Abstract
Purpose
Postprandial hyperglycemia and glycemic oscillations have been associated with increased oxidative stress. We sought to investigate the effect of two walking exercise protocols performed during lunchtime on glycemic control and oxidative stress in type 2 diabetic (T2D) patients.
Methods
Nine T2D patients participated in three randomized crossover trials; a control trial (Con), with participants having a standard lunch followed by their normal daily activities and two exercise trials (ContEx and Splitex). In ContEx, subjects performed 40 min of brisk walking 40 min after lunch, whereas in SplitEx the walking exercise was divided in two 20-min isoenergetic bouts, before and 40 min after meal. 24-h glycemic control was monitored by continuous glucose monitoring. 24-h urinary levels of 8-iso PGF2ɑ were measured as a marker of oxidative stress.
Results
SplitEx resulted in less time spent in moderate hyperglycemia after lunch vs ContEx (42.4 ± 38.7 % vs 68.2 ± 32.7 %, P = 0.04). ContEx reduced hyperglycemic time after breakfast consumed the morning after the exercise session (58.3 ± 29.6 Con vs 40.2 ± 33.4 % ContEx, P = 0.02). Compared with Con, 24-h urinary isoprostanes were decreased both in ContEx (−68 %, P = 0.02) and SplitEx (−63 %, P = 0.04).
Conclusions
Splitting an exercise session into two bouts, pre- and post-lunch, affects mainly the glycemic response to lunch, while a single-continuous isoenergetic session exerts its effect later in the 24-h period. Both exercise modalities effectively attenuate systemic oxidative stress with similar overall benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is characterized by fasting and postprandial hyperglycemia (Ceriello 2005). Glycated hemoglobin is a marker of mean glycemic control in the last 3 months and is widely used as a target for diabetes therapy. For values above 6.5 %, postprandial glycaemia appears to play a major role in the glycation process (Monnier et al. 2007), which is particularly true following lunch (Monnier et al. 2002). Postprandial glycemic variations have been demonstrated to increase nitrosative stress and metabolic derivatives such as nitrotyrosine, which in turn induce endothelial damage (Ceriello et al. 2002, 2008) and increase the risk of atherosclerosis (O’Keefe and Bell 2007; Blaak et al. 2012). Obese diabetic patients have reduced antioxidant capacity, and the imbalance between free radicals and antioxidant defense plays an important role in the generation of diabetic complications, particularly those of cardiovascular nature (Brownlee 2005; Rains and Jain 2011). Thus, in addition to hemoglobin A1c and mean glucose concentrations, postprandial hyperglycemia and acute glucose swings have been suggested as novel targets for therapy of type 2 diabetes (Monnier et al. 2006; Nathan et al. 2009).
Exercise has long been defined as one of the mainstays of diabetes therapy (Colberg et al. 2010). Many studies have demonstrated the beneficial effects of exercise of different intensity, duration or modality on 24-h glycemic control, and the postprandial glycemic response to meals (Manders et al. 2010; Little et al. 2011; Van Dijk et al. 2012). Apart from the traditional aspects of exercise prescription, some investigations have focused on the timing of the exercise session in relation to meals (Poirier et al. 2000, 2001; Gaudet-Savard et al. 2007; Colberg et al. 2009) as it appears that the nutritional state in which exercise is performed modulates the response to a meal. It has been suggested that exercising after meals is more beneficial in terms of postprandial glycemic control than exercising before meals (Larsen et al. 1997; Haxhi et al. 2013; Chacko 2014). Furthermore, splitting the daily exercise into several small bouts has been demonstrated to be more effective, and potentially more time-efficient for glycemic control than a single longer bout (Eriksen et al. 2007; Francois et al. 2014). Indeed, a second bout of exercise shortly after the first one appears to decrease blood glucose more than a single-continuous isoenergetic bout in healthy adults (Goto et al. 2011). However, such effect of repeated bouts of exercise has not yet been tested in diabetic patients.
