Abstract
Objective
Although neural adaptations from strength training are known to occur, the acute responses associated with heavy-strength (HST) and hypertrophy training (HYT) remain unclear. Therefore, we aimed to compare the acute behaviour of corticospinal responses following a single session of HST vs HYT over a 72-h period.
Methods
Fourteen participants completed a random counterbalanced, crossover study that consisted of a single HST session [5 sets × 3 repetition maximum (RM)], a HYT session (3 sets × 12 RM) of the leg extensors and a control session (CON). Single- and paired-pulse transcranial magnetic stimulation (TMS) was used to measure changes in motor-evoked potential (MEP) amplitude, corticospinal silent period (CSP), intra-cortical facilitation (ICF), short-interval intra-cortical inhibition (SICI) and long-interval intra-cortical inhibition (LICI). Additionally, maximal muscle compound wave (M MAX) of the rectus femoris (RF) and maximal voluntary isometric contraction (MVIC) of the leg extensors were taken. All measures were taken at baseline, immediately post and 2, 6, 24, 48 and 72 h post-training.
Results
A significant condition x time interaction was observed for MVIC (P = 0.001), M MAX (P = 0.003), MEP amplitude (P < 0.001) and CSP (P = 0.002). No differences were observed between HST and HYT for all neurophysiological measures. No changes in SICI, ICF and LICI were observed compared to baseline.
Conclusion
Our results suggest that: (1) the acute behaviour of neurophysiological measures is similar between HST and HYT; and (2) the increase in corticospinal excitability may be a compensatory response to attenuate peripheral fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different modes of resistance training (RT) are applied in athletics, conditioning rehabilitation and general RT practice to promote targeted gains in neuromuscular performance (Ratamess et al. 2009). Hypertrophy training (HYT), characterised by higher volume and low-to-moderate intensity loads (67–75% of 1 RM, 6–15 repetitions) is commonly employed during the early phase of a training program to increase muscle mass (Bompa and Haff 2009; Haff and Triplett 2016). On the other hand, heavy-strength training (HST) is characterised by low repetition and high intensity loads (≥80% of 1 RM, 1–6 repetitions) intended to increase maximal strength (Ratamess et al. 2009). HST is often introduced after the hypertrophy phase or where specific strength adaptations are required (Bompa and Haff 2009).
While the neuromuscular adaptations from repeated HST are well-established (Latella et al. 2012; Hendy and Kidgell 2013; Kidgell and Pearce 2010; Carroll et al. 2011; Selvanayagam et al. 2011; Folland and Williams 2007), the acute intra-cortical and corticospinal responses have seldom been directly compared with other RT modes such as HYT. Furthermore, previous investigations into HYT have primarily focused on physiological changes (Schoenfield 2014; Phillips et al. 2002; Kim et al. 2005; Walker et al. 2011), whilst the neural basis of HYT has not been well-established. We have previously reported that the MEP amplitude and M MAX is impaired following and acute session of HST in the elbow flexors (Latella et al. 2016). No intra-cortical changes were observed following the protocol indicating that the responses were modulated downstream of the primary motor cortex. Contrarily, Roustsalianen et al. (2014) also showed that an initial increase in MEP amplitude and CSP duration occurred following an acute session of HYT. Increases in corticospinal excitability and decreases in SICI have also been shown with various contraction types (ballistic and slow ramp isometric, dynamic metronome paced) of the elbow flexors (Nuzzo et al. 2016a; Leung et al. 2015). At the peripheral level, changes in peripheral nerve excitability are also known to occur with fatiguing exercises such that both reductions (Behm and St-Pierre 1997; Sacco et al. 1997; Nuzzo et al. 2016c) and increases (Behm and St-Pierre 1997; Nuzzo et al. 2016b) in M MAX have been shown following resistance exercise. These studies suggest that dynamic RT duration may have a differential effect on peripheral nerve excitability (Behm and St-Pierre 1997), while others have showed no differences between 2 and 12 sets of isometric training (Nuzzo et al. 2016b). Despite these suggestions, and to the best of our knowledge, there have been no direct investigations comparing the acute central and peripheral neural responses following a single session of applied HST and HYT.
From a neuromuscular standpoint, acute impairments in force production have been investigated extensively following strength, hypertrophy and power training (Walker et al. 2012; Howatson et al. 2016; Brandon et al. 2015; Nicholson et al. 2014). However, due to differences in exercise selection and training parameters (i.e., load and volume), the literature has produced conflicting results. Nicholson et al. (2014) showed no differences in reduction in peak force of the lower limbs following HST or HYT squat training when the load was equal between conditions. However, volume equated loads are not traditionally used for HST and HYT protocols in applied settings. The similarities in the reduction of maximal force production of the lower limbs following HST and HYT protocols has also demonstrated in later studies (Brandon et al. 2015; Howatson et al. 2016). Conversely, Walker et al. (2012) showed impairment of the MVIC to be greater following (5 × 10 RM) compared to (15 × 1 RM) leg presses. However, some evidence has suggested that shorter rest period durations can impair in session neuromuscular performance (Scudese et al. 2015) with less direct evidence on the subsequent effect of altered exercise volume and intensity. However, it must be acknowledged that this comparison was using the same volume and intensity between conditions and may not accurately reflect the changes associated with typical HST and HYT. Therefore, based upon previous findings it remains unclear whether the force generating capacity of a joint is differentially affected by the combination of intrinsic session variables (i.e., volume, intensity and rest period duration) typically seen in separate RT modalities.
