participants to the protocols, which may have attenuated the acute metabolic stress and resultant magnitude of EPOC. Nevertheless, we believe that our protocol as completed induced vigorous exercise, as represented by substantial BLa accumulation (6–9 mM, Fig. 2) and intensities above 80%HRmax which are characteristic of HIFT and KC.
Our EPOC magnitude of ~ 11 L is greater than that reported in response to 10 bouts of HIIT at 85%PPO ~ 6 L (Matthews et al. 2020), yet lower than a value equal to ~ 15 L demonstrated in response to Wingate-based sprint interval exercise (Tucker et al. 2016). In healthy men completing circuit RE at a load equal to 75% of 20 repetition maximum, two sets of 20 repetitions of eight whole-body exercises interspersed with 20 s rest elicited an EPOC of ~ 10 L (Haltom et al. 1999), similar to our value. One study in men (João et al. 2021) using heavy RE (90% of 1 repetition maximum) demonstrated an EPOC equal to 11 L. Comparisons of EPOC across studies is challenging due to dissimilar participant characteristics and exercise protocols used, different approaches used to estimate EPOC, as well as a lower exercise volume in the current study (6 min of intense exercise) and relatively low load used (body weight and 25% body mass for HIFT and KC, respectively). However, it appears that these two protocols requiring only 12 min of time elicit similar EPOC as other RE regimens requiring a greater training volume. It is apparent that overall exercise intensity of RE is a primary mediator of EPOC magnitude, and additional study is needed to assess if heavier loads used for KC may serve to optimize the EPOC (Farinatti et al. 2012).
Our results showed no association between VO2max and EPOC, which opposes prior work and our hypothesis. Matsuo et al. (2012) exhibited a significant inverse association between VO2max and EPOC determined for 3 h after exercise. However, this study was conducted in healthy men who underwent vigorous aerobic exercise (three sets of 3 min intervals at 80–90% VO2max), so it is possible that discrepancies in exercise modality across studies explain our opposing results. We hypothesized that participants having higher VO2max would exhibit a smaller magnitude of EPOC due to a purported superior oxidative capacity and in turn, faster recovery post-exercise. Nevertheless, our results showed that GS and PU performance were inversely associated with EPOC from KC and HIFT, suggesting that participants having lower muscular fitness exhibit a more pronounced post-exercise VO2. HRmean and BLa acquired 5 min post-exercise as well as BLa and VE explained 37 and 52% of the variance in EPOC in response to KC and HIFT, respectively. This suggests that the magnitude of acute metabolic and cardiorespiratory stress of both bouts is significantly and strongly associated with the post-exercise metabolic response. Prior data (Brooks et al. 1973) suggest that a substantial amount of lactate is oxidized post-exercise and converted into glycogen, which would contribute to the magnitude of EPOC. Protracted increases in ventilation have also been considered as a potential predictor of EPOC (Moniz et al. 2020), which is also supported by our results.
Our data show no sex differences in the EPOC response; however, women exhibit a significantly higher post-exercise HR and VE versus men (Figs. 3, 4). Townsend et al. (2014) showed no effect of sex on the EPOC response, yet their study included eight men and eight women completing sprint cycling and running and not RE. One characteristic of prior studies examining EPOC is that sample sizes are typically small (~ 10), and in turn, are not adequately powered to detect differences between men and women. In seven men and seven women with matched VO2max, Lamont et al. (2020) measured VO2 for 1 h after 60 min of exercise at 50%VO2max, and results showed significantly higher resting VO2 and absolute EPOC (mL/min) in men compared to women, although when EPOC was expressed according to FFM, this difference was not significant despite sizable discrepancies in their EPOC values (0.62 ± 0.15 vs. 0.31 ± 0.26 mL/kg FFM/min). Women have less muscle mass than men, and it is possible that this anatomical difference requires women to generate greater force per muscle fiber versus men and thus induces a greater metabolic stress in response to RE, which may result in a more substantial EPOC. It is possible that the load used for KC exercise or traditional RE be prescribed according to LBM rather than body mass or percent one-repetition maximum, which may attenuate effects of these inherent differences in body composition on post-exercise recovery. The higher HR is likely attributed to the smaller heart size and lower blood volume in women versus men (Bassareo & Crisafulli 2020). Moreover, women exhibited a higher post-exercise VO2 and BLa, although these differences were not significant (p = 0.10). Additional study is needed using larger sample sizes to determine sex differences in post-exercise metabolic responses. Overall, our data suggest that HR and VE recovery from these dynamic, whole body vigorous exercise bouts may be dissimilar between men and women, which should be considered by clinicians who seek to optimize development of exercise programs for all adults.
