Abstract
Purpose
It is usually stated that glycogen is stored in human muscle bound to water in a proportion of 1:3 g. We investigated this proportion in biopsy samples during recovery from prolonged exercise.
Methods
On two occasions, nine aerobically trained subjects (\(\dot{V}{\text{O}}_{2\hbox{max} }\) = 54.4 ± 1.05 mL kg−1 min−1; mean ± SD) dehydrated 4.6 ± 0.2 % by cycling 150 min at 65 % \(\dot{V}{\text{O}}_{2\hbox{max} }\) in a hot-dry environment (33 ± 4 °C). One hour after exercise subjects ingested 250 g of carbohydrates in 400 mL of water (REHLOW) or the same syrup plus water to match fluid losses (i.e., 3170 ± 190 mL; REHFULL). Muscle biopsies were obtained before, 1 and 4 h after exercise.
Results
In both trials muscle water decreased from pre-exercise similarly by 13 ± 6 % and muscle glycogen by 44 ± 10 % (P < 0.05). After recovery, glycogen levels were similar in both trials (79 ± 15 and 87 ± 18 g kg−1 dry muscle; P = 0.20) while muscle water content was higher in REHFULL than in REHLOW (3814 ± 222 vs. 3459 ± 324 g kg−1 dm, respectively; P < 0.05; ES = 1.06). Despite the insufficient water provided during REHLOW, per each gram of glycogen, 3 g of water was stored in muscle (recovery ratio 1:3) while during REHFULL this ratio was higher (1:17).
Conclusions
Our findings agree with the long held notion that each gram of glycogen is stored in human muscle with at least 3 g of water. Higher ratios are possible (e.g., during REHFULL) likely due to water storage not bound to glycogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During prolonged exercise in the heat, muscle glycogen is oxidized at a higher rate than when exercising in a thermoneutral environment (Febbraio et al. 1994). During recovery from muscle-glycogen-depleting exercise, 40 % of muscle glycogen is restored in the first four hours if enough carbohydrates are provided soon after exercise ceases (Ivy et al. 1988a, b). Furthermore, during prolonged exercise in the heat, muscle water content is maintained but declines when fluid compartments equilibrate soon after exercise [i.e., 1 h (Mora-Rodriguez et al. 2014)]. We have recently reported that muscle water deficit could be restored within 4 h after exercise when enough fluid is provided (Fernandez-Elias et al. 2014a). Thus both, muscle glycogen and water can be restored after prolonged depleting exercise. It has been proposed that the post-exercise recovery of water and glycogen in human muscle obeys a coordinated response (Olsson and Saltin 1970). Specifically, it is commonly stated that each gram of glycogen is stored in human muscle with at least 3 g of water.
It was first observed in rabbits undergoing starvation-refeeding diets that liver glycogen increased along with liver weight in a 1:4 ratio. Others found similar results when measuring both water and glycogen directly in rodent livers (MacKay and Bergman 1932; Puckett and Wiley 1932), while Bridge and Bridges found no relationship (Bridge and Bridges 1931). Thus, the ratio of liver water to glycogen contents during periods of changing nutrition remained controversial. Geddes and co-workers (Geddes et al. 1977) analysed liver glycogen molecular size in vitro and found that each gram of glycogen had 1.1 g of water associated. However, they confessed that their hydration conditions increased the effective radius of the particles by 40 %, and thus the amount of water associated with glycogen under different hydrodynamic conditions may be different.
Hepatocytes and myocytes comprise the main glycogen depots in rodents and mammalians. However, being structurally and metabolically different tissues, it is unclear if they store water and glycogen in similar fashions. In 1982, Sherman and co-workers (1982) carried out an experiment in rats’ muscles to ascertain if the glycogen-to-water relationship was consistent with the proposed 1:3 ratio. They compared a control group with an exhausted group (i.e., exercise, glycogen-depleted) and an exhausted-recovered group (3 days recovery with food and water ad libitum). They reported a lack of relationship between glycogen and water in rat skeletal muscle of different fiber composition (i.e., gastrocnemius, red vastus and white vastus).