The aim of this study was to directly compare the acute changes in central and peripheral neural responses, and neuromuscular torque between HST and HYT in the leg extensor muscles over a 72-h period post-training. Specifically, we aim to determine differences in corticospinal excitability, short-/long-interval intra-cortical inhibition and facilitation, and maximal M-wave responses following HST and HYT. Given the variance in load and intensity between both training modalities, we hypothesised that the change in central and peripheral neural excitability would be greater with HST due to the proposed demands placed on the central and peripheral nervous system. The results from our study will be important to understand the underlying neurophysiological responses to different applied RT modalities used by strength and conditioning professionals aimed at targeting specific performance adaptations in athletes.
Methods
Participants
Fourteen (M = 9, F = 5) healthy individuals (26.2 ± 3.1 years, 81.3 ± 9.6 kg, 174.2 ± 10.5 cm) with no reported incidence of neuromuscular injury to the lower limb completed a randomised, counterbalanced crossover study comparing HST and HYT, and a control (CON) conditions. All participants were recreationally resistance-trained (6–12 months experience) and reported training at least twice a week. Written informed consent was obtained for each participant prior to the start of the study. To determine limb dominance, the Waterloo Footedness Questionnaire (Elias et al. 1998) was administered and only participants that were right foot dominant were included in this study. Prior to TMS, all participants were screened using a TMS safety questionnaire to exclude potential participants with contraindications to TMS (Rossi et al. 2009). Female participants were screened to ensure they were not undertaking any part of the protocol during menstruation. All procedures used in this study were approved by the Deakin University Human Research Ethics Committee (Project ID: 2013-198) and conducted to the standards set by the Declaration of Helsinki.
Transcranial magnetic stimulation and surface electromyography recording
Single- and paired-pulse TMS was used to measure changes in corticospinal excitability and cortical inhibition and facilitation. All TMS measurements were taken with the participant seated upright with their right knee flexed at a 45° angle (full knee extension equates to 0 degrees), hip flexed at 90° and was conducted on the same chair as used for MVIC measurements. Surface electromyography (sEMG) was recorded from the RF muscle in the dominant leg using Ag–AgCL electrodes. Two electrodes were placed 20 mm apart on the midpoint of the belly of RF, with the ground electrode placed over the patella according to SENIAM guidelines (Hermens et al. 2000). The skin was prepared by removing any hair and cleaned with 70% isopropyl alcohol swabs prior to the placement of the electrodes. Surface electromyography signals were amplified (1000×) with bandpass filtering between 20 Hz and 1 kHz and digitised at 10 kHz for 500 ms, recorded and analysed using PowerLab 4/35 (ADinstruments, Australia).
To ensure consistent delivery of TMS stimuli within and between testing sessions, all participants wore a custom-made snug-fitted cap (EasyCap, Germany) positioned in relation to nasion–inion and inter-aural lines. The cap was marked with points at 1 cm intervals in a longitude–latitude matrix to allow repeated stimuli to be performed at the same point over the primary motor cortex (M1) each time. Single and paired-pulse TMS were applied over the cortical motor representation of the RF on the M1, using a double cone 110-mm coil attached to a BiStim 2002 magnetic stimulator (Magstim Co., Dyfed, UK). The double cone coil was placed over the vertex of the scalp and the ‘optimal site’ that elicited the largest and most consistent motor-evoked potentials (MEP) from the RF, determined through initial exploration in areas surrounding the vertex for each individual. Once the optimal site was located, the resting motor threshold (RMT), used for calculating paired-pulse stimulation of the RF muscle was determined by the lowest TMS intensity at which an MEP could be obtained with at least 5 of the 10 stimuli with peak-to-peak amplitude of 50–100 µV at rest (Rothwell et al. 1999; Westin et al. 2014). Determination of the active motor threshold (AMT), used for single-pulse stimulation, required the participant to hold a steady contraction at 10% MVIC and defined as the lowest TMS intensity at which a MEP could be obtained with at least 5 of the 10 stimuli with peak-to-peak amplitude being greater than 200 µV (Rothwell et al. 1999). Ten single-pulse TMS were applied at 20% above AMT and were administered with a randomly chosen 5–8 s intervals between stimuli. To account for any differences in peripheral nerve excitability, all measures of single-pulse MEP amplitude were normalised to M MAX (MEP amplitude/M MAX = Normalised MEP). The CSP duration was calculated as the time from the onset of the MEP to the return of the sEMG signal in (ms). Paired-pulse TMS was conducted with the muscle at rest as used in other exercise fatigue studies (Verin et al. 2004; Benwell et al. 2006) using RMT for the calculation of stimulation intensities.
Paired-pulse was used to measure changes in intra-cortical measures of SICI, LICI and ICF. Paired-pulse TMS consisted of a conditioning (CS) and test stimulus (TS) separated by a specified interstimulus intervals (ISI) and the configuration for SICI, LICI and ICF were as follows; SICI (CS = 90% RMT, TS = 120% RMT, ISI = 3 ms) (Kujirai et al. 1993), LICI (CS = 120% RMT, TS = 120% RMT, ISI = 100 ms) (Du et al. 2014; McNeil et al. 2011) and ICF (CS = 90% RMT, TS = 120% RMT, ISI = 12 ms) (Kobayashi & Pascual-Leone 2003; Kujirai et al. 1993). Both SICI and ICF were expressed as a percentage of the unconditioned single-pulse MEP amplitude, while LICI was calculated and expressed as a percentage of the test to conditioning MEP amplitude for each individual paired stimuli.