Among all the different indices of glycemic control, glycemic variability measured by MAGE (Mean Amplitude of Glycemic Excursions) and glycemic response to meals measured by the incremental AUC (Area Under the Curve) were found to be the most predictive for urinary levels of 8-iso Prostaglandin F2α (8-iso PGF2α) (Monnier et al. 2006), an isoprostane which is a well-recognized marker of oxidative stress (Basu 2008), and have been correlated with major factors contributing to cardiovascular disease (Patrono et al. 2005). Therefore, it could be possible that by improving glycemic control, exercise may decrease the oxidative damage related to hyperglycemia. However, there is still no clear consensus and the evidence in the literature is limited. Results from existing studies vary from an increase to no effect (Brinkmann et al. 2012), or a decrease in oxidative stress (Campbell et al. 2010; Arikawa et al. 2013). The lowering effect of exercise in healthy adults appears to be evident only in those with higher basal levels of 8-iso PGF2α (Arikawa et al. 2013). As diabetic patients have been demonstrated to have elevated levels of 8-iso PGF2α (Davì et al. 1999), exercise may limit the effect of oxidative stress in this population group. Despite the fact that the beneficial effects of exercise have been well established, adherence to exercise remains low among diabetic patients (Morrato et al. 2007) and lack of time has been identified as one of the reasons for this poor adherence (Trost et al. 2002). Therefore, the challenge in exercise prescription is not only designing a protocol that is effective, but also making it fit in the everyday busy schedules. Different ways of performing exercise, such as interval training or splitting the exercise session into more than one bout, have being recently proposed as a way to overcome some of the barriers related to physical inactivity (Francois et al. 2014). The effectiveness of different protocols has generally been determined in very well-controlled laboratory conditions (Manders et al. 2010; Van Dijk et al. 2013; Oberlin et al. 2014) accounting usually for the effect of breakfast only. However, in order to be fully relevant and applicable to patient care, these results might need to be tested in real-life conditions. As sequential meal intake is typical of most diets and most of the day is spent in the postprandial state, we sought to investigate the effect of walking exercise during a lunch break as an effective modality of daily exercise. There are two main reasons for choosing lunch break: first, because lunchtime is the time of the day when glycemic values are highest and contribute majorly to HbA1c (Monnier et al. 2002) and exercising at this time might potentially counteract late morning and lunchtime hyperglycemia; second, because this time frame is usually one of the few feasible moments of the day when exercise can be easily incorporated in the daily routine. In addition, considering the evidence supporting the benefits associated with repeated bouts of exercise, we compared two 20-min bouts of brisk walking performed before and after a standard lunch, with 40 min of continuous isoenergetic postprandial walking in patients with type 2 diabetes. We hypothesized that splitting the daily exercise session into two bouts around lunch could improve the glycemic profile and whole-body oxidative state in type 2 diabetes patients.
Methods
Participants
Nine sedentary, overweight/obese type 2 diabetic male patients on oral hypoglycemic medications, participated in the study. All volunteers underwent medical screening in order to ascertain the absence of liver, renal, and cardiopulmonary disease and diseases contraindicating physical activity. Exclusion criteria included the use of exogenous insulin, weight instability (>3 kg/6 months), regular physical activity (>150 min/week), chronic complications of diabetes where exercising is contraindicated, and lack of ability to perform brisk walking on a treadmill. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Approval by the Ethics Committee of the University of Rome Sapienza and informed written consent from all participants was obtained before starting the experimental sessions.
Preliminary testing
During the first visit, venous blood samples were drawn for the assessment of fasting blood glucose, HbA1c, and lipid profile. Maximal oxygen consumption (\({\dot{\text{V}}\text{O}}_{{2 {\text{max}}}}\)) was assessed at the treadmill, using the modified Balke and Ware protocol (Blair et al. 1989) The test was terminated when the criteria for achievement of \({\dot{\text{V}}\text{O}}_{{2 {\text{max}}}}\) were met (Howley et al. 1995) or at volitional exhaustion. The ventilatory and gas exchange variables were measured using a breath-by-breath gas analyzer (Quark b2 Cosmed, Rome, Italy), which was calibrated with gases of known concentration before the test. Cardiac function was monitored at rest and during exercise using a 12-lead electrocardiogram.