This study has a few limitations. First, data cannot be applied to other regimens of KC or HIFT characterized by different modalities, intensity, or duration. Second, our results do not apply to less fit adults or those with obesity or chronic disease. Therefore, additional studies are needed which examine EPOC in distinct populations undergoing unique KC and HIFT regimens. Third, we only measured post-exercise VO2 for 60 min and it is possible that more protracted increases in VO2 could occur with a longer period of data collection. We elected to measure resting VO2 during our baseline session rather than including an additional non-exercise control condition, with the goal to reduce participant attrition by requiring three sessions rather than four. Moniz et al. (2020) suggested that measuring resting VO2 on a different day does not impact the resultant estimation of EPOC. Fourth, Burt et al. (2014) showed significant increases in energy expenditure for 48 h after 100 repetitions of Smith Machine squatting, which was explained by the need to repair damaged muscle fibers. Measurement of creatine kinase would shed light on onset of muscle damage which may contribute to long-term elevations in VO2 after KC or HIFT. Fifth, we did not consider potential impact of the menstrual cycle on EPOC magnitude, yet prior data (Ishikawa et al. 2023) show no differences in EPOC between the follicular and luteal phase in women. Sixth, this is an acute study, so additional investigation is needed to assess long-term responses to KC and HIFT in different participants. Lastly, we used a kettlebell equal to 25% body mass which is a heavier load than used in prior studies (Fortner et al. 2014; Chan et al. 2020). Therefore, additional investigation is needed to determine if different kettlebell loads impact EPOC magnitude.
Overall, our data show that KC and HIFT elicit a similar EPOC and elevation in VO2 which is sustained for approximately 30–60 min post-exercise, leading to 55 extra calories expended. The magnitude of EPOC is similar to that of traditional circuit training and greater than that generated from cycling-based HIIT. Our results oppose prior data and show no association between aerobic fitness and EPOC, although significant associations were revealed for indices of muscular fitness, indicating that individuals with lower muscular fitness may exhibit a greater post-exercise increase in VO2. BLa, VE, and exercise HR accounted for a sizable portion of the EPOC, suggesting that RE protocols eliciting substantial cardiometabolic stress may potentiate the magnitude of EPOC. In addition, women revealed a markedly higher post-exercise metabolic response to KC and HIFT versus men, likely associated with their small heart size and lower muscle mass. Further work is merited to employ different exercises, exercise volumes, and loads to maximize the post-exercise metabolic response to dynamic, whole-body RE such as KC and HIFT.
Data availability
All data relevant to this study are included in the manuscript.
Abbreviations
- BLa:
-
Blood lactate concentration
- BMI:
-
Body mass index
- EE:
-
Energy expenditure
- EPOC:
-
Excess post-exercise oxygen consumption
- HIFT:
-
High intensity functional training
- HR:
-
Heart rate
- KC:
-
Kettlebell complex exercise
- LBM:
-
Lean body mass
- PPO:
-
Peak power output
- RE:
-
Resistance exercise
- RER:
-
Respiratory exchange ratio
- BMR:
-
Basal metabolic rate
- V̇E :
-
Ventilation
- V̇O2 :
-
Oxygen consumption
- V̇O2max:
-
Maximal oxygen uptake
References
Astorino TA, White AC, Dalleck LC (2009) Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int J Sports Med 30(4):279–284
Bassareo PP, Crisafulli A (2020) Gender differences in hemodynamic regulation and cardiovascular adaptations to dynamic exercise. Curr Cardiol Rev 16(1):65–72. https://doi.org/10.2174/1573403X15666190321141856
Beltz, N., Erbes, D., Porcari, J., Martinez, R., Doberstein, S., & Foster, C. (2013). Kettlebells Kick Butt. ACE. 1–3
Bennie JA, Lee D-C, Khan A, Wiesner GH, Bauman AE, Stamatakis E, Biddle SJH (2018) Muscle-strengthening exercise among 397,423 U.S. adults: prevalence, correlates, and associations with health conditions. Am J Prev Med 55(6):864–874. https://doi.org/10.1016/j.amepre.2018.07.022
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nature Rev Endocrinol 15(5):288–298. https://doi.org/10.1038/s41574-019-0176-8
