Abstract
As part of a series of reviews aimed at providing historical context to the study of whole-body metabolism, this article focuses on the technique of closed-circuit respirometry. Developed by nineteenth century physiologists Henri-Victor Regnault and Jules de Reiset, a constant-pressure closed-circuit calorimeter capable of measuring oxygen consumption and carbon dioxide production in small animals became the framework for future experiments on whole-body metabolism in humans. The volume-loss and volume-replenishment techniques can be used to indirectly assess energy expenditure using an oxygen reservoir; spirometers are simplistic in design but difficult to operate. Leaks, calibration errors, equilibration of gases and dead space are some of the major limitations of the methodology. Despite operational difficulties, closed-circuit respirometry is highly accurate and reproducible. Due to the bespoke nature of many closed-circuit systems, maintenance and repair is often troublesome. Compounded by technological advancement, closed-circuit techniques have become progressively outdated. Nevertheless, the classical experiments in whole-body metabolism played a pivotal role in furthering our understanding of basic human physiology and paved the way for current methodologies used in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic processes in humans result in the production of heat where the rate of heat production defines energy metabolism. Heat production is expressed as the calorie, whilst calorimetry refers to the measurement of heat transfer. Direct calorimetry measures the body’s rate of energy metabolism/heat production in a chamber where small changes in temperature can be accurately detected. Kenny et al. (manuscript in this EJAP series) provide a detailed treatment on the topic of direct calorimetry. Indirect calorimetry, on the other hand, does not involve the direct measurement of heat production and is based on the premise that energy-releasing reactions depend upon the usage of oxygen. An important distinction between direct and indirect calorimetry is the consideration of heat produced by mechanical work. Where direct calorimetry may underestimate energy expenditure when mechanical work is done on the environment within the measuring chamber, indirect calorimetry estimates total heat production and work done from measurements of oxygen consumption (\(\dot{V}{\text{O}}_{2}\)) and carbon dioxide production (\(\dot{V}{\text{CO}}_{2}\)). There are two overarching methods used to assess heat production indirectly and involve ‘open’ and ‘closed’ circuits. In the present review, we are concerned with closed-circuit systems, which were first used to measure whole-body metabolic rate of endothermic animals through the analysis of \(\dot{V}{\text{O}}_{2}\) and \(\dot{V}{\text{CO}}_{2}\) in a hermetically sealed chamber for a given time period. Human experiments that followed from earlier animal work were instrumental to our basic understanding of metabolism.
Through the work of the seventeenth century British scientists Robert Boyle (1627–1691) and John Mayow (1643–1679), the properties of air were beginning to be unravelled—essential not only to life, but possessing elements necessary for the chemical processes of combustion and respiration. From these early experiments, Mayow developed and built a calorimetric prototype centred on the principles of flow, volume and pressure. Mayow’s original prototype was built in 1674 and featured a mouse placed inside a bell jar, inverted above a water seal. Consumption of air decreased pressure inside the jar, which allowed water to flow in and its volume to rise (Frankenfield 2010). The discovery of respiratory gases shortly followed.
Oxygen was isolated from air by heating substances such as mercuric oxide, first by Carl Wilhelm Scheele (1742–1786) between 1770 and 1773 (based on translated notes from experiments conducted within this time period; work not published until 1777) (Scheele 1777) and soon thereafter by Joseph Priestley (1733–1804) in 1774 (Priestley 1774). The resulting gas (termed ‘fire-gas’ by Scheele) was odourless and able to sustain the combustion of candle more than air (West 2014). In the late eighteenth century, inspired by these and the discoveries of Joseph Black (1728–1799) and Henry Cavendish (1731–1810), Antoine-Laurent Lavoisier (1743–1794) related respiration to combustion and the concept that when animals breathe, they are “[(…) burning and consuming themselves]” (West 2013). This notion took Lavoisier and his colleague Pierre-Simon Laplace (1749–1827) to design and build a triple-chamber ice calorimeter large enough to fit a small animal inside. A guinea pig was placed in the innermost chamber surrounded by two layers of ice (the outer layer served to insulate the middle). The volume of water collected as the ice melted was believed to be proportional to the heat generated by respiration. However, the ice caused respiratory rate to increase as core temperature dropped, leading to overestimations of heat production (Lodwig and Smeaton 1974). By replacing the middle layer of ice with water and the outer insulating layer with down, Adair Crawford (1748–1795) rectified some of the inaccuracies of the technique (Bernstein 2010). Some years later (1790), Lavoisier and Armand Séguin (1767–1835) carried out the very first assessment of oxygen consumption in humans. Reports suggest that Lavoisier and Séguin used a closed-circuit system in which expired air was collected in a reservoir for analysis (Frankenfield 2010; Karamanou and Androutsos 2013; West 2013). Unfortunately, it seems that no original written records from these experiments survived the French Revolution. However, Lavoisier’s experiments are considered instrumental to the study of human respiratory physiology, influencing many generations of future scientists.