Study design and experimental sessions
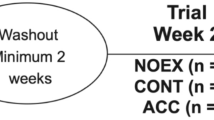
The experimental sessions (Fig. 1) were performed in a randomized crossover design with every subject undergoing three different experimental days, i.e., one control day (CON), in which subjects received a standard meal and performed no exercise, and two exercise days—split exercise (SplitEx) and continuous exercise (ContEx). The three experimental trials were separated by at least 1 week between them. In the SplitEx day, participants were asked to walk for a total of 40 min with the first 20 min occurring immediately prior to the standard lunch and the second 20-min period occurring 40 min after the meal. In ContEx, the 40-min walking session was performed 40 min after the same standard lunch. Exercise intensity was set at 50 % of the heart rate reserve (HRR), with all sessions completed on a treadmill (intensity was set by modulating both treadmill speed and gradient). Moderate intensity was chosen as it allows a substantial increase in energy expenditure while still being feasible to complete around meal time. For the same reason, 40 min of exercise meets the guidelines on the recommended amount of daily physical activity. A heart rate monitor was used to monitor the exercise sessions. In each experimental day, participants were offered the same standard mixed meals composed of 55–60 % carbohydrate, 25 % fat, and 15–20 % protein, for a total caloric intake of 30 kJ kg−1 each. Meals were prepared using the same ingredients and by the same investigator. The caloric content and composition of the meal were set and assessed by a nutritionist using a software for nutritional analysis (Opera, DS Medica, Italy).
Schematic overview of the experimental periods. On day 1, participants underwent one of the interventions, Control (panel Con), SplitEx (panel SplitEx), or ContEx (panel ContEx). On each experimental day, patients were provided with a standard lunch consumed in the experimental site (black fork and knife in a white background). The rest of the meals were consumed outside of the experimental site (white knife and fork in a black background). On the exercise days, a walking session was performed close to lunch (walking symbol), in either two small 20-min bouts or a single 40-min bout
Physical activity and diet
Patients could pursue their normal daily life but were instructed to refrain from unusual strenuous physical activity during the study period. Activity outside the experimental sessions was monitored by an activity monitor (BodyMedia SenseWear armband body monitoring system; BodyMedia Inc., Pittsburgh, PA) worn concomitantly with the Continuous Glucose Monitoring System (CGMS). Intake of all medications was continued normally throughout the experimental sessions.
On each experimental day, participants were provided with standardized meals prior to arrival at the laboratory. On arrival, they were offered a standard lunch to be consumed in the laboratory, in addition they were also provided with standardized dinner and breakfast to be consumed as appropriate on completion of each trial. Specific instructions were given to the patients on the timing of the meals in the experimental days. To ensure that the diet was matched between experimental days, the participants were required to keep a food diary for 24 h prior and after their first experimental day and then consume the same diet in the days preceding and following the second and third study days.
Continuous glucose monitoring
The day before each experimental session, a Continuous Glucose Monitoring System (CGMS® iPRO TM—Medtronic, Northridge, CA, USA) sensor was implanted in the anterior abdominal wall. Participants were instructed in the use of the device and were asked to measure capillary blood glucose four times daily, before the three main meals and before bed. These measurements were used to calibrate the sensor. Interstitial glucose was continuously measured in the subcutaneous tissue every 5 min. Data were downloaded to a computer and a total of 24 consecutive hours, starting from lunch of the trial day to lunch of the post-trial day, were considered for evaluation of glucose variations and responses to meals. The 24-h period was then divided in smaller time frames of 3 h each, namely breakfast, lunch, and dinner postprandial periods and night time (from midnight till 06:00 in the morning), for further analysis.