Maximal M-wave measurements
Maximal M-wave (M MAX) responses, measured as the peak-to-peak amplitude of the wave-form were obtained from sEMG recording of the right RF muscle by direct supramaximal electrical stimulation (pulse duration 100 ms) of the femoral nerve under resting conditions using a high-voltage constant current electrical stimulator (Nihon Khoden, Japan). Nerve stimulation was delivered using bipolar electrodes positioned over the right femoral nerve in the femoral triangle approximately 3–5 cm below the inguinal ligament (Doguet and Jubeau 2014) along the right inguinal fold. The nerve stimulation current intensity was progressively increased until there was no further increase in sEMG amplitude. To ensure maximal responses were obtained, the maximal current intensity was further increased by 20% and the highest M MAX obtained from 5 stimuli was recorded.
Maximal voluntary isometric contraction of the leg extensor muscles
Maximal torque of the RF muscle was measured using a 5 s MVIC (2 s ramp up followed by 3 s maximal effort). Three MVIC trials, separated by a 60 s rest period, were conducted with the participants seated upright on a Cybex dynamometer (Cybex, USA) and strapped across the chest and hips to prevent extraneous movements of the upper body. The ankle of the right foot was strapped to the immovable leg extension arm of the dynamometer, approximately 7.5 cm proximal to the medial malleolus (Krishnan et al. 2011). The hip was positioned at 90° of flexion with a 45° flexion angle of the right knee (Krishnan et al. 2011). Verbal encouragement and real-time visual force feedback were provided for each effort. All torque signals were sampled at 1000 Hz, with additional filtering not required. The maximal recorded peak torque (Nm) of the three trials was reported as MVIC.
Experimental protocol
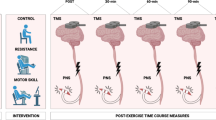
Figure 1 shows the setup and timeline for each testing session. The study consisted of a familiarisation session prior to testing, to reduce the potential of any learning effects on the outcome measures of the study (described in the subsequent sections), followed by three testing conditions (HST vs HYT vs CON) performed in a counterbalanced randomised order. During the familiarisation session, the outcome measures included maximal voluntary isometric contraction on dynamometer (Cybex Humac Norm, USA), single- and paired-pulse TMS using a (Bistim 2002, magnetic stimulator (Magstim Co., Wales, UK) and peripheral nerve stimulation using a constant direct current electric stimulator (Nihon Koden, Japan) were recorded. Each participant’s 1 RM single leg extension strength was also measured on a leg extension machine (Nautilus Pin Loaded Leg Extension, Canada) and recorded to determine the training load intensity for the subsequent HST and HYT sessions. A 1-week inter-protocol period was implemented between each of the four visits (familiarisation, HST, HYT and CON). Participants were instructed to refrain from any strenuous lower body exercise 72 h prior to and during all conditions, asked to refrain from stretching, active recovery and asked to maintain usual dietary and sleeping habits throughout the testing period. During the 1-week intermissions, participants were allowed to continue usual training as long as this did not interfere with the 72-h period prior to the subsequent testing protocol. All testing sessions and associated outcome measures for each session were tested on the same time-of-day in a shielded laboratory to account for any circadian fluctuations in neuromuscular performance (Teo et al. 2011) and effects on arousal from external distractions.
The training load intensity for HST and HYT of the leg extensor muscles was set at the participant’s calculated 3 RM (94% of 1 RM), and 12 RM (67% of 1 RM), respectively, that was derived from the 1 RM obtained in the familiarisation session using the formula developed by Brzycki (1993). The HST protocol consisted of 5 working sets consisting of 3 RM with 180 s recovery in between (total volume 15 repetitions). The HYT protocol consisted of 3 working sets consisting of 12 RM with 60 s recovery in between (total volume 36 repetitions). The differences in training volume between HST and HYT were acknowledged as an important factor in maintaining the integrity of real-world RT programs which have a disparate training volumes and intensities. The training load was increased if the researcher (a certified strength and conditioning practitioner) deemed that extra repetitions could be performed, and likewise, lowered if there was failure to complete the repetitions with proper form. The contraction tempo for the leg extension exercise was set at 3 s eccentric phase, 0 s pause, 3 s concentric phase (Ackerley et al. 2011; Latella et al. 2012; Hendy and Kidgell 2013). Prior to the resistance exercise, all participants performed a 5 min warm up on a cycle ergometer at 60% estimated maximum predicted heart rate, and 2 warm up sets of leg extensions at 12 and 10 repetitions at an increasing weight. During the control session, all participants performed the warm ups on the cycle ergometer sat quietly for 15 min (average training time of HST and HYT conditions) between pre and post neurophysiological measures. All outcome measures were assessed at baseline (prior to the warm up of each testing session), immediately after and at 2, 6, 24 and 48 and 72 h post-training. These specified time points corresponded to the fatigue (post-training—2 h), recovery (6, 24 and 48 h) and adaptation (72 h) phases as reported by the super-compensation theory (Bompa and Haff 2009).