In humans, Olsson and Saltin (1970) found a parallel increase in muscle glycogen and whole body water after muscle-glycogen-depleting exercise when exercise was followed by a high-carbohydrate diet to refill muscle glycogen stores. Their calculations corroborated the data in rat liver since there was a relationship between glycogen resynthesis and whole body water gains of 1–4 g. However, water was not measured directly in the muscle but at the whole body level using tritium-labelled water. In humans Neufer and co-workers (1991) found that dehydrated muscle resynthesized glycogen at the same rate than when muscle is rehydrated. They concluded that muscle glycogen resynthesis during recovery from exercise is not limited by a reduction in muscle water content and thus their data argues against a fixed ratio between glycogen and water in human muscle.
Despite contradictory study results, the ratio of 1:3 g of glycogen to water recovery is well accepted (Brooks and Fahey 1984). Furthermore, given that muscle glycogen recovery determines performance during aerobically intense exercise (Bergström et al. 1967) it is commonly advised the ingestion of sufficient amount of fluid along with solid carbohydrates to enhance muscle glycogen recovery (Coggan and Swanson 1992). The 1:3 relationship between glycogen and water has been used to explain mismatch between estimates and actual results in weight-loss programs (Kreitzman et al. 1992). The loss of water associated with glycogen could explain the fast reduction in body weight without a parallel body fat loss when people undergo a hypocaloric low-carbohydrate diet. Likewise, the rapid weight regain when discontinuing the low-carbohydrate diet could be explained by the restoration of glycogen and its associated water (Kreitzman et al. 1992).
The purpose of this study was to investigate the relationship between water and glycogen restoration following prolonged exercise in the heat designed to reduce both muscle glycogen and muscle water content. This information is important to re-address current nutritional advices regarding glycogen recovery to improve athletic performance as well as to explain body weight fluctuations in people who adopt a low-carbohydrate diet. To achieve this goal we measured the recovery of muscle glycogen and water 4 h after prolonged exercise in the heat that reduced both muscle glycogen and muscle water content. Importantly, in order to avoid estimations errors when extrapolating to the whole body level, both water and glycogen changes were measured in the same muscle biopsy in each assessment. A randomized, cross-over study design was used to investigate the role of rehydration in muscle glycogen recovery in subjects who underwent two trials with the same provision of carbohydrate but very different volumes of water ingested.
Methods
Participants
Nine endurance-trained male cyclists, who routinely cycled at least 2 h day−1, 4–7 days week−1 during the last 3 years, were recruited. Participant’s physical characteristics are shown in Table 1. Subjects were fully informed about the experimental procedures and the possible risks and discomforts associated with the experiment before they gave their written informed consent to participate. This study was approved by the Virgen de la Salud Hospital Research Ethics Committee in accordance with the latest version of the Declaration of Helsinki.
Preliminary testing
One week prior to their participation in the study, subjects underwent a physical examination including rest and exercise ECG (Cosmed T12, Italy). Subjects underwent graded exercise testing (GXT) in an electromagnetically braked cycle ergometer (Cardiotest 100, Seca, Germany) to determine their maximal aerobic capacity (i.e., \(\dot{V}{\text{O}}_{2\hbox{max} }\)). After a 5-min warm-up at 100 W, participants began cycling at 125 W with increments of 25 W each minute until exhaustion. \(\dot{V}{\text{O}}_{2}\) and \(\dot{V}{\text{CO}}_{2}\) were measured using an automated breath by breath indirect calorimetry system (Quark b2, Cosmed, Italy) and averaged every 10 s. \(\dot{V}{\text{O}}_{2\hbox{max} }\) was defined as the highest oxygen consumption value that occurred despite increases in workload. Maximal heart rate (from ECG recordings) and maximal power output (W max; Arts et al. 1993) were determine as the highest values recorded during the GXT.