Urine collection and F2 isoprostane measurement
Thermal bags and urine containers were provided to the patients for the 24-hr urine collection. Urinary 8-iso PGF2ɑ was measured using an enzyme-linked immunosorbent assay (CEA701Ge, USCN Life Science Inc.) according to manufacturer’s instructions. Briefly, urine samples were centrifuged for 20 min at 1000 g to remove particulates and immediately stored at −20 °C. To avoid variability due to methodological reasons, all samples were analyzed in triplicate on the same day. The plates were analyzed with a microplate reader (BIO-RAD Model 680) at 450 nm wave length. The assay detection range was 24.69–2,000 pg/mL with a sensitivity typically less than 9.15 pg/mL. F2 isoprostane values have been expressed relative to urinary creatinine concentration. Urinary creatinine levels were determined using the Jaffe kinetic method.
Statistics and data analysis
The data from the CGMS were downloaded to a personal computer and converted into glycemic values using capillary blood glucose measurements as calibration values. As the intervention in the present study took place at lunchtime, the 24-h glycemic control was derived from the glycemic profiles obtained between lunchtime of the intervention day and lunchtime of the day after. Total and incremental areas under the glycemic curve (AUCs and iAUCs) were calculated for the 24-h period (lunch–lunch) and for each postprandial period following interventions (lunch, dinner, and breakfast) using the trapezoid method. Prevalence of hyperglycemia was calculated as the percentage of time spent with glycemic values over the normal range. Glycaemia values of 126 and 150 mg/dl were defined as thresholds for calculations of moderate hyperglycemia and hyperglycemia, respectively.
Glycemic variability, which reflects glycemic fluctuations, was assessed by the Standard Deviation of the mean 24-h glucose (SD), the mean amplitude of glycemic excursions (MAGE), the continuous overlapping net glycemic action for 1-, 2-, and 3 h time differences (CONGA1, CONGA2, CONGA4) and the Standard Deviation of Blood Glucose Rate of Change (SD-BGRC). SD-BGRC has been suggested as a measure of stability of glucose fluctuation, with larger variations of BG Rate of Change indicating a less stable system (Clarke and Kovatchev 2009). SD, MAGE, and CONGA were calculated using the EasyGV© software (www.easygv.co.uk). Matlab 2014b (Mathworks Inc. Torino, Italy) was used for calculations of the SD-BGRC, AUCs, and Prevalence of hyperglycemia.
Statistical analysis was performed with Statistical Package for Social Sciences version 20.0 (SPSS Inc, Chicago, IL, USA). All data were tested for normality using the Kolmogorov-Smirnov and the Shapiro–Wilk test and for the non-normally distributed data normality was achieved by log or square root transformation. Intervention main effect was assessed by repeated measures analysis of variance (RM-ANOVA) with exercise as the within-subject factor and medication (metformin only vs combined metformin and incretins) as a between-subject factor. Duration of diabetes, HbA1c, and fasting glycaemia were considered as potential influencing factors and were further inserted in the analysis as covariates. Significance level was set at α < 0.05. Data are reported as mean ± SD.
Results
Participant characteristics and medications are presented in Table 1. Mean treadmill speed and inclination were 4.6 ± 0.7 km/h and 4.4 ± 1.1 %, respectively, which corresponded to 52.2 ± 5.9 % of HRR. Energy expenditure was similar between the two exercise bouts (323.6 ± 66 vs 310.7 ± 72.1 kcal, P = 0.4). Active energy expenditure calculated by the accelerometer was 363.3 ± 198.8 kcal during the control day and 683.8 ± 543.5 kcal and 655.3 ± 367.2 kcal in ContEx and SplitEx, respectively, with no statistical difference between days, although a trend toward significance (P = 0.09) was found comparing Control with ContEx.
Glycemic control
Glycemic profiles in the 24 h following the lunch in the three experimental days are depicted in Fig. 2. Mean 24-h glycaemia, total and incremental AUCs, and variability parameters (SD, MAGE, and CONGA) were similar between the Control, SplitEx, and ContEx days (Table 2). However, during the 3 h following lunch, SD of the BG rate of change was significantly higher in the ContEx, whereas it was comparable in the other two conditions (Con: 0.6 ± 0.2 vs ContEx: 0.7 ± 0.2, P = 0.03). SplitEx resulted in less time spent in moderate hypeglycemia after the standard lunch compared to both Con and ContEx (P < 0.05) (Table 2).
