Abstract
Objectives
The traditional view of differential left ventricular adaptation to training type has been questioned. Right ventricular (RV) data in athletes are emerging but whether training type mediates this is not clear. The primary aim of this study was to evaluate the RV phenotype in endurance- vs. resistance-trained male athletes. Secondary aims included comparison of RV function in all groups using myocardial speckle tracking, and the impact of allometric scaling on RV data interpretation.
Methods
A prospective cross-sectional design assessed RV structure and function in 19 endurance-trained (ET), 21 resistance-trained (RT) and 21 sedentary control subjects (CT). Standard 2D tissue Doppler imaging and speckle tracking echocardiography assessed RV structure and function. Indexing of RV structural parameters to body surface area (BSA) was undertaken using allometric scaling.
Results
A higher absolute RV diastolic area was observed in ET (mean ± SD: 27 ± 4 cm2) compared to CT (22 ± 4 cm2; P < 0.05) that was maintained after scaling. Whilst absolute RV longitudinal dimension was greater in ET (88 ± 9 mm) than CT (81 ± 10 mm; P < 0.05), this difference was removed after scaling. Wall thickness was not different between ET and RT and there were no between group differences in global or regional RV function.
Conclusion
We present some evidence of RV adaptation to chronic ET in male athletes but limited structural characteristics of an athletic heart were observed in RT. Global and regional RV functions were comparable between groups. Allometric scaling altered data interpretation in some variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The traditional interpretation of cardiac morphological adaptation to exercise is that a dichotomous athletic heart phenotype exists in endurance- and resistance-trained athletes (Morganroth et al. 1975) linked to differential haemodynamic loading during acute exercise (George et al. 1991). Recent evidence has cast doubt on this in regard to the left ventricle (LV) with limited adaptation of any form noted in resistance-trained athletes (Haykowsky et al. 2000; Utomi et al. 2013, 2014).
A smaller right ventricular (RV) database exists, in comparison to the LV, but great RV size has been fairly consistently described in endurance-trained athletes (Baggish et al. 2008; Teske et al. 2009b; Oxborough et al. 2012b; D’Andrea et al. 2013) and supported by a recent prospective study (Baggish et al. 2008) that reported RV eccentric hypertrophy after 12 months of endurance training (ET) (Arbab-Zadeh et al. 2014). Interest in the RV adaptation to exercise has also been prompted by the demonstration of a disproportionate increase in haemodynamic afterload on the RV, compared to the LV, during intense ET (La Gerche et al. 2011). If differences in haemodynamic loading exist between the ventricles during the same sporting activity, it is plausible that their adaptive response may be different.
There are few comparisons of RV structure and function in athletes with divergent training stimulus and little RV data in resistance-trained athletes (Utomi et al. 2013). Those data that are available are limited by the application of scaling or indexing (none used or ratio scaling adopted) and an inconsistent range of echocardiographic imaging modes have been employed (Koc et al. 2007; D’Andrea et al. 2013; King et al. 2013; Pagourelias et al. 2013).
Non-invasive imaging of the RV has been viewed as complex, but continuing technical developments have resulted in a standardised assessment protocol for the RV (Rudski et al. 2010). These protocols have facilitated renewed interest in the RV of athletes and the ability to differentiate RV physiological hypertrophy from pathological changes associated with congenital diseases such as arrhythmogenic RV cardiomyopathy (ARVC) (Maron et al. 2007). Likewise, newer imaging modes assessing global and regional RV functional data in athletes are receiving some attention. RV strain (ε) data in athletes are controversial with reduced resting deformation in some (Teske et al. 2009a; La Gerche et al. 2012; King et al. 2013) but not all studies (Oxborough et al. 2012b; Pagourelias et al. 2013). Given the potential value of deformation parameters to improve the discrimination of normal and pathologic adaptation (Teske et al. 2009a; Marcus et al. 2010), further evaluation in different athlete groups will be informative.