Experimental design
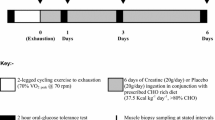
All participants underwent two experimental trials in a random counterbalanced order separated 1 week between them. Each trial started by dehydrating subjects with 150 min of continuous cycling at 65 % \(\dot{V}{\text{O}}_{2\hbox{max} }\) in a hot-dry environment (33 ± 4 °C, 25 % relative humidity, air flow 2.5 m s−1) without fluid replacement. After dehydration, subjects rested supine in a thermoneutral environment (22 ± 2 °C and 40 % relative humidity) while ingesting 250 g of carbohydrate (i.e., 250 gr glucose plus fructose with 125 mg of Na+, 30 mg of K+ and 1.5 mg of Cl−; Powerade®) dissolved in 400 mL of water (i.e., REHLOW trial), or ingested the same amount of carbohydrate syrup (i.e., 250 g) plus a volume of water that matched their individual fluid losses (i.e., ~3170 mL; REHFULL trial).
Experimental protocol
The day before the onset of each experimental trial, participants refrained from exercising, followed a diet rich in carbohydrates (6–9 g kg−1 per day) and drank liberally to ensure full glycogen storage. Experimental trials started at the same time-of-day to avoid circadian variation in physiological variables (Krauchi and Wirz-Justice 1994). Participants arrived at the laboratory after an overnight fast and were provided with a standardized breakfast (i.e., 3 g kg−1 of body mass consisting of milkshake, bread and cacao) and 500 mL of water to ensure euhydration. One hour after breakfast subjects voided and their nude body weight was measured using a sensitive scale (i.e., ±0.05 kg; Seca 764, Germany). Urine was analysed for specific gravity (USG) to confirm euhydration [i.e., <1.020; (Fernandez-Elias et al. 2014b; Sawka et al. 2007)]. Then, subjects rested on a stretcher and a muscle biopsy was obtained from the vastus lateralis. Following the muscle biopsy, participants dressed in shorts and cleated cycling shoes and entered in the climatic chamber where they started cycling. Upon completion of the dehydrating exercise, participants toweled dry and their post-exercise nude body mass was measured again. Then, participants lay down on a stretcher for an hour to ensure that body fluids were evenly redistributed among fluid compartments (Mora-Rodriguez et al. 2014) and subsequently a second muscle biopsy was obtained (i.e., post-exercise biopsy). Then participants drank either 250 g of carbohydrates in 400 mL of water (REHLOW) or the same syrup plus as much water as needed to replace body fluids losses (REHFULL). Subjects were encouraged to drink it within 20 min. Subjects rested supine for 3 h after which a third biopsy was obtained. During recovery, urine was collected and its volume measured using a graduated cylinder (Symax, Proton, Spain). At the end of the recovery period, participant’s body weight was measured again. A timeline of the trials is shown in Fig. 1.
Muscle biopsy
Muscle biopsy samples were taken from the vastus lateralis using the suction-modified Bergstrom technique (Tarnopolsky et al. 2011). Skin was prepared with Povidone-iodine (Betadine, MEDA, France), followed by injection of 2 % lidocaine without epinephrine (Braun 2 %, Braun Medical, Spain). Then, the skin and underlying tissues were surgically opened (scalpel blade number 10, Braun, Germany) and muscle tissue obtained using a 5 mm internal diameter Bergstrom biopsy needle. Samples were immediately cleaned of connective tissue and rapidly frozen in liquid nitrogen for subsequent analysis of water and glycogen. The incision was closed using adhesive strips (Steri-StripTM, 3M, USA) covered with an adhesive dressing pad (TegadermTM + Pad, 3M, USA) and compressive dressing (IcoVenda, Novico medica, Spain) during the next 24 h.
To limit participants’ burden in terms of number of biopsies (three per trial) only one pre-exercise biopsy was obtained from each participant out of the two trials. Since we standardized diet and physical activity before trials, and because trials were separated one week to allow full recovery, we assumed that participants started both trials with the same muscle glycogen and water level. In each trial all biopsies were taken from the same leg using a different incision for each biopsy. The incisions were spaced ~5 cm in between and were taking advancing distally (Costill et al. 1988).