When considering the prevalence of hyperglycemia, there was no main effect of exercise in the 24 h of experimental days (P > 0.05). When assessing the effect of medication on the prevalence of hyperglycemia, those taking only metformin spent significantly less time in hyerglycemia after lunch compared to patients on combination therapy (metformin: 12.4 ± 27.1 % vs combination: 49.9 ± 24.2 %, P = 0.02). Both exercise days produced lower prevalence of hyperlgycemia in the 3 h following breakfast on the morning after intervention, compared to the control day (Fig. 3). Although this parameter was nearly identical in the two experimental days (SplitEx: 40.2 % ± 33.4 vs ContEx: 40.2 ± 33 %, P = 0.998), differences with the Con resulted in statistical significance only for the ContEx (ContEx: 40.2 ± 33.0 % vs Con: 58.3 ± 29.7 %, P = 0.02).
Urinary isoprostanes
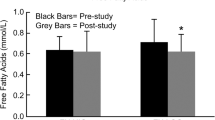
Urinary 8-Epi-PGF2α was significantly lower in the two exercise days compared to control (SplitEx: 166.0 ± 108.1 pg/mg creatinine; ContEx: 143.5 ± 47.9 pg/mg creatinine vs Con: 451.9 ± 400.7 pg/mg creatinine, SplitEx vs Con: P = 0.04; ContEx vs Con: P = 0.02) with no difference between exercise days (Fig. 4). Statistical significance persisted after controlling for duration of diabetes (P < 0.01).
Discussion
The present study investigated the effect of two different sessions of brisk walking administered during lunchtime in type 2 diabetes patients. The two isoenergetic sessions were both effective in terms of improving glycemic control and 24-h oxidative stress, although not significantly different between them. Split exercise resulted in less post-lunch variability, whereas the continuous exercise had a greater impact on the glycemic response to breakfast on the morning following exercise.
Lunchtime was chosen because of the higher glycemic values during this period (Monnier et al. 2002) and because the lunch break may often be the only time during the day when exercise can become part of the daily routine. For this reason, we proposed the insertion of a session of moderate—intensity walking either after lunch or divided in two smaller bouts before and after lunch. Splitting the exercise session allowed our participants to spend less time in above normal glycemic values 3 h following lunch compared to the more classical postprandial continuous exercise. Post-lunch glucose AUCs did not differ between experimental days. These data are in line with those of Francois and colleagues, who employed a similar design and compared different exercise modalities in relation to standard meals, resulting in significantly different AUCs between protocols for breakfast and dinner, but not for lunch (Francois et al. 2014).
During the night following intervention, SplitEx resulted in longer time spent above normal glucose values compared to the continuous exercise, but this difference did not reach statistical significance, probably due to higher variability in this time frame. The reasons for such variability are not clear. The possible factors that normally could alter glycemic levels during night, like physical activity, dinner and medication, were not different between the three experimental days. Indeed, participants strictly followed the instructions provided by the investigators and consumed meat or fish with vegetables and bread for dinner, thus excluding any relevant impact on night-time glycemia.
In the 3 h following breakfast, ContEx was associated with lower prevalence of hyperglycemia compared to the control day, with the two exercise days being the same. Such extended effect of exercise on the meals following the exercise session is not unexpected, and it recalls results from Manders and colleagues who found that the effect of exercise performed before breakfast was evident up until the following dinner (Manders et al. 2010).
Variability parameters in the 24 h did not differ between experimental days. Few studies have investigated the effect of exercise on glycemic variability. It has been suggested that the conventional methods of assessing glycemic variability is not specific enough to detect possible differences, which in fact were evidenced by nonconventional methods (Figueira et al. 2013). In the present study, we used only the conventional methods to calculate such parameters, which might have limited the possibility of identifying differences between days.