Statistical analysis
All data were analysed using IBM SPSS Statistics v.22 (IBM, USA). Data were screened for outliers followed by a Shapiro–Wilk test and found to be normally distributed prior to further analysis. A 3 × 7 repeated measures analysis of variance (ANOVA) with factors CONDITION (HST, HYT and CON) and TIME (Pre, post, 2, 6, 24, 48 and 72 h) were used to compare changes in MVIC, MMAX, MEP amplitude, CSP, SICI, LICI and ICF between conditions and across time. Where statistical significance was detected between conditions, post-hoc paired t tests with a Bonferroni correction were conducted to test for differences between individual groups (Field 2013). For all tests, the Greenhouse–Geisser correction was applied if the assumption of sphericity was violated. Alpha level was set at P < 0.05, and all results are displayed as mean ± SE. Within participant reliability data was calculated for MEP, ICF and SICI using intra-class correlation coefficients (ICCs) and Pearson’s product-moment coefficient (r) at baseline for each condition across time for the CON condition. ICC’s were classified as poor (<0.40), fair (0.40–0.59), good (0.60–0.74) and excellent (≥0.75) as used in previous TMS research (Temesi et al. 2017). In addition, the within participant coefficient of variation (CV) was expressed as a percentage derived from the formula (poolSD/poolMean) × 100 where the SD and mean is a pooled value of the sample with ≤10% indicating low variability. Absolute reliability was calculated to establish the variability of repeated measurements (Atkinson and Nevill 1998) using the standard error of the mean (SEM) = SD √(1 − ICC) and the minimal detectable change at the 95% confidence interval (MDC95) = SEM × √(2) × 1.96 was also calculated as similarly displayed in other physical research studies (Overend et al. 2010). All reliability data has been reported in (Tables 1 and 2).
Results
Neuromuscular, corticospinal and intra-cortical data
The raw data for (Torque, M MAX, MEP/M MAX and CSP) is displayed in Table 1 and for each (ICF, SICI and LICI) displayed in Table 2 for each condition across time points.
Maximal voluntary isometric contraction
Figure 2 shows the percentage change in torque for MVIC following HST, HYT and CON conditions from baseline to 72 h post-training. Repeated measures ANOVA showed a significant CONDITION x TIME interaction (F 12,132 = 3.188, P < 0.001). Post-hoc analyses revealed MVIC was significantly reduced immediately post-training for HST (p = 0.004) and HYT (p < 0.001), and at 2 h for HST (p = 0.024), when compared to CON. No differences were observed between HST and HYT immediately post-training (p = 0.086), at 2 h (p = 0.242) and at all other time points (p > 0.05). A significant main effect of TIME (F6,66 = 11.534, P < 0.001) was also observed. Post-hoc analyses revealed a significant reduction compared to baseline at immediately post-training (p = 0.001) for HST and HYT and at 2 h (p = 0.003) for HST.
MVIC as a percentage of baseline values for HST, HYT and CON. Asterisk indicates a significant interaction between HST or HYT and CON while hash tag indicates a significant decrease from baseline. No differences were observed between HST and HYT immediately post-training (p = 0.086) or at 2 h (p = 0.242)
Peripheral nerve excitability
Figure 3 shows the percentage change in mV for M MAX following HST, HYT and CON conditions from baseline to 72 h post-training. Repeated measures ANOVA showed a significant CONDITION x TIME interaction (F 12,132 = 2.684, P = 0.003). Post-hoc analyses revealed M MAX was significantly reduced immediately post-training for HST (p = 0.001) and HYT (p = 0.004), and 2 h for HYT (p = 0.010), when compared to CON. No differences were observed between HST and HYT immediately post-training (p = 0.831) and at all other time points (p > 0.05). A significant main effect of TIME (F 6,66 = 5.965, P < 0.001) was also observed. Post-hoc analyses revealed a significant reduction compared to baseline at immediate post-training (p < 0.001) for.HST and HYT and at 2 h (p = 0.001) for HYT.
Corticospinal excitability
Figure 4a shows the percentage change in mV for MEP following HST, HYT and CON conditions from baseline to 72 h post-training. Repeated measures ANOVA showed a CONDITION × TIME interaction (F 12,132 = 3.213, P < 0.001) was observed. Post-hoc analyses revealed MEP was significantly increased immediately post-training for HST (p = 0.044), and HYT (p = 0.005) when compared to control. No differences were observed immediately post-training between HST and HYT (p = 0.468) and at all other time points (p < 0.05). A significant main effect of TIME (F 6,66 = 9.890, P < 0.001) was also observed. Post-hoc analyses revealed a significant increase compared to baseline immediately post-training (p = 0.001) and at 2 h (p = 0.006) for HYT. Figure 4b shows the percentage change from baseline in CSP for HST, HYT and CON conditions from baseline to 72 h post-training. Repeated measures ANOVA showed a significant CONDITION × TIME interaction (F 12,132 = 2.755, P = 0.002). Post-hoc analyses revealed CSP was significantly shorter immediately post-training for HST (p < 0.001) and HYT (p < 0.001), 2 h for HST (p = 0.023) and HYT (p = 0.041), 6 h for HYT (p = 0.013) and at 24 h for HYT (p = 0.017) when compared to CON. No differences were observed between HST and HYT immediately post-training (p = 0.598) and at all other time points (p > 0.05). A significant main effect of TIME (F 6,66 = 11.958, P < 0.001) was also observed. Post-hoc analyses revealed a significant increase compared to baseline immediately post-training (p = 0.001) and at 2 h (p = 0.006) for HST and HYT and at 6 h (p = 0.003) and 24 h (p = 0.004) for HYT only.
a MEP as a percentage of baseline values for HST, HYT and CON. Asterisk indicates a significant interaction between HST and CON while hash tag indicates a significant increase from baseline. No differences were observed immediately post training between HST and HYT (p = 0.468). b CSP as a percentage of baseline values for HST, HYT and CON. Asterisk indicates a significant interaction between HST and CON while hash tag indicates a significant decrease from baseline. No differences were observed immediately post training between HST and HYT (p = 0.598) and across all time points (p > 0.05)
Figure 5 shows raw sEMG taken from the RF muscle displaying MEP and CSP in response to single-pulse TMS stimulation of the motor cortex for (a) HST, (b) HYT, and (c) CON from a single participant at pre, post-training and 2 h. A decrease in CSP, denoted by the arrows can be observed post training for HST and HYT and an increase in MEP amplitude at post-training and 2 h for HST and HYT, respectively.