The French chemist and physicist Henri-Victor Regnault (1810–1878) made important strides in the study of thermodynamics throughout the nineteenth century. In 1849, with the help of Jules de Reiset (1818–1896), Regnault described and built the first known closed-circuit calorimeter, which served as the blueprint for closed-circuit systems over the next 70 years. Regnault’s name is inscribed on the Eiffel Tower among other brilliant scientists and his initial R is purported to be the inspiration for the universal gas constant symbol (Jensen 2003). An overview of the major historical events with regard to closed-circuit respirometry is provided in Table 1. The present article reviews the methods used in the assessment of energy expenditure, placing an emphasis on closed-circuit designs from a historical perspective.
Regnault and the advancement of closed-circuit respirometry
Regnault made many contributions to the scientific fields of chemistry and physics, his work characterised by attention to detail and precision. In 1841, Regnault was appointed experimental physics Professor at the Collège de France. There, he published several manuscripts on thermodynamics, including expansion, density and elastic force of gases, temperature measurement, vapour characteristics, and heat of compounds and gases. Regnault’s meticulously controlled experiments shed light on some weaknesses in Gay-Lusaac’s laws, in particular that all gases do not dilate equally.
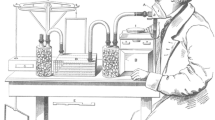
In 1849, Regnault and Reiset built a closed-circuit calorimeter capable of measuring energy expenditure in small animals (Fig. 1). In brief, the animal (in this instance, a small dog) was placed inside a water-sealed jar; O2 was supplemented as CO2 was absorbed by burettes of potassium hydroxide, thus preventing non-physiological stimulation of central chemoreceptors. A manometer was used to control pressure within the system, such that pressure remained constant. Oxygen consumption was assessed directly by the amount of oxygen required to maintain a constant pressure. Carbon dioxide production was measured by the change in weight of the absorption vessel. Temperature was also tightly regulated. The principles of measurement provided a foundation for later closed-circuit calorimeters that were refined over time.
Original figure from Regnault and Reiset’s (1849) respiratory physiology apparatus (see description in text). Public domain
Years later, Wilbur Olin Atwater (1844–1907) and Edward Bennett Rosa (1873–1921) built a calorimeter for humans that incorporated direct and indirect (open-circuit) techniques. In 1905, Atwater and his trainee, Francis Gano Benedict (1870–1957), redesigned the previous model and built a respiration chamber (Fig. 2) utilising Regnault and Reiset’s closed-circuit principles, mixing direct and indirect (closed-circuit) calorimetry into a hybrid system suitable for human experiments.
Atwater and Benedict’s calorimeter for man (from Bell et al. 1965). Numbers 1–5 represent the direct calorimetry portion of the chamber. Water flows through the system from 1 to 4, being collected in a reservoir at 5. Thermometers were placed at 2 and 3 to determine changes in water temperature between the inlet and outlet. The subject enters/exits the chamber via number 6 (door), whilst 7 represents a glass window. The closed-circuit respirometer is represented by 8 and 9. The air in the chamber is collected through 8, enters the system and undergoes oxygen replacement before returning to the chamber through 9. Image is reproduced with permission from Elsevier
Air was drawn from the chamber into two containers of sulphuric acid and through a soda lime reservoir to absorb water vapour and carbon dioxide, respectively. The systems pressure was detected by a membrane made of rubber that moved according to the tension of gases that passed through it. This movement was suppressed by the opening of an O2 cylinder, equalising its tension. Thus, O2 consumption was calculated from the weight of the cylinder before and after the experiment (McLean and Tobin 1987). Benedict substituted the rubber membrane for a spirometer in 1912, which allowed greater control over measurements. Using this system, Atwater and Benedict were able to make accurate estimates of \(\dot{V}{\text{O}}_{2}\) and \(\dot{V}{\text{CO}}_{2}\) in various subjects for prolonged periods of time. Despite its accuracy, many difficulties were encountered, rendering the chamber incompatible for day-to-day use. Gradually, closed-circuit calorimeters were replaced by easier-to-operate open-circuit systems.