Finally, it is well established that taking into account differences in body size can alter the interpretation of LV athletic heart data (Dewey et al. 2008). Despite this little attention has been given to appropriate scaling of RV dimensions (Oxborough et al. 2012b). Prakken et al. (2010) and Luijkx et al. (2013) applied simple ratio scaling to magnetic resonance imaging-derived RV parameters to determine reference values for athletes. Although informative, empirical studies demonstrate that body size does not relate to cardiac dimensions in a linear fashion (Batterham et al. 1999; George et al. 2001; Dewey et al. 2008) and thus we must interpret data from Prakken and Luijkx carefully. The impact of an allometric approach to scaling of RV data on interpretation of outcomes could extend our understanding of the athletic heart phenotype.
This study tests the following primary hypothesis: global RV adaptation to exercise is mediated by training type. Secondary hypotheses are (1) global and regional RV function will be related to training type, and (2) the allometric scaling of RV structural data will alter data interpretation.
Methods
Study population
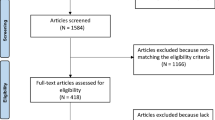
After institutional Ethics Committee approval, 61 males provided written informed consent to participate in the study. We recruited elite athletes (competitive at a national or international level) with either a predominant endurance- (ET) or resistance-training (RT) focus. Specifically, 19 were elite ET recruited from an international field at the 100-mile Western States Endurance Run (CA, USA) and 21 were elite RT recruited from the British national weightlifting and Aikido squad. The ET and RT were matched for accumulated mean training years, training hours per week and training days per week of (ET: 11 years, 12 h/weeks and 6 days/weeks; RT: 12 years, 11 h/weeks, 6 days/weeks, respectively).
All athletes reported a minimum of 6 years continuous national and international level participation and were competing at the highest competitive sporting level in their respective specialties. In addition, 21 sedentary controls (CT) were recruited from the university student population in a similar age range. The CT were healthy individuals who were not engaged in systematic sport-related training and engaged in less than 3-h recreational activity per week. All participants self-reported as healthy, free from known cardiovascular disease and were not currently taking any form of prescribed medication. All participants were Caucasian, non-smokers, 18–45 years of age and reported no early family history of cardiovascular disease.
The study was guided by the “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement”: guidelines for reporting observational studies (von Elm et al. 2008) and conformed to the Declaration of Helsinki. The nature of LV structure and function in these subjects was recently published (Utomi et al. 2014). The demographic characteristics of all participants are presented in Table 1.
Study design and procedures
A prospective cross-sectional study design was employed with data acquired in a resting state at a single testing session. All subjects were advised to abstain from exercise training, caffeine and alcohol consumption at least 3 h prior to the investigation. After a detailed explanation of the test protocol, subjects were initially issued with a medical questionnaire and then assessed for basic anthropometrics. Height and body mass were assessed using a stadiometer and digital weighing machine (SECA 764, Birmingham, UK) and body surface area (BSA) was calculated. After 5 min of supine rest, brachial artery systolic and diastolic blood pressures as well as resting heart rate were assessed with an automated sphygmomanometer (DINAMAP 300, GE Medical Systems, Milwaukee, USA).
Echocardiographic assessment
Standard echocardiographic investigation was performed using a Vivid Q ultrasound machine (GE Medical System, Horten, Norway) with a 1.7- to 4-MHz transducer. All acquisitions were made with the subject lying in the left lateral decubitus position by the same experienced echocardiographer using a standard echo-protocol in accordance with the American Society of Echocardiography (ASE) (Lang et al. 2005). Standard 2D echocardiographic parameters were obtained from parasternal and modified apical acoustic windows (Rudski et al. 2010). Images were recorded to DVD in a raw digital format and offline analysis was performed using commercially available software (EchoPAC Version 7.0; GE Vingmed Ultrasound, Horten, Norway).
Conventional 2D and Doppler/tissue Doppler echocardiography
In accordance with ASE guidelines, RV size was measured at end diastole from the proximal RV outflow tract at the level of the aortic valve (PLAX RVOT and RVOT1) and at the pulmonary valve annulus (RVOT2) using a parasternal short-axis (PSAX) orientation. RV free wall was measured in diastole from the subcostal view and RV cavity dimensions at the basal RV inflow (RVD1); mid level (RVD2) and RV length (RVD3) were measured from the modified apical four-chamber orientation. RV diastolic and systolic areas were calculated by tracing around the endocardium from a modified apical four-chamber orientation, and RV fractional area change (RV FAC) was calculated (Rudski et al. 2010).