Intra-cortical facilitation and inhibition
Figure 6 shows the percentage change in mV for (a) ICF (b) SICI and (c) LICI following HST, HYT and CON conditions from baseline to 72 h. There were no significant CONDITION × TIME interactions observed for ICF (F 11,132 = 0.907, P = 0.478), SICI (F 11,132 = 0.849, P = 0.066) or LICI (F 11,143 = 1.225, P = 0.288).
Discussion
The primary aim of this study was to investigate the acute behaviour of neurophysiological and neuromuscular responses between a single session of HST and HYT of the leg extensors. Our results showed that the neurophysiological changes in maximal isometric torque, peripheral nerve and corticospinal excitability and intra-cortical facilitatory and inhibitory responses were similar for both training paradigms. Specifically, the post-exercise decrease in MVIC torque, M MAX and CSP, coupled with an increase in MEP were similar between HST and HYT. No changes were observed from baseline in SICI, ICF and LICI for either condition. Collectively, the results suggested that post-exercise neurophysiological responses to HST and HYT were not altered by training modality.
Changes in peripheral measures of MVIC and M MAX
The results showed that HST and HYT resulted in an immediate reduction in maximal torque production and peripheral nerve excitability that lasted up to 2 h post-training. However, the reductions were no different between training modes. It is likely that training to repetition maximum close to the point of momentary voluntary muscular failure may be an important factor in this finding. The variation in MVIC of the leg extensors observed in this study (ICC 0.65 between conditions and 0.91 across time) has also been demonstrated in other studies (ICC of 0.64–0.91) (Christ et al. 1994). Given the time of testing was kept consistent across conditions, the variability is more likely to be attributed to motor unit activity. Factors such as organization of the motor unit pool, recruitment and rate-coding and activation patterns are all thought to contribute to the variability of force during isometric contractions (Taylor et al. 2003). Comparatively the reliability of M MAX displayed in this study between conditions and across days was high (ICC 0.91–0.99) as similarly reported recent studies of the RF muscle (ICC 0.88–0.90) (Balshaw et al. 2017). Impairment of M MAX is thought to occur from processes at the neuromuscular junction via changes in membrane potentials and reduced efficacy of sodium/potassium pump (Nielsen and Clausen 2000; Kirkendall 1990; Mileva et al. 2012; Tucker et al. 2005; Deschenes et al. 1994; Behm & St-Pierre 1997). Nuzzo et al. (2016a) also showed a suppression in M MAX amplitude from the biceps brachii muscle following 12 sets of 8 maximal ballistic isometric elbow flexion contractions separated by 4 s rest. Interestingly, the authors reported a similar M MAX response between 2 and 12 sets of isometric training (Nuzzo et al. 2016b) suggesting that discrepancies in exercise volume to not have a differential effect on peripheral nerve excitability. However, these findings have not always been shown with Behm and St-Pierre (1997) reporting different effects on M MAX depending on the duration of resistance exercise. The impairment of M MAX may at least in part explain the reductions observed in maximal torque production. Similar reductions in neuromuscular torque and force production between training conditions have also been reported in other studies. Nicholson et al. (2014) showed no change for peak force between HST and HYT squat protocols using equated loads between conditions. Howatson et al. (2016) also reported significant reductions in MVIC of the knee extensors following strength (4 × 5 RM) and power (5 × 5 repetitions at 30% of the strength condition) training, and also with heavy (85% of 1 RM), moderate (75% of heavy condition) and light (50% of heavy condition) back squat exercise to repetition maximum (Brandon et al. 2015). Conversely, a reduction in MVIC was shown to be greater with HYT loading strategies compared to HST in the leg extensors (Walker et al. 2012). However, the large discrepancy in volume between protocols HST (15 × 1 RM) or HYT (5 × 10 RM) may reflect the disparity in this findings by Walker et al. (2012) compared to Nicholson et al. (2014) and the current findings of this study.
Changes in corticospinal excitability and voluntary activation following HST and HYT
An immediate increase in MEP amplitude and decrease in the CSP duration was observed following training, which was not different between HST and HYT. The low variability of the CSP baseline between conditions (CV 8.0%) and across time for CSP (4.61%) provides feasibility for changes in inhibition to be evaluated. Although MEP was more reliable at baseline (CV 12.46%), it was more less stable between days (CV 37.72%) with a higher variability also demonstrated in other studies (CV 25.0%) (Orth & Rothwell 2004). Kiers et al. (1993) highlight that the variability of the MEP can be caused by fluctuations in corticospinal and segmental motor neuron excitability. Although this may potentially affect the ability to detect differences in corticospinal excitability following HST and HYT, the findings of the current study were in line with recent research showing a greater MEP amplitude and cervicomedullary MEP following slow ramp or ballistic isometric exercise of the elbow flexors (Nuzzo et al. 2016a). An increase in MEP amplitude was similarly reported by Ruotsalainen et al. (2014) following HYT protocol of the elbow flexors. Interestingly, these findings have not always been reported following acute resistance or fatiguing exercise. Several authors have reported a decrease in MEP amplitude immediately following fatiguing quadriceps exercise (Gruet et al. 2014) and HST in the elbow flexors (Latella et al. 2016). The discrepancies observed between the current study and Latella et al. (2016) may be representative of the differences in facilitatory and inhibitory control that existed in the musculature of the upper and lower limbs. The upper limbs are more commonly used in the control of fine motor tasks compared to the lower limbs. Therefore, the responses of the corticospinal tract may in part be reflective of the functional differences in the musculature. Similarly, CSP duration has been shown to increase within an exercise session (Ruotsalainen et al. 2014) and during fatigue (Gruet et al. 2014; Gandevia et al. 1994; Sacco et al. 1997) fatiguing RT of the upper and lower limbs. Conversely, the current findings suggest that acute HST and HYT results in a temporary release of inhibition within the corticospinal tract, and have similarly been observed with short-term training of the lower limbs (Christie and Kamen 2014).