Measurement of water and glycogen in biopsy samples
All samples from a given subject were analyzed in the same assay batch. Frozen samples were weighed on an electronic balance with a sensitivity of 0.1 µg (XB220A, Precisa, Switzerland). Elapsed time from sample removal from the freezer until weighing was recorded to permit correction for tissue water evaporation. Samples were freeze dried in a thermoelectric freeze-dryer (Cryodos-50, Telstar, Spain) for 6 h at −50 °C and at a vacuum of 10−2 Torr. In brief, this apparatus freezes the liquid in the sample to then sublimate it with a potent vacuum pump at a high flux rate (83 L min−1). Samples were then re-weighed in the same precision scale and the difference in weight (i.e., water) was expressed per 100 g of dry tissue. Data from one subject that underwent 10 resting biopsies within 8 weeks in a euhydrated state (Usg < 1.020 and body weight ±0.25 kg) revealed a high reproducibility (i.e., 5 % CV) in the measurement of muscle water content using this technique in our laboratory.
Glycogen content was determined from the measurement of glucose after acid hydrolysis analysis (Passonneau and Lauderdale 1974). Briefly, muscle samples (~20 mg) were homogenized using a glass-on-glass system on ice with deionized water. Then, samples were hydrolyzed in 2 N hydrochloric acid and heated for 2 h at 100 °C (Tembloc, JP Selecta, Spain). Finally, samples were neutralized to pH 6.5–7.5 with 1 N sodium hydroxide, and glucose concentration was analyzed by standard enzymatic assay. Glycogen from bovine liver (Sigma, Spain) was used as internal standard.
Statistical analysis
Data is presented as mean ± SD. Shapiro–Wilk test was used to confirm normal distribution of data. Differences between treatments were identified by using student’s paired t test. Data collected repeatedly over time were analyzed using two-way (time × treatment) repeated measures ANOVA. After a significant F ratio (Greenhouse–Geisser adjustment for sphericity), pair-wise differences were identified using Tukey’s (HSD) post hoc procedure. The level of significant was set at P < 0.05. Cohen’s formula for effect size (ES) was used and the results were based on the following criteria: >0.70 large effect, 0.30–0.69 moderate effect, and <0.30 small effect (Cohen 1988). Data analysis was performed using SPSS software v.19 (IBM, USA). Data are presented as mean ± SD.
Results
Body weight loss and fluid replacement
Dehydrating exercise resulted in similar body weight loss in both trials (3.33 ± 0.26 vs. 3.17 ± 0.17 kg; P = 0.48; Table 2). During the 4 h after exercise, subjects recovered 2.01 ± 0.87 kg in the REHFULL trial. However, as expected, body weight remained at dehydrated levels (65.9 ± 6.7 kg) in the REHLOW trial since the 400 mL ingested were calculated to offset the loss of insensible sweat and respiratory water during the 4 h recovery. Urine production during recovery was 186 ± 65 and 1027 ± 356 mL for REHLOW and REHFULL, respectively (P < 0.05; Table 2).
Muscle glycogen and muscle water content changes
One hour after the 150 min of dehydrating exercise, muscle glycogen and muscle water were reduced to the same extent in both trials (Fig. 2). Muscle glycogen decreased by 42 ± 11 and 46 ± 12 % during REHLOW and REHFULL, respectively (lower than pre-exercise values; P < 0.05; Fig. 2). Muscle water decreased by 13 ± 6 and 13 ± 7 % during REHLOW and REHFULL (lower than pre-exercise values; P < 0.05; Fig. 2). Hence, in both trials, the participants had similar depletion of glycogen and water content in their vastus lateralis before the experimental intervention began (i.e., LOW or FULL rehydration).
After the 3 h of rehydration-recovery period, muscle glycogen increased to a similar value in both trials (79 ± 15 and 87 ± 18 g kg−1 dm, for REHLOW and REHFULL respectively; Fig. 2). Net glycogen resynthesis was not different between trials, although it tended to be higher in REHFULL than in REHLOW (i.e., 11 ± 8 vs. 23 ± 22 g kg−1 dm, respectively; P = 0.15; ES = 0.79). As intended, muscle water content after the recovery period was significantly lower in REHLOW compared to REHFULL (3459 ± 324 vs. 3814 ± 222 g kg−1 dm respectively; P < 0.05; Fig. 2). Net water recovery was significantly lower in REHLOW (i.e., 34 ± 46 g kg−1 dm) than in REHFULL (i.e., 391 ± 249 g kg−1 dm; P < 0.05).