Likewise, mean glycaemia, total, and incremental AUCs were similar between experimental days and appear to not have been affected by the exercise intervention. Most of the previous studies that showed decrease of these parameters were performed in strict laboratory conditions (Praet and van Loon 2007; Manders et al. 2010). It could be argued that, although strict laboratory conditions eliminate interference of possible confounding events, ecological validity could be compromised. Our study was implemented in a real-life setting, in which patients could pursue their normal daily physical activities. Such conditions might have blunted differences in glycemic responses that would normally be more easily evidenced in the laboratory. Although most of the 24-h variability parameters remained unaltered with the exercise intervention, SD of BG rate of change did change with exercise. More glycemic stability was observed following SplitEx than ContEx, as demonstrated by lower values of this parameter. It appears that splitting the exercise session in a “sandwich mode” provides a more immediate effect on the adjacent meal. At present, it is difficult to compare this specific result with existing studies as such parameter of glycemic variability/stability has not been described in relation to exercise previously. SD-BGRC has been considered similar to another measure of variability, CONGAn (Clarke and Kovatchev 2009), which in the present study did not differ between experimental days. We might speculate that this rather new measure of variability could be a sensitive measure of glucose fluctuations related to exercise but more research is certainly required to investigate such speculation.
Interestingly, our results seem to suggest that medication may play a separate role on glycemic control. In the present study, patients prescribed metformin, spent less time in postprandial hyperglycemia, and showed greater glycemic stability, independent of the exercise intervention. This result is in contrast with what reported in a previous study from Guerci et al. (2012). When assigned to either the metformin-only or one of the metformin plus incretin groups, adding an incretin proved superior in terms of glycemic variability and postprandial response. The study was performed in a randomized control fashion, which implies random assignment of patients to either groups. However, in clinical practice and according to official guidelines (Nathan et al. 2009), incretins can be added as second-line agents when lifestyle plus metformin fail to achieve glycemic control. It could be possible that in patients selected from the clinical setting, those who are on more than one hypoglycemic agent are also in a more advanced stage of diabetes, although their levels of HbA1c might be comparable to those on metformin only as a direct result of a more robust therapy.
Therefore, our finding of greater stability and less prevalence of hyperglycemia with metformin only may indicate that these patients are in a less advanced stage of the disease. We acknowledge that this specific result is certainly of limited generalizability due to the small number of patients on each group of hypoglycemic agents. Comparing the effect of different medication regimens on glycemic responses to exercise was not the primary aim of the present study, and it was not designed in such a way as to properly evaluate these differences with sufficient statistical power. However, these pilot results may advocate for further research addressing the interaction of diabetes medications, especially new classes of hypoglycemic agents, with glycemic responses to exercise and meals.
Exercise decreased the 24-h level of 8-Epi-PGF2α, irrespective of whether it was performed continuously or split in two bouts. This effect appears to be independent of 24-h glycemic control and duration of the disease. It might be suggestive to postulate about an exercise-specific effect on this oxidative stress biomarker, but more research and larger sample size might be necessary for confirmation. In non-exercise conditions, urinary isoprostanes have been associated with glycemic variability (Monnier et al. 2006) as well as platelet activation (Davì et al. 1999) and consequently, atherothrombosis (Patrono et al. 2005). Moreover, urinary 8-Epi-PGF2α levels have been advocated as a sensitive biochemical endpoint for dose-finding studies of antioxidant interventions (Patrono et al. 2005). In our case, exercise acts as an antioxidant and as few as 40 min of brisk walking at lunchtime are enough to lower lipid peroxidation thus holding the potential to counteract the atherogenesis process associated with diabetes. Our data do not allow us to provide an assertive statement on the mechanisms behind such effect. Further dedicated studies are needed to investigate whether exercise has an effect per se or if its effect is dependent on the associated metabolic alterations. It has been previously shown that, in erythrocyte membranes, a bout of exercise causes a transitory increase in lipid peroxidation with a subsequent decrease after recovery (Brinkmann et al. 2012). Such an after-exercise decrease might compensate for the initial increase and when considering the 24-h time frame, exercise may lead to a net reduction of oxidative damage. This hypothesis is supported by the urinary levels of 8-iso PGF2α measured in the present study. This marker is considered the most accurate method to measure in vivo oxidative stress (Kadiiska et al. 2005) and urinary markers have been suggested to better reflect 24-h fluctuations due to a greater stability compared to the plasmatic counterpart (Monnier et al. 2006). Particularly in diabetic patients, in vivo formation of this specific isoprostane is increased (Davì et al. 1999; Sampson et al. 2002) and its excretion rate decreases with improvements in diabetes control (Davì et al. 1999), which makes urinary 8-iso PGF2α a potentially good marker to monitor the effect of therapies for diabetes.