Further support for the similarities observed between RT modalities comes from similar changes in muscular activation between a 5, 10 or 20 RM elbow flexion protocol (Behm et al. 2002) suggesting that maximal effort close to momentary voluntary muscular failure rather than repetition selection was an important factor in acute neural behaviour. Less direct evidence has been provided by Sacco et al. (2000) showing a similar reduction in the MEP amplitude between maximal and submaximal sustained contractions of the biceps brachii. The current findings suggested that the increase in excitability and decrease in inhibition may be a compensatory mechanism in an attempt to attenuate the concurrent peripheral fatigue observed with a reduction in MMAX and MVIC. Despite the work by Ruotsalainen et al. (2014), Nuzzo et al. (2016a) and Nuzzo et al. (2016b), this was the first study to show similarity of corticospinal behaviour following HST and HYT protocols reflective of intrinsic session parameters (intensity, repetition and volume) recommended in current RT guidelines (Ratamess et al. 2009).
Changes in intra-cortical inhibition and facilitation following HST and HYT
Furthermore, we have presented evidence for similar neuromodulation between HST and HYT with intra-cortical facilitation and inhibition. The findings showed that SICI, ICF and LICI were not different between training paradigms or control across time. Although variations in paired-pulse TMS responses can occur, the consideration of time of day, footedness and phase of menstrual cycle in this study rules out several confounding factors. The influence of gender has also been shown to have little effect on paired-pulse measures with De Gennaro et al. (2003) showing low reliability for both females and males. Although the variability in LICI in this study was large (CV 38.84%), between conditions at baseline the reliability of SICI and ICF between conditions was higher (CV 12.89 and 15.30%, respectively) compared to previous reports (31.0 and 20.0%, respectively) (Orth et al. 2003) providing support that intra-cortical differences were unlikely to occur between conditions. This indicated that changes in the responsiveness of the corticospinal pathway following one session of acute HST or HYT were likely to be modulated downstream of the M1 (Nuzzo et al. 2016a). These findings were also in line with Latella et al. (2016), which showed that HST of the arm was primarily modulated by changes in corticospinal and peripheral excitability in the absence of any intra-cortical changes following training. Recently (Hunter et al. 2016) showed a decrease in ICF and increase in SICI during and 2 min following a sustained submaximal contraction of the biceps brachii, which suggested that intra-cortical facilitatory and inhibitory networks become less excitable during fatigue. However, to date measures of intra-cortical facilitation and inhibition have seldom been reported with a single session of applied HST or HYT in the leg extensors. Based upon the findings, it is suggested that intra-cortical facilitatory and inhibitory circuits are not affected by intermittent, dynamic contractions typically used in applied RT and are rather modulated during the maintenance of force required during sustained voluntary efforts.
Limitations
In light of the findings, it is acknowledged that several limitations existed in the current experimental design. First, the acute training session design was in line with current RT guidelines (Ratamess et al. 2009), however, the interaction of several other variables such as exercise selection (gross vs isolated motor task) and movement velocity could have influenced the neurophysiological outcomes. Second, the measures were taken from the RF muscle and may not provide a global representation of the other leg extensor muscles involved in the exercise, with intense leg extension exercise shown to recruit both slow and fast twitch fibre types and activate all portions of the quadriceps group (Staron et al. 2000; Krustrup et al. 2004), therefore the neurophysiological behaviour under fatigue may be differently modulated in fast or slow twitch dominant muscle groups. Furthermore, population was sampled using convenience-sampling relying on participants who had easy access to the university which fitted the selection criteria and was not designed to detect gender differences that may occur across the super-compensation cycle. Although the sample size was similar to other studies investigating strength and neural responses from RT (Nuzzo et al. 2016a; Howatson et al. 2016) an increased sample size would have provided better power for the study and considering the higher variability of single and paired-pulse TMS measurements across time should be considered in future research studies investigating neurophysiological outcomes across different days.
Conclusion
The findings of the current study showed that the neurophysiological responses were similar between real-world HST and HYT. Both HST- and HYT-modulated neural adaptations support the idea that training to repetition maximum may be an important factor in activating neural mechanisms in both heavy and moderate load RT. Second, the acute increases in corticospinal excitability appear to be a compensatory mechanism in an attempt to regulate neuromuscular performance in the presence of peripheral fatigue. Strength and conditioning professionals should consider the potential impact of both HST and HYT on the central and peripheral nervous system. Acute neural responses should be acknowledged as an important part of regulating fatigue and recovery in both high and moderate load resistance training when designing resistance training programs.