Measurement techniques
Representing the measurement of an animal’s heat production, calorimetry derives from the Latin word calor, which means heat or warmth. Indirectly, this heat (or energy) can be estimated via the assessment of pulmonary gaseous exchange. Thus, indirect calorimetry (or respirometry) consists of quantifying the energy production/expenditure of an animal with respect to the analysis of its \(\dot{V}{\text{O}}_{2}\) and \(\dot{V}{\text{CO}}_{2}\) during resting or exercising conditions. The respiratory quotient (RQ) is taken as the ratio of \(\dot{V}{\text{CO}}_{2}\) to \(\dot{V}{\text{O}}_{2}\). Until the advent and technological advancement of modern gas analysers, insight into whole-body metabolism was achieved by closed-circuit techniques.
Differences between open- and closed-circuit respirometry
Open-circuit respirometry estimates the volume of gas exchanged at the lung by measuring the concentration of inspired and expired O2 and CO2. In contrast, closed-circuit respirometry does not require O2 or CO2 analysers once the system is sealed and changes in volume can be determined. This approach may be especially suitable for patients that require controlled supplemental O2, such that the inspired fraction of oxygen (FIO2) can be regulated to maintain blood–gas homoeostasis. Early application of closed-circuit respirometry was in the management of mechanically ventilated patients. Open-circuit respirometers may be used for the same purpose, but require adaptation (Branson 1990). Inherent errors associated with high FIO2 measurements should also be considered (Ultman and Bursztein 1981; Hieronymi and Mrochen 1989). To date, few studies have compared the two types of respirometry; nevertheless, it appears that values are similar between the two approaches (Branson et al. 1989; Raurich et al. 1989).
Volume-loss and volume-replenishment techniques
Considered for many years as the ‘gold-standard’, the volume-loss technique was used frequently until the mid–late twentieth century, when the Haldane transformation enabled experimenters to estimate expired ventilation from inspired gases by virtue of the inert nature of nitrogen. The volume-loss technique (Fig. 3a) is based on the quantitative measurement of a known volume oxygen reservoir as the subject breathes through a closed circuit during rest or exercise. Carbon dioxide and water vapour are absorbed by the system, whilst the displacement of gas in the oxygen reservoir indicates the volume of oxygen consumed. The change in volume as a function of time allows \(\dot{V}{\text{O}}_{2}\) to be calculated in mL/min (Branson and Johannigman 2004). The Harris and Benedict (1918) equations (developed from 136 men and 103 women) were formulated from the aforementioned technique to provide an estimate of basal metabolic rate (BMR), taking into account sex, body mass (range 25.0–124.9 kg), height (range 151–200 cm) and age (range 21–70 years). The multiple regression equations were validated to be within 5% of measured BMR in the early 1950s (Fleisch 1951; Robertson and Reid 1952). However, with changes in lifestyle and improved capabilities of measuring apparatus, these equations have since been found to overestimate BMR by 10–15% (Daly et al. 1985). Consequently, multiple alternative predictive equations have been developed [e.g. Mifflin et al. 1990; World Health Organisation (WHO) 1985]. Simplified versions of the original Harris–Benedict equations developed for men (Eq. 1) and women (Eq. 2) are provided below:
Volume-loss (a) and volume-replenishment (b, c) techniques for measurement of energy expenditure (from Branson 1990). Image is reproduced with permission from the American Association for Respiratory Care
The volume-replenishment technique (Fig. 3b, c) differs from volume loss by maintaining a constant spirometer volume. A bellows filled with oxygen or an oxygen reservoir may be used. The amount of oxygen reinserted into the system after each breath cycle is equivalent to the volume of oxygen consumed (Branson 1990). Closed-circuit respirometers are now rarely used as open-circuit designs are smaller, easier to operate and more accessible, whilst retaining highly reliable and accurate results.