Ratio of glycogen to water recovery
The ratio of glycogen resynthesis to water restoration in muscle biopsies was 1:3 in the REHLOW and 1:17 in REHFULL, a difference that almost reached statistical significance (P = 0.059; ES = 0.057; Fig. 3).
Discussion
The main purpose of this study was to determine the relationship between glycogen and water recovery after dehydrating and glycogen-depleting exercise in skeletal muscle. It is commonly stated that 3–4 g of water are bound to each gram of glycogen in skeletal muscle (Olsson and Saltin 1970). Our direct measurement of both water and glycogen recovery in muscle biopsies revealed that this ratio is variable and seems to depend on the amount of fluid provided. In fact, we found that the ratio of glycogen and water storage in muscle can be reduced almost sixfold (from 1:17 to 1:3; Fig. 3) when rehydration is insufficient (i.e., REHLOW trial) resulting in a ratio similar to what Olson and Saltin proposed back in 1970 (i.e., 1:3 glycogen to water).
Initially, our data could be interpreted to support that these two phenomena (i.e., muscle glycogen and water replenishment) occur at different rates during recovery from prolonged exercise and, therefore, are variable and independent of each other. However, when fluid was given to only offset respiratory and insensible water losses during the 3 h of recovery and subjects remained dehydrated (i.e., REHLOW; Table 2) the glycogen-to-water recovery ratio was 1:3 (Fig. 3). The ingested solution provided during REHLOW contained a 1:1.6 glucose-to-water ratio (i.e., 250 g of carbohydrate in 400 mL of water) and thus passive transport of fluid and carbohydrate into the muscle does not explain the 1:3 ratio found. We think that this 1:3 ratio during REHLOW is not an accidental finding, but rather suggests that each gram of glycogen is bound to at least 3 g of water in muscle. Then, the 1:17 ratio measured during REHFULL reflects that possibility of higher water storage not associated with glycogen.
Olsson and Saltin (1970) proposed in 1970 that 3–4 g of water are bound to each gram of glycogen when stored in human skeletal muscle. In that study, subjects underwent glycogen-depleting exercise followed by 2 days of a low-carbohydrate diet switching over to a high-carbohydrate diet from day 3 to 5. During the high-carbohydrate diet (day 3–5) they found a parallel increase in muscle glycogen (i.e., 500 g) and whole body water (i.e., 2.2 L measured with tritium-labelled water) corresponding to a 1:3 ratio. Nonetheless, water recovery was measured at the whole body level while glycogen resynthesis was measured in a small portion of the musculature (i.e., biopsy sample). The authors extrapolated glycogen resynthesis to all the musculature in the body estimating muscle mass from potassium excretion. Furthermore, it was assumed that all muscles lost the same amount of glycogen than the sampled muscles. Although these assumptions could have distorted the calculation of this ratio, our data measuring both water and glycogen in the same muscle sample coincides with their data in the 1:3 ratio (i.e., only in REHLOW).
The ratio of 1:3 in glycogen to water content was first found in livers of rodent undertaking nutritional manipulations (fast and refeeding). From its proposal, the concept of a coordinated storage of water and glycogen has raised debate among researchers (Peters and Lavietes 1933). Beyond liver data, this ratio has been investigated in rat muscles (Sherman et al. 1982). Sherman and co-workers measured water and glycogen in muscles of a group of rats 45 min after exhausting exercise (i.e., glycogen depleted) and compared it to another group that was allowed to consume water and food ad libitum during 3 days (i.e., recovered). Although muscle glycogen was different between the exhausted and the recovered rats, muscle water was not different. Unlike humans, rats do not rely on sweat to dissipate the heat produced by exercise but rather they regulate their temperature by constricting or expanding blood vessels in their tails (Vanhoutte et al. 2002). In this light, their lack of difference in muscle water between the glycogen-depleted and the glycogen-recovered rats is not that surprising, since none of the groups may have incurred significant fluid deficit.
The second objective of our study was to address if muscle water restoration would limit the rates of muscle glycogen recovery. This question has been previously investigated in humans. Neufer and co-workers (Neufer et al. 1991) used prolonged exercise to lower muscle glycogen and sauna to reduce muscle water inducing similar dehydration level to the ones reported presently (5 vs. 4.6 %). They also measured glycogen and water content in muscles biopsies and were first to find that water deprivation does not prevent normal muscle glycogen resynthesis. We corroborated their findings, since there was no significant reduction in the amount of glycogen stored when subjects remained dehydrated (11 ± 8 vs. 23 ± 22 g kg−1 dm; P = 0.15).