Abbreviations
- CSP:
-
Corticospinal silent period
- HST:
-
Heavy-strength training
- HYT:
-
Hypertrophy training
- TMS:
-
Transcranial magnetic stimulation
- ICF:
-
Intra-cortical facilitation
- LICI:
-
Long interval cortical inhibition
- MEP:
-
Motor evoked potential
- MVIC:
-
Maximal voluntary isometric contraction
- MMAX :
-
Maximal compound wave
- RF:
-
Rectus femoris
- RM:
-
Repetition maximum
- RT:
-
Resistance training
- sEMG:
-
Surface electromyography
- SICI:
-
Short interval cortical inhibition
References
Ackerley SJ, Stinear CM, Byblow WD (2011) Promoting use-dependent plasticity with externally-paced training. Clin Neurophysiol 122(12):2462–2468
Atkinson G, Nevill AM (1998) Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 26(4):217–238
Balshaw TG, Fry A, Maden-Wilkinson TM, Kong PW, Folland JP (2017) Relaibility of quadriceps surface electromyography measurements is improved by two vs. single site recordings. Eur J Appl Physiol 117(6):1085–1094
Behm DG, St-Pierre DMM (1997) Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J Appl Physiol 82:1654–1661
Behm DG, Reardon G, Fitzgerald J, Drinkwater E (2002) The effect of 5, 10, and 20 repetition maximums on the recovery of voluntary and evoked contractile properties. J Strength Cond Res 16(2):209–218
Benwell NM, Sacco P, Hammond GR, Byrnes ML, Mastaglia FL, Thickbroom GW (2006) Short-interval cortical inhibition and corticomotor excitability with fatiguing hand exercise: a central adaptation to fatigue? Exp Brain Res 170:191–198
Bompa T, Haff G (2009) Periodization, theory and methodology of training, 5th edn. Human kinetic, Champaign
Brandon R, Howatson G, Strachan F, Hunter AM (2015) Neuromuscular differences to power vs strength back squat exercise in elite athletes. Scand J Med Sci Sports 25:630–639
Brzycki M (1993) Strength testing—predicting a one-rep max from reps to fatigue. JOPERD 64:88–90
Carroll TJ, Selvanayagam VS, Riek S, Semmler JG (2011) Neural adaptations to strength training: moving beyond transcranial magnetic stimulation and reflex studies. Acta Physiol (Oxf) 202(2):119–140. doi:10.1111/j.1748-1716.2011.02271.x
Christ CB, Slaughter MH, Stillman RJ, Cameron J, Boileau RSA (1994) Reliability of selected parameters of isometric muscle function associated with testing 3 days × 3 trials in women. J Str Cond Res 8:65–71
Christie A, Kamen G (2014) Cortical inhibition is reduced following short-term training in young and older adults. Age (Dodr.) 36(2):749–758
De Gennaro L, Ferrara M, Bertini M, Pauri F, Cristani R, Curcio G, Romei V, Fratello F, Rossini PM (2003) Reproducibility of callosal effects of transcranial magnetic stimulation (TMS) with interhemispheric paired pulses. Neurosci Res 46:219–227
Deschenes M, Maresh C, Kraemer W (1994) The neuromuscular junction: structure, function, and its role in excitation of the muscle. J Strength Cond Res 8(2):103–109
Doguet V, Jubeau M (2014) Reliability of H-reflex in vastus lateralis and vastus medialis muscles during passive and active isometric conditions. Eur J Appl Physiol. doi:10.1007/s00421-014-2969-8
Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE (2014) Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J Mot Behav 46:39–48. doi:10.1080/00222895.2013.850401
Elias LJ, Bryden MP, Bulman-Fleming MB (1998) Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologica 36(1):37–43
Field A (2013) Discovering statistics using IBM SPSS statistics, 3rd edn. SAGE publications, London, p 459
Folland JP, Williams AG (2007) Morphological and neurological contributions to increased strength. Sports Med 37(2):145–168
Gandevia SC, Butler JE, Allen GM, Taylor JL (1994) Prolongation of the ‘silent’ period following transcranial magnetic stimulation during human muscle fatigue. J Physiol 480:110
Haff GG, Triplett TN (2016) Essentials of strength training and conditioning. NSCA-National Strength and Conditioning Association, 4th edn, 5th edn. Human kinetic, Champaign
Gruet M, Temesi J, Rupp T, Levy P, Verges S, Millet GY (2014) Dynamics of corticospinal changes during and after high-intensity quadriceps exercise. Exp Physiol 99(8):1053–1064
Hendy AM, Kidgell DJ (2013) Anodal tDCS applied during strength training enhances motor cortical plascticity. Med Sci Sports Exerc 45(9):1721–1729
Hermens HJ, Fririks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyograph Kinesiol 10(5):361–374
Howatson G, Brandon R, Hunter AM (2016) The response to and recovery from maximum-strength and -power training in elite track and field athletes. Int J Sports Physiol Peform 11:356–362
Hunter SK, McNeil CJ, Butler JE, Gandevia SC, Taylor JL (2016) Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res 234(9):2541–2551
Kidgell DJ, Pearce AJ (2010) Corticospinal properties following short-term strength training of an intrinsic hand muscle. Hum Mov Sci 29:631–641. doi:10.1016/j.humov.2010.01.004
Kiers L, Cros D, Chiappa KH, Fang J (1993) Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89(6):415–423
Kim PL, Staron RS, Phillips SM (2005) Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568(1):283–290
Kirkendall DT (1990) Mechanisms of peripheral fatigue. Med Sci Sports Exerc 22(4):444–449
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2(3):145–156
Krishnan C, Allen EJ, Williams GN (2011) Effect of knee position on quadriceps muscle force steadiness and activation strategies. Muscle Nerve 43(4):563–573
Krustrup P, Soderlund K, Mohr M, Gonzalez-Alonso J, Bangsbo J (2004) Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. J Physiol 449(1):56–65
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471(1):501–519
Latella C, Kidgell DJ, Pearce A (2012) Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. Eur J Appl Physio 112:3097–3310. doi:10.1007/s00421-011-2289-1