The present results might be of limited generalizability due to the rather small sample size involved in the experiments, but results could be encouraging for future investigations on both the acute and the chronic effects of exercise on glycemia and oxidative stress. It would be of particular interest to investigate the long-term effects of regular exercise on oxidative stress and atherosclerosis in diabetic patients. Our data suggest that even though the hypoglycemic effect of exercise was not very clear, exercise produced a marked effect on the 24-h oxidative stress. Glycemic variability does not seem to determine the levels of PGF2α as the two parameters do not go in the same direction in the present study. This suggests that different mechanisms may be involved in oxidative stress reduction other than altered glycemic control.
In conclusion, both modalities of exercise at lunchtime effectively decrease systemic markers of oxidative cellular damage. The overall effect on 24-h glycemia is similar between split and continuous exercise, with an evident glucose-lowering effect late in the 24-h time frame. Therefore, we recommend brisk walking during lunchtime as an effective way to enhance postprandial glycemic control in T2D patients.
Abbreviations
- AUC:
-
Total area under the curve
- Con:
-
Control trial
- CGM:
-
Continous glucose monitoring
- CONGA:
-
Continuous overlapping net glycemic action
- ContEx:
-
Continous exercise
- HbA1c:
-
Glycated hemoglobin
- HRmax:
-
Maximal heart rate
- HRR:
-
Heart rate reserve
- iAUC:
-
Integrated area under the curve
- MAGE:
-
Mean amplitude of glycemic excursions
- RER:
-
Respiratory exchange ratio
- RM-ANOVA:
-
Repeated measures analysis of variance
- SplitEx:
-
Split exercise
- SD-BGRC:
-
Standard deviation of blood glucose rate of change
- T2D:
-
Type 2 diabetic patients
- 8-iso PGF2ɑ:
-
8-iso prostaglandin F2α
- VO2 max:
-
Maximal oxygen uptake
References
Arikawa AY, Thomas W, Gross M et al (2013) Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp Clin Trials 34:212–217
Basu S (2008) F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal 10:1405–1434
Blaak EE, Antoine JM, Benton D et al (2012) Impact of postprandial glycaemia on health and prevention of disease. Obes Rev 13:923–984
Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KHGL (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262:2395–23401
Brinkmann C, Blossfeld J, Pesch M et al (2012) Lipid-peroxidation and peroxiredoxin-overoxidation in the erythrocytes of non-insulin-dependent type 2 diabetic men during acute exercise. Eur J Appl Physiol 112:2277–2287
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Campbell PT, Gross MD, Potter JD et al (2010) Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc 42:1448–1453
Ceriello A (2005) Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54:1–7
Ceriello A, Quagliaro L, Catone B et al (2002) Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care 25:1439–1443
Ceriello A, Esposito K, Piconi L et al (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354
Chacko E (2014) Timing and intensity of exercise for glucose control. Diabetologia 57:2425–2426
Clarke W, Kovatchev B (2009) Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther 11(Suppl 1):S45–S54
Colberg SR, Zarrabi L, Bennington L et al (2009) Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc 10:394–397
Colberg SR, Albright AL, Blissmer BJ et al (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42:2282–2303
Davì G, Ciabattoni G, Consoli A et al (1999) In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99:224–229
Eriksen L, Dahl-Petersen I, Haugaard SB, Dela F (2007) Comparison of the effect of multiple short-duration with single long-duration exercise sessions on glucose homeostasis in type 2 diabetes mellitus. Diabetologia 50:2245–2253
Figueira FR, Umpierre D, Casali KR et al (2013) Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS One 8:e5773