In contrast to the data in humans (Neufer et al. 1991), data in animals suggest that fluid modulates the rate of glycogen restoration in muscle. In rat myotubes, glycogen synthesis is reduced when cell water is reduced by exposure to a hyperosmotic media. Furthermore, glycogen synthesis is increased after swelling induced by exposure to hyposmotic media (Low et al. 1996). In horses, fluid ingestion after exercise affects glycogen restoration. Waller and co-workers found that glycogen recovery is accelerated in Standardbred horses when after depleting-dehydrating exercise (70 min intervallic exercise) horses are provided with an amount of water and electrolytes similar to the volume (8000 mL) and composition of sweat lost during exercise (Waller et al. 2009). Thus, more studies in humans are needed to confirm the lack of effect of muscle hydration on the rates of glycogen resynthesis.
Our study has several limitations. First our sample size (n = 9) was perhaps concealing a real difference in glycogen recovery between REHLOW and REHFULL (i.e., type II error) since we found a large effect size (i.e., 0.79). Due to the variable response of one subject (higher glycogen recovery in REHLOW than REHFULL) a sample of 58 individuals would have been needed to reach statistical significance. Second, to avoid subject attrition due to the excessive number of muscle biopsies we collected only one pre-exercise sample biopsy and assumed that due to our careful standardization of diet and exercise, the measured levels of muscle water and glycogen could be extrapolated to both trials. Data in one subject who underwent 10 resting biopsies within 8 weeks in a euhydrated, well-fed rest state, revealed a 5 % CV in muscle water and a 4 % CV in muscle glycogen. Nevertheless, it is possible that this low variability was not present in all subjects.
Finally, our water-measuring technique does not allow differentiation between the water stored associated (linked) with glycogen and the gains in water not associated with glycogen. It has been long argued that water could be linked to other solutes in muscle overestimating the relationship between water and glycogen recovery (Peters and Lavietes 1933). After endurance exercise consisting of 2 h of pedalling, whole body amino acid net balance is slightly negative when carbohydrates are provided (Howarth et al. 2009). Thus, it is unlikely that protein synthesis and its associated water in muscle were causing water overestimation in our short-term recovery study (i.e., 4 h). Nevertheless, we recognize that the ratios between glycogen and water presented are only estimations of the coordinated response of the restoration of these substrates in human skeletal muscle.
In conclusion, the results of our short-term recovery (i.e., 4 h) experiment sampling human skeletal muscle do not oppose the frequently quoted statement that with every gram of glycogen 3 g of water are stored. Although we have not directly measured the water bound to the skeletal muscle glycogen molecule, we have measured the coordinated increases in both water and glycogen in the same muscle biopsy piece. When we restrict oral rehydration after prolonged dehydrating exercise the ratio of vastus lateralis glycogen to water recovery is 1:3. Our data suggest that at least 3 g of water are stored for each gram of glycogen in muscles previously depleted of both by prolonged dehydrating exercise.