Tricuspid annular plane excursion (TAPSE) was obtained as a measure of RV longitudinal function. RV stroke volume (RVSV) was calculated using the general formula RVSV = π (RVOT/2) × 2 × VTI; RV velocity time integral (VTI) was measured from the Doppler spectral envelop derived from a 4-mm sample volume placed at the RV outflow in the PSAX view (Lang et al. 2005; Rudski et al. 2010).
To accommodate for individual differences in body size, and any consequent impact on interpretation of between group differences in RV structure, empirically supported (Oxborough et al. 2012a, b) allometric power function relationships were applied in an allometric scaling model (Y/X b; where Y is the cardiac variable, a is the y-axis intercept, X is the body size variable, and b is the slope of the log-transformed data plot). This technique is theoretically and empirically superior to ratio standard scaling (Y/X) which is very common in clinical cardiology but whose basic flaw is a priori assumption of a linear relationship (Batterham et al. 1999). This is not the case, unless Tanner’s special circumstance is met (Batterham et al. 1999), and likely invalidates most data interpretation (Dewey et al. 2008). Specifically, RV structural variables were allometrically scaled for individual differences in BSA according to laws of geometric similarity (Batterham et al. 1999; George et al. 2001; Dewey et al. 2008). The Law of Geometric similarity suggests that simple ratio scaling of the form Y/X is only theoretically appropriate when both body size and cardiac parameters share the same number of structural dimensions (e.g. LV mass and body mass are both three-dimensional constructs). In cases where variables of different dimensions are coupled (e.g. LV mass and BSA), the ratio of their dimensions (3/2 = 1.5) is used as the b exponent.
Pulsed-wave TDI was used to assess RV myocardial velocities. The transducer was orientated more medially, to align the longitudinal movement of the RV lateral wall with the ultrasound beam to ensure that the angle of isonation was as close to zero as possible. A 4-mm sample volume was positioned in the lateral aspect of the tricuspid annulus of the RV lateral wall and peak velocities in systole (RVS′), early diastole (RVE′) and late diastole (RVA′) were measured. Tissue velocity data were scaled to RV length (Batterham et al. 2008).
Speckle tracking echocardiography (STE)
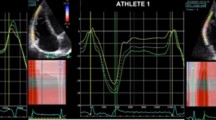
The modified apical four-chamber orientation was used for the assessment of longitudinal RV ε and strain rate (SR). During offline analysis, the region of interest (ROI) was placed around the RV lateral wall from the base to apex. A priori, each segment was visually inspected, and the ROI adjusted to track in synchrony before approval. Regional peak values were obtained at the base, mid and apical RV wall, and a base-to-apex gradient was calculated. Global peak values were calculated as an average of all three myocardial segments. Indices obtained included peak RV ε and SR during ventricular systole (SRS) and during early and late ventricular diastole (SRE and SRA) (Fig. 1). Intra-observer variation for RV structural and functional data in our laboratory demonstrated no systematic bias for peak ε or indices of SR (P < 0.05) with intraclass correlation coefficients of 0.834 and 0.610, respectively (Oxborough et al. 2012a).
Data analysis and statistics
As an important exploration of our athlete data we applied the revised ARVC taskforce quantitative diagnostic criterion (major and minor) to structural features of the RV in our participants (Marcus et al. 2010). It should be noted that a key requirement of all the major and minor criteria is that regional wall motion abnormality must also be present. All further analyses were performed using SPSS, version 20.0, for Windows (SPSS, Chicago, IL, USA) and the critical alpha was set at P ≤ 0.05. Data are presented as mean ± SD. Normality of data distribution was assessed with the Kolmogorov–Smirnov test and analysed between groups using one-way ANOVA with Bonferoni post hoc tests for multiple comparison to estimate differences between groups where appropriate.
Results
Demographic characteristics of the study population
The three groups were comparable for body mass, height, BSA and resting blood pressure (Table 1). ET athletes were slightly older than CT as well as having a lower resting HR compared to RT.