Francois ME, Baldi JC, Manning PJ et al (2014) “Exercise snacks” before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 57:1437–1445
Gaudet-Savard T, Ferland A, Broderick TL et al (2007) Safety and magnitude of changes in blood glucose levels following exercise performed in the fasted and the postprandial state in men with type 2 diabetes. Eur J Cardiovasc Prev Rehabil 14:831–836
Goto K, Tanaka K, Ishii N et al (2011) A single versus multiple bouts of moderate-intensity exercise for fat metabolism. Clin Physiol Funct Imaging 31:215–220
Guerci B, Monnier L, Serusclat P et al (2012) Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: results from the randomized Optima study. Diabetes Metab 38:359–366
Haxhi J, Scotto Di Palumbo A, Sacchetti M (2013) Exercising for metabolic control: is timing important? Ann Nutr Metab 62:14–25
Howley ET, Basset DR, Welch HG (1995) Criteria for maximal oxygen uptake: review and commentary. Med Sci Sport Exerc 27:1292–1301
Kadiiska MB, Gladen BC, Baird DD et al (2005) Biomarkers of Oxidative Stress Study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 38:698–710
Larsen JJ, Dela F, Kjaer M, Galbo H (1997) The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 40:447–453
Little JP, Gillen JB, Percival ME et al (2011) Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 111:1554–1560
Manders RJF, Van Dijk JWM, Van Loon LJC (2010) Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 42:219–225
Monnier L, Colette C, Rabasa-Lhoret R et al (2002) Morning hyperglycemic excursions. Diabetes Care 25:737–741
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687
Monnier L, Colette C, Dunseath GJ, Owens DR (2007) The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 30:263–269
Morrato EH, Hill JO, Wyatt HR et al (2007) Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 30:203–209
Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
O’Keefe JH, Bell DSH (2007) Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 100:899–904
Oberlin DJ, Mikus CR, Kearney ML et al (2014) One bout of exercise alters free-living postprandial glycemia in type 2 diabetes. Med Sci Sports Exerc 46:232–238
Patrono C, Falco A, Davì G (2005) Isoprostane formation and inhibition in atherothrombosis. Curr Opin Pharmacol 5:198–203
Poirier P, Tremblay A, Catellier C et al (2000) Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab 85:2860–2864
Poirier P, Mawhinney S, Grondin L et al (2001) Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc 33:1259–1264
Praet SFE, van Loon LJC (2007) Optimizing the therapeutic benefits of exercise in type 2 diabetes. J Appl Physiol 103:1113–1120
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50:567–575
Sampson MJ, Gopaul N, Davies IR et al (2002) Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care 25:537–541
Trost S, Owen N, Bauman A et al (2002) Correlates of adults’ participation in physical activity: review and update. Med Sci Sport Exerc 34:1996–2001
Van Dijk JW, Manders RJF, Tummers K et al (2012) Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 55:1273–1282
Van Dijk JW, Venema M, Van Mechelen W et al (2013) Effect of moderate-intensity exercise versus activities of daily living on 24-h blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 36:3448–3453
Acknowledgments
We thank Doctor Federico Quinzi for the help in data analysis and Doctor Tittania Musella for helping with patient recruitment and Medtronic Inc. for kindly providing the CGM devices. The study was supported by a grant from the University of Rome “Foro Italico” (112013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Fabio Fischetti.
L. Di Luigi and M. Sacchetti contributed equally.
Rights and permissions
About this article
Cite this article
Haxhi, J., Leto, G., di Palumbo, A.S. et al. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur J Appl Physiol 116, 573–582 (2016). https://doi.org/10.1007/s00421-015-3317-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3317-3