Abbreviations
- \(\dot{V}{\text{O}}_{2\hbox{max} }\) :
-
Maximal oxygen consumption
- h:
-
Hours
- yrs:
-
Years
- Usg:
-
Urine specific gravity
References
Arts FJ, Kuipers H, Jeukendrup AE, Saris WH (1993) A short cycle ergometer test to predict maximal workload and maximal oxygen uptake. Int J Sports Med 14:460–464
Bergström J, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Bridge EM, Bridges EM (1931) The relation of glycogen to water storage in the liver. J Biol Chem 96:381–386
Brooks G, Fahey T (1984) Exercise Physiology. Macmillan Publishing Co, New York
Coggan AR, Swanson SC (1992) Nutritional manipulations before and during endurance exercise: effects on performance. Med Sci Sports Exerc 24:S331–S335
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum, New Jersey
Costill DL, Pearson DR, Fink WJ (1988) Impaired muscle glycogen storage after muscle biopsy. J Appl Physiol 64:2245–2248
Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF (1994) Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol 77:2827–2831
Fernandez-Elias VE, Hamouti N, Ortega JF, Mora-Rodriguez R (2014a) Hyperthermia but not muscle water deficit increases glycogen use during intense exercise. Scand J Med Sci Sports (in press)
Fernandez-Elias VE, Martinez-Abellan A, Lopez-Gullon JM, Moran-Navarro R, Pallares JG, De la Cruz-Sanchez E, Mora-Rodriguez R (2014b) Validity of hydration non-invasive indices during the weightcutting and official weigh-in for Olympic combat sports. PLoS One 9:e95336
Geddes R, Harvey JD, Wills PR (1977) The molecular size and shape of liver glycogen. Biochem J 163:201–209
Howarth KR, Moreau NA, Phillips SM, Gibala MJ (2009) Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol 106:1394–1402
Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF (1988a) Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol 64:1480–1485
Ivy JL, Lee MC, Brozinick JT Jr, Reed MJ (1988b) Muscle glycogen storage after different amounts of carbohydrate ingestion. J Appl Physiol 65:2018–2023
Krauchi K, Wirz-Justice A (1994) Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 267:R819–R829
Kreitzman SN, Coxon AY, Szaz KF (1992) Glycogen storage: illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition. Am J Clin Nutr 56:292S–293S
Low SY, Rennie MJ, Taylor PM (1996) Modulation of glycogen synthesis in rat skeletal muscle by changes in cell volume. The Journal of physiology 495(Pt 2):299–303
MacKay EM, Bergman MC (1932) The relation between glycogen and water storage in the liver. J Biol Chem 96:373–380
Mora-Rodriguez R, Fernandez-Elias VE, Hamouti N, Ortega JF (2014) Skeletal muscle water and electrolytes following prolonged dehydrating exercise. Scand J Med Sci Sports (Epub ahead of print)
Neufer PD, Sawka MN, Young AJ, Quigley MD, Latzka WA, Levine L (1991) Hypohydration does not impair skeletal muscle glycogen resynthesis after exercise. J Appl Physiol (1985) 70:1490–1494
Olsson KE, Saltin B (1970) Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand 80:11–18
Passonneau JV, Lauderdale VR (1974) A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60:405–412
Peters JP, Lavietes PH (1933) The nature of “preformed water”. J Clin Investig 12:695–712
Puckett HL, Wiley FH (1932) The relation of glycogen to water storage in the liver. J Biol Chem 96:367–371
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39:377–390
Sherman WM, Plyley MJ, Sharp RL, Van Handel PJ, McAllister RM, Fink WJ, Costill DL (1982) Muscle glycogen storage and its relationship with water. Int J Sports Med 3:22–24
Tarnopolsky MA, Pearce E, Smith K, Lach B (2011) Suction-modified Bergstrom muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve 43:717–725
Vanhoutte G, Verhoye M, Raman E, Roberts M, Van der Linden A (2002) In-vivo non-invasive study of the thermoregulatory function of the blood vessels in the rat tail using magnetic resonance angiography. NMR Biomed 15:263–269
Waller AP, Heigenhauser GJ, Geor RJ, Spriet LL, Lindinger MI (2009) Fluid and electrolyte supplementation after prolonged moderate-intensity exercise enhances muscle glycogen resynthesis in Standardbred horses. J Appl Physiol 106:91–100
Acknowledgments
We truly thank the participants for their dedication and effort. We thank Rafael Urrialde health and nutrition director from Coca Cola Iberia for the gracious donation of the carbohydrate powder (Powerade®). We thank Dr. Jeffrey F. Horowitz from the University of Michigan for his advice and guidance during pilot data collection. V.E.F-E. was supported by a predoctoral fellowship from the Junta de Comunidades de Castilla-La Mancha. This study was partially supported by a grant from the Junta de Comunidades de Castilla-La Mancha (PEII-2014-004-A).
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Fernández-Elías, V.E., Ortega, J.F., Nelson, R.K. et al. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur J Appl Physiol 115, 1919–1926 (2015). https://doi.org/10.1007/s00421-015-3175-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3175-z