Bocalini DS, Lima LS, de Andrade S, Madureira A, Rica RL, dos Santos RN, Serra AJ, Silva JA, Rodriguez D, Figueira A, Pontes FL (2012) Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin Intervent Aging 7:551–556. https://doi.org/10.2147/CIA.S33893
Børsheim E, Bahr R (2003) Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med (auckland, n.z.) 33(14):1037–1060. https://doi.org/10.2165/00007256-200333140-00002
Brooks GA, Brauner KE, Cassens RG (1973) Glycogen synthesis and metabolism of lactic acid after exercise. Am J Physiol 224(5):1162–1166. https://doi.org/10.1152/ajplegacy.1973.224.5.1162
Budnar RG, Duplanty AA, Hill DW, McFarlin BK, Vingren JL (2014) The acute hormonal response to the kettlebell swing exercise. J Str Cond Res 28(10):2793–2800. https://doi.org/10.1519/JSC.0000000000000474
Burleson MAJ, O’Bryant HS, Stone MH, Collins MA, Triplett-McBride T (1998) Effect of weight training exercise and treadmill exercise on post-exercise oxygen consumption. Med Sci Sports Exerc 30(4):518–522
Burt DG, Lamb K, Nicholas C, Twist C (2014) Effects of exercise-induced muscle damage on resting metabolic rate, sub-maximal running, and post-exercise oxygen consumption. Eur J Sports Sci 14(4):337–344. https://doi.org/10.1080/17461391.2013.783628
Chan M, MacInnis MJ, Koch S, MacLeod KE, Lohse KR, Gallo ME, Sheel AW, Koehle MS (2020) Cardiopulmonary demand of 16-kg kettlebell snatches in simulated girevoy sport. J Str Cond Res 34(6):1625–1633. https://doi.org/10.1519/JSC.0000000000002588
Chooi YC, Ding C, Magkos F (2019) The epidemiology of obesity. Metab Clin Exper 92:6–10. https://doi.org/10.1016/j.metabol.2018.09.005
Farinatti P, Castinheiras Neto AG, da Silva NL (2012) Influence of resistance training variables on excess postexercise oxygen consumption: a systematic review. Int Scholarly Res Not. https://doi.org/10.1155/2013/825026
Feito Y, Heinrich KM, Butcher SJ, Poston WSC (2018) High-intensity functional training (HIFT): definition and research implications for improved fitness. Sports 6(3):76. https://doi.org/10.3390/sports6030076
Fortner HA, Salgado JM, Holmstrup AM, Holmstrup ME (2014) Cardiovascular and metabolic demands of the kettlebell swing using Tabata interval versus a traditional resistance protocol. Int J Exerc Sci 7(3):179–185
Fung BJ, Shore SL (2010) Aerobic and anaerobic work during kettlebell exercise: a pilot study. Med Sci Sports Exerc 42(5):834. https://doi.org/10.1249/01.MSS.0000386575.49791.be
Greer BK, O’Brien J, Hornbuckle LM, Panton LB (2021) EPOC comparison between resistance training and high-intensity interval training in aerobically fit women. Int J Exerc Sci 14(2):1027–1035
Haltom RW, Kraemer RR, Sloan RA, Hebert EP, Frank K, Tryniecki JL (1999) Circuit weight training and its effects on excess postexercise oxygen consumption. Med Sci Sports Exerc 31(11):1613–1618. https://doi.org/10.1097/00005768-199911000-00018
Hargreaves M, Spriet LL (2020) Skeletal muscle energy metabolism during exercise. Nature Metab 2(9):817–828. https://doi.org/10.1038/s42255-020-0251-4
Ishikawa A, Matsuda T, Ikegami N, Funaki A, Yamada M, Kamemoto K, Sakamaki-Sunaga M (2023) Effects of the menstrual cycle on EPOC and fat oxidation after low-volume high-intensity interval training. J Sports Med Phys Fit 63(11):1165–1174
Jackson AS, Pollock ML (1978) Generalized equations for predicting body density of men. Br J Nutr 40:497–504
Jackson AS, Pollock ML, Ward A (1980) Generalized equations for predicting body density of women. Med Sci Sports Exerc 12:175–181
João GA, Almeida GPL, Tavares LD, Kalva-Filho CA, Carvas Junior N, Pontes FL, Baker JS, Bocalini DS, Figueira AJ (2021) Acute behavior of oxygen consumption, lactate concentrations, and energy expenditure during resistance training: comparisons among three intensities. Front Sports Active Living 3:797604. https://doi.org/10.3389/fspor.2021.797604
Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR (2005) Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Amer J Clin Nutr 82(5):941–948. https://doi.org/10.1093/ajcn/82.5.941
Kaminsky LA, Imboden MT, Arena R, Myers J (2017) Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the fitness registry and the importance of exercise national database (FRIEND) registry. Mayo Clin Proc 92(2):228–233. https://doi.org/10.1016/j.mayocp.2016.10.003
Kang DH, Kim Y-N (2022) The effect of a kettlebell complex program according to the support surface on lower extremity muscle activity and balance in baseball players. J Korean Phys Ther 34(6):312–316