2D-Echocardiography: RV structure
RV structural data (absolute and allometrically scaled) are contained in Table 2. Higher absolute values for RVD1, RVD3, RV areas and RV free wall thickness were observed in the ET compared to CT. Absolute RVD1 was also higher in ET than RT. When these variables were scaled allometrically, all remained significant except RVD3 (between ET and CT) and RVD1 (between ET and RT). RT also had a higher RVWT than CT which was maintained on allometric scaling, although there were no differences in RVWT between ET and RT. There were no differences in other absolute or scaled RV structural data (RVD1, RVD2, RVD3, RVD area) between RT and CT (Fig. 2).
Global and regional RV function
Global RV functional data are presented in Table 3. There was no significant difference (P < 0.05) in standard RV functional parameters between all groups. Global RV ε, SRS as well as segmental ε did not significantly differ between the three groups (Fig. 3). The base-to-apex gradient for ε was not different between groups.
Application of the revised Task Force criteria for the diagnosis of ARVC
We did not observe any regional wall motion abnormality in any the subjects included in this study. Table 4 presents the comparison of participant’s structural RV data against the Revised Task Force criteria.
Discussion
The key findings from this study were that (1) various measures of RV structure were larger in ET compared to CT, but limited differences were noted between RT and CT; (2) RV global and regional function was not different between groups; and (3) allometric scaling or indexation of RV structural data for individual differences in BSA altered data interpretation in only two variables.
RV morphology
This study provides additional evidence that physiological remodelling can occur in the RV of ET (Oxborough et al. 2012b; D’Andrea et al. 2013) as well as during prospective studies (Baggish et al. 2008; Arbab-Zadeh et al. 2014), where a progressive increase in endurance training load resulted in evidence of RV dilatation. RV wall thickness was higher in ET than CT although all RV wall thickness values were within the normal physiologic range (Lang et al. 2015). This finding confirms some previous data (Koc et al. 2007; King et al. 2013).
The putative mechanism(s) involved in RV adaptation to ET remains controversial and poorly understood. Haemodynamic theories of adaptation previously applied to the LV have been purported to play a similar role in the RV such that ET activity places a prolonged but intermittent haemodynamic volume overload that initiates structural adaptation to normalise end-diastolic wall stress by increasing chamber diameter and to a smaller extent wall thickness (Grossman et al. 1975). Between group differences in resting HR could only explain c. 10 % of the shared variance in the larger RV morphology in ET. It was demonstrated recently that a disproportionate haemodynamic afterload is placed on the RV, compared with the LV, during intense ET due to a greater relative increase in pulmonary artery systolic pressure vs. systemic pressure (La Gerche et al. 2011) and this may partially explain the increase in RV wall size in the ET vs CT.
There were no differences in RV chamber dimensions between RT and CT, which likely reflects the limited haemodynamic volume overload experienced during intermittent resistance training and this mirrors recent RV data (D’Andrea et al. 2013; Pagourelias et al. 2013) as well as an athlete-control cross-sectional examination of the LV in RT athletes in a recent meta-analysis (Utomi et al. 2013). RV free wall thickness was significantly higher in RT than CT and thus the RT present with a mild wall thickening in the absence of changes in RV chamber dimensions. The interpretation of causation/mechanisms with respect to RVWT data in RT is speculative but worthy of some discussion. The small increase in RVWT in RT compared to CT may reflect an elevated haemodynamic pressure overload associated with acute RT where significant increases in systemic arterial pressure have been observed (MacDougall et al. 1985) although data for pulmonary artery blood pressure changes with such exercise are not available. Increased blood pressure with RT has been linked to a dichotomous presentation of the athlete’s heart dating all the way back to Morganroth’s seminal paper in 1975 as wall thickening would serve to normalise an elevated wall stress. Potential reasons for the fact that RVWT was not larger in RT than ET, as was the case in Morganroth et al. (1975), may relate to the limited time exposure to any haemodynamic overload, compared to the long-term steady-state haemodynamic conditions associated with endurance training. Of note, the presence of a haemodynamic pressure overload on the LV during resistance training has been challenged when a brief phase 1 Valsalva manoeuvre is performed (Haykowsky et al. 2001), although similar data are not available for RV and pulmonary artery pressures during resistance training. Finally, we should note that the differences in RVWT between groups are small (1 mm), all data were within normal ranges (Lang et al. 2015) and the nature of cross-sectional studies cannot discount genetic and selection bias issues in cardiac data. By comparison, a lack of RVWT adaptation was noted in prospective data collected before and after a progressive increase in RT training load (Baggish et al. 2008).