Spirometers
Spirometers were originally incorporated into closed-circuit designs in the form of an oxygen reservoir. However, spirometers can also be used in closed-circuit systems for the measurement of \(\dot{V}{\text{O}}_{2}\). Simple in design, spirometers consist of an inverted bell-shaped chamber (approximately, 6–14 L) and a container filled with water to provide a seal. A counterweight provides the bell with buoyancy in the water. Subjects breathe through a mouthpiece or facemask into the bell. Respiratory cycles appear as oscillations. The bell lowers into the water as oxygen is consumed; the height of the bell (implying volume) is read off a scale attached to the spirometer or displayed on a kymograph. Krogh’s spirometer (1913, Fig. 4a) has a rectangular base and pivots on one edge. A pen is fixed to the opposite side of the bell. The Benedict–Roth spirometer (1922, Fig. 4b) is cylindrical in shape and uses a pulley system to provide buoyancy. The change in volume of the bell is a product of its cross-sectional area and the change in height. Original spirometers designed for the measurement of energy expenditure were of such size that a 1 mm change in height was equal to a 20.73 mL change in volume (or 0.1 kcal at an RQ of 0.82). An example of a typical recording using spirometers is shown in Fig. 4c.
Comparisons have been made between the effectiveness of spirometers and Fick-derived estimates of \(\dot{V}{\text{O}}_{2}\). In the reverse Fick equation, \(\dot{V}{\text{O}}_{2}\) is calculated as cardiac output multiplied by the arterial–venous O2 difference. The Fick method (Fick 1870) has the advantage of yielding estimates of O2 delivery, but excludes \(\dot{V}{\text{O}}_{2}\) of the lung. Studies have shown that \(\dot{V}{\text{O}}_{2}\) calculated using the Fick principle underestimates \(\dot{V}{\text{O}}_{2}\) measured by a water-sealed spirometer (Stock and Ryan 1996). Importantly, spirometers have a fourfold greater repeatability compared to Fick (Smithies et al. 1991).
Should one be interested in building a closed-circuit respirometer for the purposes of measuring energy expenditure, we direct the reader to the work of Bartels et al. (1963). The authors excellently outline the construction process of various spirographs, highlighting the advantages and disadvantages of each model along with their most suitable application.
Limitations and sources of error
A number of errors and limitations regarding closed-circuit respirometry have been identified and resolved over the years, including the effects of FIO2, leakages, duration of the measurement, assessment of mechanically ventilated patients, ambient temperature and size/mobility of the systems (Branson 1990). Some of these errors were minimised by the skill and knowledge of the operator, whereas others were avoided through technical and theoretical improvements in design and careful consideration of the gas laws.
Concentration of gases
One of the advantages related to closed-circuit systems is the possibility of analysing patients that require a FIO2 >21%, such that the reservoir content can be filled with the necessary fraction of oxygen. However, if the spirometer is filled with ambient air, the concentration of oxygen in the reservoir will decline over time, eventually leading to the delivery of hypoxic gas to the patient. This issue was common in volume-loss systems, but negated in volume-replenishment systems, because oxygen is reinserted into the system as the assessment is ongoing. If performed on a mechanically ventilated patient, the ventilator would be used only as a pressure generator.
Equilibration and saturation of gases
Another important consideration regards the equilibration of gases. If FIO2 given to a patient equals 100%, alveolar partial pressure of O2 increases in accordance with Dalton’s law, whilst the partial pressure of nitrogen decreases. Consequently, the collected values do not represent true O2 uptake until steady state is achieved, assumed by Krogh and Rasmussen (1922) to occur within 5 min. Moreover, assuming saturation of gases in the system, gas volumes should be corrected to standard temperature and pressure, dry (STPD), to avoid erroneous measurements (Lewis et al. 1937; Pappenheimer et al. 1950). The spirometer units must be multiplied by a factor representing the STPD adjusted volume of O2 per centimetre change in height of the spirometer bell, based on Eq. 3 (below), where V s is the physical volume per centimetre travel of the bell, T s the mean spirometer temperature and P s the pressure of water vapour at T s:
Leaks
The circuit should be hermetically sealed and the temperature of gas regulated to make correct measurements. Any leak within the circuit or interface with the patient (e.g. facemask, mouthpiece) could influence the collected data, as the magnitude of error is directly related to the leakage size. The presence of leaks from the spirometer bell can be checked by filling the bell with water, closing all inlet and outlet valves and determining if a change in the height of the bell occurs when a weight is placed on top. Leaks around the mouth and nose are most difficult to control. Later systems include a leak test to circumvent the issue.
Temperature changes
Charles’ law describes how an ideal gas tends to expand when it is heated and contract when cooled. Therefore, gas volumes are influenced by temperature. Although temperature is unlikely to change during the duration of observations, a thermometer placed inside the bell solves any issues that may arise.