Lachman ME, Lipsitz L, Lubben J, Castaneda-Sceppa C, Jette AM (2018) When adults don’t exercise: behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innov Aging. https://doi.org/10.1093/geroni/igy007
Lamont LS, Romito R, Rossi K (2010) Fat-free mass and gender influences the rapid-phase excess post exercise oxygen consumption. Appl Physiol Nutr Metab 35:23–26
Matsuo T, Ohkawara K, Seino S, Shimojo N, Yamada S, Ohshima H, Tanaka K, Mukai C (2012) Cardiorespiratory fitness level correlates inversely with excess post-exercise oxygen consumption after aerobic-type interval training. BMC Res Notes 5:646. https://doi.org/10.1186/1756-0500-5-646
Matthews ARD, Astorino TA, Crocker GH, Sheard AC (2020) Acute effects of high-intensity interval exercise while wearing a sauna suit on energy expenditure and excess post-exercise oxygen consumption. J Strength Cond Res 34:2427–2433
McDougle JM, Mangine GT, Townsend JR, Jajtner AR, Feito Y (2023) Acute physiological outcomes of high-intensity functional training: a scoping review. PeerJ 11:e14493. https://doi.org/10.7717/peerj.14493
McRae G, Payne A, Zelt JGE, Scribbans TD, Jung ME, Little JP, Gurd BJ (2012) Extremely low volume, whole-body aerobic–resistance training improves aerobic fitness and muscular endurance in females. Appl Physiol Nutr Metab 37(6):1124–1131. https://doi.org/10.1139/h2012-093
Moniz SC, Islam H, Hazell TJ (2020) Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand J Med Sci Sports 30(4):638–651. https://doi.org/10.1111/sms.13610
Otto WHI, Coburn JW, Brown LE, Spiering BA (2012) Effects of weightlifting vs kettlebell training on vertical jump, strength, and body composition. J Str Cond Res 26(5):1199–1202. https://doi.org/10.1519/JSC.0b013e31824f233e
Schuenke MD, Mikat RP, McBride JM (2002) Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: implications for body mass management. Eur J Appl Physiol 86(5):411–417. https://doi.org/10.1007/s00421-001-0568-y
Short KR, Sedlock DA (1997) Excess postexercise oxygen consumption and recovery rate in trained and untrained subjects. J Appl Physiol (1985) 83(1):153–159. https://doi.org/10.1152/jappl.1997.83.1.153
Speakman JR, Selman C (2003) Physical activity and resting metabolic rate. Proc Nutr Soc 62(3):621–634. https://doi.org/10.1079/PNS2003282
Townsend LK, Couture KM, Hazell TJ (2014) Mode of exercise and sex are not important for oxygen consumption during and in recovery from sprint interval training. Appl Physiol Nutr Metab 39(12):1388–1394. https://doi.org/10.1139/apnm-2014-0145
Tucker WJ, Angadi SS, Gaesser GA (2016) Excess postexercise oxygen consumption after high-intensity and sprint interval exercise, and continuous steady-state exercise. J Str Cond Res 30(11):3090–3097. https://doi.org/10.1519/JSC.0000000000001399
Vancini RL, Andrade MS, Rufo-Tavares W, Zimerer C, Nikolaidis PT, de Lira CAB (2019) Kettlebell exercise as an alternative to improve aerobic power and muscle strength. J Hum Kinet 66:5–6. https://doi.org/10.2478/hukin-2018-0062
Williams BM, Kraemer RR (2015) Comparison of cardiorespiratory and metabolic responses in kettlebell high-intensity interval training versus sprint interval cycling. J Str Cond Res 29(12):3317–3325. https://doi.org/10.1519/JSC.0000000000001193
Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, Houmard JA, Kraus WE (2012) Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (bethesda, Md.: 1985) 113(12):1831–1837. https://doi.org/10.1152/japplphysiol.01370.2011
Acknowledgements
The authors thank the participants for their dedication to complete this study. This project was partially funded by a Research and Scholarly Activity grant.
Author information
Authors and Affiliations
Contributions
BS and TA had the following roles in creation of the study: BS and TA conceived the study idea, conducted pilot testing, recruited participants, collected data, and analyzed data. BS and TA wrote the initial and final versions of this manuscript. BS and TA approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in executing this study.
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sturdy, R.E., Astorino, T.A. Post-exercise metabolic response to kettlebell complexes vs. high intensity functional training. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05579-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05579-z