The implications of these data are that a dichotomous athletic heart phenotype, based on training background and haemodynamic stimulus, is not as clear as the classical studies from Morganroth et al. (1975) but cannot be completely disregarded on the basis of this single cross-sectional analysis. Despite this, the current data still have an important role in informing the likely upper normal limits of RV structures in different athlete groups and their use in cardiovascular pre-participation screening.
Global and regional RV function
Similar to the study by King et al. (2013), RV FAC and TAPSE as well as a range of other global parameters were not different between ET, RT and CT (King et al. 2013). Conversely, Teske et al. (2009a, b) reported a higher TAPSE in ET (26 ± 5 mm) than CT (24 ± 3 mm) (Teske et al. 2009a). Whilst the absolute mean data are not dissimilar to our study (ET: 24 ± 3 mm; CT 25 ± 2 mm) the heterogeneity in statistical outcomes between studies may be due to sample size, differences in age, training levels and echocardiographic vendor/quality. We also observed no between group differences in RV diastolic Doppler-flow parameters which confirms past work (Oxborough et al. 2012b; Pagourelias et al. 2013; Prakken et al. 2010) but disagrees with Teske et al. (2009a). Overall there seems to be little evidence to support training-induced changes in global RV systolic and diastolic function and this would suggest that resting RV function is normal in athletes.
In addition, we observed no between group differences in regional RV function using tissue Doppler imaging and STE. This confirms previous tissue Doppler data in athlete–control comparisons (D’Andrea et al. 2013; Pela et al. 2004). Of note global and regional longitudinal RV ε and SR were not different between groups and were also not diminished in any athletes. This observation contrasts with the report by Teske et al. (2009a) who demonstrated that ε and SR of the RV free wall were reduced in 63 highly trained athletes relative to controls, particularly in the basal segments of athletes with RV dilatation (Teske et al. 2009a). The authors proposed that this reflected normal physiological adaptation that resulted in a base-to-apex gradient in RV deformation (Teske et al. 2009a). Whilst we observed a base-to-apex gradient in deformation, this was not different between groups. It is speculated that because RV volume is greatest at the base, a smaller degree of deformation may be required to generate the same stroke volume, if contractility is preserved which consequently produces a base-to-apex gradient (La Gerche et al. 2012). It is also possible that any functional adaptation in the athletic heart is not apparent at rest, but may be noticeable during exercise (La Gerche et al. 2012). Further assessment of global and regional RV function at peak exercise could provide further insight to allow for distinction between athletes, control participants and those with RV pathology.
The impact of allometric scaling
The present study demonstrated that when absolute RV structural data are scaled for individual differences in body size (here BSA scaled allometrically) alterations in statistical comparisons can occur which fundamentally changes data interpretation. In the current study, this scaling approach only altered data interpretation for two variables. The importance of body size on cardiac dimension has been demonstrated in previous LV studies (George et al. 1998; Utomi et al. 2014) and a recent meta-analysis (Utomi et al. 2013). The current data add to this knowledge base specifically with respect to the interpretation of RV data in athletes. Further work should produce normative data for large athlete groups using this allometric approach.
Clinical implications of the revised Task Force criteria for the diagnosis of ARVC
We applied the revised Task Force diagnostic criteria for ARVC to RV data in all groups. First, the absence of regional wall abnormality and a normal FAC in all subjects meant that no subject met the functional component of the ARVC revised criteria (Marcus et al. 2010). Major and minor RV structural criteria were observed in all groups but with a generally higher prevalence in ET. In 50 % of ET, absolute PLAX RVOT fulfilled the major echocardiographic diagnostic criteria (≥32 mm) and a further 16 % met the minor structural criteria (≥29 mm but ≤32 mm) for ARVC (Table 4).
When these data were scaled (ratio) to BSA the prevalence dropped markedly and again reflects the importance of scaling RV structural data, although work on appropriate scaling should be factored into further developments in ARVC criteria. Also of interest was a lower expression in the major structural criteria for PSAX RVOT ≥36 mm (ET 27 %), which again reduced after ratio scaling, suggesting that the imaging window should be taken into account in any data interpretation. These findings are consistent with previous reports from our group (Oxborough et al. 2012b) and others (Teske et al. 2009a; Prakken et al. 2010). The important point, from a clinical perspective, is that no individual participant met either the major or minor ARVC criteria of poor RV function (Marcus et al. 2010).