Capacity of the system
In general, the assessment of \(\dot{V}{\text{O}}_{2}\) is limited to the volume capacity of the system. The system could be refilled; however, this would mean intermittently pausing the experiment or procedure. If the ventilation or \(\dot{V}{\text{O}}_{2}\) of the patient is increased, the time of measurement is also reduced. Volume-replenishment systems overcome this limitation, although the time frame for assessment is limited by the capability of the CO2 absorber. Furthermore, attention must be given to changes in operating lung volumes, as a fall below functional residual capacity will lead to the appearance of a decrease in \(\dot{V}{\text{O}}_{2}\). Similarly, increases in lung volume will lead to overestimation of energy expenditure.
Dead space ventilation and the work of breathing
In clinical settings, closed-circuit respirometers pose several challenges; in particular, the determination of tidal volume delivered by the ventilator must be calculated with recognition of the volume of compressible air contained within the spirometer. Moreover, incorporating a calorimeter into the ventilator circuit increases inspiratory duty cycle and consequently elevates the work of breathing (Keppler et al. 1989). The mass of the bell and resistance to gas flow should also be taken into consideration. In addition, the volume of air contributing to dead space ventilation should be minimised where possible; this includes the space between the breathing valve and spirometer bell, the soda lime chamber and facemask. The dead space can be estimated by calculation of dimensions or by admixture of a known foreign gas and application of the principle of dilution. If the volume or composition of the gas is known, then a correction factor may be applied. The spirometer can be flushed with the gas to be analysed before starting the final collection.
Calibration errors
Finally, damage to the bell or writing arm of spirometers may lead to inaccurate recordings; for this reason, spirometers must be calibrated regularly using a wet gas meter.
Conclusions
As the measurement of heat production and metabolism evolved from John Mayow’s early prototype to Henri Regnault’s closed-circuit calorimeter, the sophisticated open-circuit techniques used today are indebted to the scientific contributions and discoveries made by the exceptional minds of the last 400 years. Due to the bespoke nature of many closed-circuit systems, potential errors in measurement were significant and contributed to its progressive decline in popularity. The last commercially available device was built in the 1980s and has since been discontinued. Despite falling into obsolescence, closed-circuit respirometry remains a beautifully elegant means of assessing oxygen consumption in humans.
Abbreviations
- BMR:
-
Basal metabolic rate
- FIO2 :
-
Inspired fraction of oxygen
- RQ:
-
Respiratory quotient
- STPD:
-
Standard temperature and pressure, dry
- \(\dot{V}{\text{CO}}_{2}\) :
-
Carbon dioxide production
- \(\dot{V}{\text{O}}_{2}\) :
-
Oxygen consumption
- WHO:
-
World Health Organisation
References
Atwater WO, Benedict FG (1905) A respiration calorimeter with appliances for the direct determination of oxygen, 42nd edn. Carnegie Institution of Washington, Washington, DC
Atwater WO, Rosa EB (1899) A new respiration calorimeter and experiments on the conservation of energy in the human body. Phys Rev 9:129–166
Bartels H, Bücherl E, Hertz CW, Schwab M (1963) Methods in pulmonary physiology. Hafner Publishing Company Inc, New York
Bell GH, Davidson JN, Scarborough H (1965) Textbook of physiology and biochemistry. E & S Livingstone, Edinburgh
Bernstein LH (2010) Calorimetry and metabolic knowledge. Nutrition 26:951. doi:10.1016/j.nut.2010.01.017
Branson RD (1990) The measurement of energy expenditure: instrumentation, practical considerations, and clinical application. Respir Care 35:640–656
Branson RD, Johannigman JA (2004) The measurement of energy expenditure. Nutr Clin Pract 19:622–636
Branson RD, Hurst JM, Davis K, Bower R (1989) Comparison of open circuit and closed circuit methods for measuring oxygen consumption. J Parenter Enter Nutr 13:19S
Daly JM, Heymsfield SB, Head CA et al (1985) Human energy requirements: overestimation by widely used prediction equation. Am J Clin Nutr 42:1170–1174
Fick A (1870) Über die messung des blutquantums in herzventrikeln. Physikalisch Medizin Gesellschaft, Würzburg