Latella C, Hendy AM, Pearce AJ, VanderWesthuizen D, Teo W-P (2016) The time-course of acute changes in corticospinal excitability, intra-cortical inhibition and facilitation following a single-session heavy strength training of the biceps brachii. Front Hum Neurosci 10:607
Leung M, Rantalainen T, Teo W-P, Kidgell D (2015) Motor cortex excitability is not differentially modulated following skill and strength training. Neurosci 305:99–108
McNeil CJ, Martin PG, Gandevia SC, Taylor JL (2011) Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res 209:287–297
Mileva KN, Sumners DP, Bowtell JL (2012) Decline in voluntary activation contributes to reduced maximal performance of fatigued human lower limb muscles. Eur J Appl Physiol 112:3959–3970. doi:10.1007/s00421-012-2381-1
Nicholson G, Mcloughlin G, Bissas A, Iszpoglou T (2014) Do the acute biochemical and neuromuscular responses justify the classification of strength- and hypertrophy-type resistance exercise? J Stength Cond Res 28(11):3188–3199
Nielsen OB, Clausen T (2000) The N+/K+ pump protects muscle excitability and contractility during exercise. Exerc Sport Sci Rev 28:159–164
Nuzzo JL, Barry BK, Gandevia SC, Taylor JL (2016a) Acute strength training increases responses to stimulation of corticospinal axons. Med Sci Sports Exerc 48(1):139–150
Nuzzo JL, Barry BK, Gandevia SC, Taylor JL (2016b) Stability of the biceps brachii M MAX with one session of strength training. Muscle Nerve 54:791–793
Nuzzo JL, Trajano GS, Barry BK, Gandevia SC, Taylor JL (2016c) Arm-posture-dependent changes in corticospinal excitability are largely spinal in origin. J Neurophysiol 115:2076–2082
Orth M, Rothwell JC (2004) The cortical silent period; intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 115(5):1076–1082
Orth M, Snijders Ah, Rothwell JC (2003) The variability of intracortical inhibition and facilitation. Clin Neurophysiol 114(12):2362–2369
Overend T, Anderson C, Sawant A, Perryman B, Locking-Cusolito H (2010) Relative and absolute reliability of physical function measures in people with end-stage renal disease. Physiother Can 62(2):122–128
Phillips SM, Roy PG, Tipton KD, Wolfe RR, Tamopolsky MA (2002) Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Phsyiol Pharmacol 80(11):1045–1053
Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ, Triplett NT (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc. doi:10.1249/MSS.0b013e3181915670
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039
Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W (1999) Magnetic stimulation: motor evoked potentials. The international federation of clinical neurophysiology. Electroenceph Clin Neurophysiol Suppl 52:97–103
Ruotsalainen I, Ahtiainen J, Kidgell DJ, Avela J (2014) Changes in corticospinal excitability during an acute bout of resistance exercise in the elbow flexors. Eur J Appl Physiol 114:1545–1553. doi:10.1007/s00421-014-2884-z
Sacco P, Thickbroom GW, Thompson ML, Mastaglia F (1997) Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve 20:1158–1166
Sacco P, Thickbroom GW, Byrnes ML, Mastaglia F (2000) Changes in corticomotor excitation after fatiguing muscle contractions. Muscle Nerve 23:1840–1846
Schoenfield BJ, Ratamess NA, Peterson MD, Contreras B, Sonmez GT, ALvar BA (2014) Effects of different volume-equated resistance training loading strategies on muscular adaptations in well-trained men. J Strength Cond Res 28(10):2909–2918
Scudese E, Willardson JM, Simao R, Senna G, de Salles BF, Miranda H (2015) The effect of rest interval length on repetition consistency and perceived exertion during near maximal loaded bench press sets. J Strength Cond Res 29(11):3079–3083
Selvanayagam VS, Riek S, Carroll TJ (2011) Early neural responses to strength training. J Appl Physiol 111(2):367–375
Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K (2000) Fiber type composition of the vastus lateralis muscle of young man and women. J Histochem Cytochem 48(5):623–629
Taylor AM, Christou EA, Enoka RM (2003) Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol 90(2):1350–1361
Temesi J, Ly SN, Millet GY (2017) Reliability of single- and paired-pulse transcranial magnetic stimulation for the assessment of knee extensor muscle function. J Neurol Sci 375:442–449
Teo W-P, Newton MJ, McGuigan MR (2011) Circadian rhythms in exercise performance: implications for hormonal and muscular adaptation. J Sports Sci Med 10:600–606
Tucker KJ, Tuncer M, Turker KS (2005) A review of the H-reflex and M-wave in the human triceps surae. Hum Mov Sci 24:667–688
Verin E, Ross E, Demoule A, Hopkinson N, Nickol A, Fauroux B, Moxham J, Similowski T, Polkey MI (2004) Effects of exhaustive incremental treadmill exercise on diaphragm and quadriceps motor potentials evoked by transcranial magnetic stimulation. J Appl Physiol 1:253–259
Walker S, Taipale RS, Kai N, Kraemer WJ, Hakkinen K (2011) Neuromuscular and hormonal responses to constant and variable resistance loadings. Med Sci Sports Exerc 43(1):26–33
Walker S, Davis LD, Avela J, Hakkinen K (2012) Neuromuscular fatigue during dynamic maximal strength and hypertrophic resistance loadings. J Electromyograph Kinesiol 22:356–362
Westin GG, Bassi BD, Lisanby SH, Luber B (2014) Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin Neurophysiol 125(1):142–147
Acknowledgements
We would like to thank all participants for their contribution to this study. CL is supported by an Australian Postgraduate Award. WPT is supported by an Alfred Deakin Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Latella, C., Teo, WP., Harris, D. et al. Effects of acute resistance training modality on corticospinal excitability, intra-cortical and neuromuscular responses. Eur J Appl Physiol 117, 2211–2224 (2017). https://doi.org/10.1007/s00421-017-3709-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3709-7