Limitations and future research
This was a cross-sectional study in small but highly trained cohorts of male Caucasian athletes. Further work should expand the participant base as well as development of a prospective cohort design. Future studies should describe the upper limits of atrial structure and function in elite athlete groups.
Conclusion
This study observed greater RV dimensions in elite ET with mild RVWT thickening in both ET and RT compared to CT. Global and regional RV function was not different between both athlete groups and CT. Data interpretation was altered for two RV structural variables by the use of allometric scaling. These data will be a useful addition to the evidence base of upper normal limits of physiological adaptation in the RV that underpins data interpretation in athlete cardiovascular screening.
Abbreviations
- ARVC:
-
Arrhythmogenic right ventricular cardiomyopathy
- ASE:
-
American Society of Echocardiography
- BSA:
-
Body surface area
- CT:
-
Control subjects
- ε :
-
Strain
- ET:
-
Endurance-trained athlete
- FAC:
-
Fractional area change
- LV:
-
Left ventricular
- PASP:
-
Pulmonary artery systolic pressure
- PLAX:
-
Parasternal long axis
- PSAX:
-
Parasternal short axis
- RT:
-
Resistance-trained athlete
- RV:
-
Right ventricle
- RVD area:
-
Right ventricular end-diastolic area
- RVD1:
-
Right ventricular basal inflow
- RVL:
-
Right ventricular length
- RVOT:
-
Right ventricular outflow tract
- SR:
-
Strain rate
- SRA’:
-
Strain rate during late ventricular diastole
- SRE’:
-
Strain rate during early ventricular diastole
- SRS’:
-
Strain rate during ventricular systole
- STE:
-
Speckle tracking echocardiography
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TDI:
-
Tissue Doppler imaging
References
Arbab-Zadeh A, Perhomen M, Howden E, Peshock R, Zhang R, Adams-Huet B, Haykowsky M, Levine B (2014) Cardiac remodelling in response to 1 year of intensive endurance training. Circ J 130:2152–2161
Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AMJR, Wood MJ (2008) Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol 104:1121–1128
Batterham A, George K, Whyte G, Sharma S, McKenna W (1999) Scaling cardiac structural data by body dimensions: a review of theory, practice, and problems. Int J Sports Med 20:495–502
Batterham A, Shave R, Oxborough D, Whyte G, George K (2008) Longitudinal plane colour tissue-Doppler myocardial velocities and their association with left ventricular length, volume, and mass in humans. Eur J Echocardiogr 9(4):542–546 (Epub 2008 Mar 11)
D’Andrea A, Riegler L, Golia E, Cocchia R, Scarafile R, Salerno G, Pezzullo E, Nunziata L, Citro R, Cuomo S (2013) Range of right heart measurements in top-level athletes: the training impact. Int J Cardiol 164:48–57
Dewey F, Rosenthal D, Murphy DJ, Froelicher V, Ashley E (2008) Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 117:2279–2287
George KP, Wolfe LA, Burggraf GW (1991) The ‘athletic heart syndrome’. A critical review. Sports Med 11:300–330
George KP, Batterham AM, Jones B (1998) Echocardiographic evidence of concentric left ventricular enlargement in female weight lifters. Eur J Appl Physiol Occup Physiol 79:88–92
George K, Sharma S, Batterham A, Whyte G, McKenna W (2001) Allometric analysis of the association between cardiac dimensions and body size variables in 464 junior athletes. Clin Sci (Lond) 100:47–54
Grossman W, Jones D, McLaurin L (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Investig 56:56
Haykowsky Teo KK, Quinney AH, Humen DP, Taylor DA (2000) Effects of long term resistance training on left ventricular morphology. Can J Cardiol 16:35–38
Haykowsky M, Taylor D, Teo K, Quinney A, Humen D (2001) Left ventricular wall stress during leg-press exercise performed with a brief valsalva maneuver. Chest 119:150–154
King G, Almuntaser I, Murphy RT, la Gerche A, Mahoney N, Bennet K, Clarke J, Brown A (2013) Reduced right ventricular myocardial strain in the elite athlete may not be a consequence of myocardial damage. “Cream Masquerades as Skimmed Milk”. Echocardiography 30:929–935