Fleisch A (1951) Basal metabolism standard and its determination with the “metabocalculator”. Helv Med Acta 18:23–44
Frankenfield DC (2010) On heat, respiration, and calorimetry. Nutrition 26:939–950. doi:10.1016/j.nut.2010.01.002
Grimaux E (1899) Lavoisier, 1743–1794, d’après sa correspondance, ses manuscrits, ses papiers, ses papiers de famille et d’autres documents inédits, 3rd edn. Alcan, Paris
Harris JA, Benedict FG (1918) A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4:370–373. doi:10.1073/pnas.4.12.370
Hieronymi U, Mrochen H (1989) Errors in the determination of oxygen consumption using the analysis of respiratory gases. Anaesthesiol Reanim 14:167–174
Jensen WB (2003) The universal gas constant R. J Chem Educ 80:731. doi:10.1021/ed080p731
Karamanou M, Androutsos G (2013) Antoine-Laurent de Lavoisier (1743–1794) and the birth of respiratory physiology. Thorax 68:978–979. doi:10.1136/thoraxjnl-2013-203840
Keppler T, Dechert RE, Arnoldi DK et al (1989) Evaluations of the Water MRM-6000 and Biergy VVR closed-circuit indirect calorimeters. Respir Care 34:28–35
Krogh A (1913) A bicycle ergometer and respiration apparatus for the experimental study of muscular work. Skand Arch Physiol 30:375–394. doi:10.1111/j.1748-1716.1913.tb00681.x
Krogh A, Rasmussen O (1922) Ueber bestimmung des energieumsatzes bei patienten. Wien Klin Wochenschr 35:803–806
Lavoisier AL, Laplace PS (1783) Mémoire sur la chaleur. In: Oeuvres de Lavoisier. Imprimerie Impériale, vol 2, Paris, pp 283–333.
Lewis RC, Kinsman GM, Iliff A (1937) The basal metabolism of normal boys and girls from two to twelve years old, inclusive. Am J Dis Child 53:348–428. doi:10.1001/archpedi.1937.04140080077002
Lodwig TH, Smeaton WA (1974) The ice calorimeter of Lavoisier and Laplace and some of its critics. Ann Sci 31:1–18. doi:10.1080/00033797400200101
Mayow J (1674) Tractatus quinque medico-physici. E Theatro Sheldoniano, Edinburgh
McLean JA, Tobin G (1987) Animal and human calorimetry. Cambridge University Press, Cambridge
Mifflin MD, St Jeor ST, Hill LA et al (1990) A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51:241–247
Pappenheimer JR, Comroe JH, Cournand A (1950) Standardization of definitions and symbols in respiratory physiology. Fed Proc 9:602–605
Priestley J (1774) Experiments and observations on different kinds of air. St. Paul's Church Yard, London
Raurich JM, Ibañez J, Marse P (1989) Validation of a new closed circuit indirect calorimetry method compared with the open Douglas bag method. Intensive Care Med 15:274–278. doi:10.1007/BF00271067
Regnault V, Reiset J (1849) Recherches chimiques sur la respiration des animaux des diverses classes. Ann Chim Physiq 26:299–519
Robertson JD, Reid DD (1952) Standards for the basal metabolism of normal people in Britain. Lancet 1:940–943
Roth P (1922) Modifications of apparatus and improved technic adaptable to the Benedict type of respiration apparatus. Boston Med Surg J 186:491–501. doi:10.1056/NEJM192204131861501
Scheele CW (1777) Chemische Abhandlung von der Luft und dem Feuer: nebst einem Vorbericht. Verlegt von Magn. Swederus, Uppsala
Smithies MN, Royston B, Makita K et al (1991) Comparison of oxygen consumption measurements: indirect calorimetry versus the reversed Fick method. Crit Care Med 19:1401–1406
Stock MC, Ryan ME (1996) Oxygen consumption calculated from the Fick equation has limited utility. Crit Care Med 24:86–90
Ultman JS, Bursztein S (1981) Analysis of error in the determination of respiratory gas exchange at varying FIO2. J Appl Physiol 50:210–216
West JB (2013) The collaboration of Antoine and Marie-Anne Lavoisier and the first measurements of human oxygen consumption. Am J Physiol Lung Cell Mol Physiol 305:L775–L785. doi:10.1152/ajplung.00228.2013
West JB (2014) Carl Wilhelm Scheele, the discoverer of oxygen, and a very productive chemist. Am J Physiol Lung Cell Mol Physiol 307:L811–L816. doi:10.1152/ajplung.00223.2014
WHO (1985) Energy and protein requirements. World Health Organisation technical report series, no. 724, Geneva
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Archiza, B., Welch, J.F. & Sheel, A.W. Classical experiments in whole-body metabolism: closed-circuit respirometry. Eur J Appl Physiol 117, 1929–1937 (2017). https://doi.org/10.1007/s00421-017-3681-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3681-2