Koc M, Bozkurt A, Akpinar O, Ergen N, Acarturk E (2007) Right and left ventricular adaptation to training determined by conventional echocardiography and tissue Doppler imaging in young endurance athletes. Acta Cardiol 62:13–18
la Gerche A, Burns AT, D’hooge J, Macisaac AI, Heidbüchel H, Prior DL (2012) Exercise strain rate imaging demonstrates normal right ventricular contractile reserve and clarifies ambiguous resting measures in endurance athletes. J Am Soc Echocardiogr 25(253–262):e1
La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL (2011) Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 43:974–981
Lang RM, Bierig M, Devereux RB, Flachskamp FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. J Am Soc Echocardiogr 18:1440–1463
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39
Luijkx T, Cramer M, Buckens C, Zaidi A, Rienks R, Mosterd A, Prakken N, Dijkman B, Mali W, Velthuis B (2013) Unravelling the grey zone: cardiac MRI volume to wall mass ratio to differentiate hypertrophic cardiomyopathy and the athlete’s heart. Brit J Sports Med. doi:10.1136/bjsports-2013-092360
MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR (1985) Arterial blood pressure response to heavy exercise. J Appl Physiol 58:785–790
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP (2010) Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia Proposed Modification of the Task Force Criteria. Eur Heart J 31:806–814
Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, Dimeff R, Douglas PS, Glover DW, Hutter AM (2007) Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 115:1643–1655
Morganroth J, Maron BJ, Henry WL, Epstein SE (1975) Comparative left ventricular dimensions in trained athletes. Ann Intern Med 82:521–524
Oxborough D, George K, Birch K (2012a) Intraobserver reliability of two-dimensional ultrasound derived strain imaging in the assessment of the left ventricle, right ventricle, and left atrium of healthy human hearts. Echocardiography 29:793–802
Oxborough D, Sharma S, Shave R, Whyte G, Birch K, Artis N, Batterham A, George K (2012b) The right ventricle of the endurance athlete: the relationship between morphology and deformation. J Am Soc Echocardiogr 25:263–271
Pagourelias ED, Kouidi E, Efthimiadis GK, Deligiannis A, Geleris P, Vassilikos V (2013) Right atrial and ventricular adaptations to training in male caucasian athletes: an echocardiographic study. J Am Soc Echocardiogr 26:1344–1352
Pela G, Bruschi G, Montagna L, Manara M, Manca C (2004) Left and right ventricular adaptation assessed by Doppler tissue echocardiography in athletes. J Am Soc Echocardiogr 17:205–211
Prakken NH, Velthuis BK, Teske AJ, Mosterd A, Mali WP, Cramer MJ (2010) Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil 17:198–203
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713 (quiz 786–788)
Teske A, Cox M, de Boec B, Doevendans P, Hauer R, Cramer M (2009a) Echocardiographic tissue deformation imaging quantifies abnormal regional right ventricular function in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Soc Echocardiogr 22:920–927
Teske A, Prakken N, de Boeck B, Velthuis B, Doevendans P, Cramer M (2009b) Effect of long term and intensive endurance training in athletes on the age related decline in left and right ventricular diastolic function as assessed by Doppler echocardiography. Am J Cardiol 104:1145–1151
Utomi V, Oxborough D, Whyte GP, Somauroo J, Sharma S, Shave R, Atkinson G, George K (2013) Systematic review and meta-analysis of training mode, imaging modality and body size influences on the morphology and function of the male athlete’s heart. Heart 99:1727–1733. doi:10.1136/heartjnl-2012-303465
Utomi V, Oxborough D, Ashley E, Lord R, Fletcher S, Stembridge M, Shave R, Hoffman M, Whyte G, Somauroo J, Sharma S, George K (2014) Predominance of normal left ventricular geometry in the male ‘athlete’s heart’. Heart. doi:10.1136/heartjnl-2014-305904
von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting of observational studies. Internist (Berl) 49:688–693
Acknowledgments
Study participants, coaches, British Aikido Association National Squad/team members, UK; Western States Endurance Run Foundation, USA and Dr Nick Sculthorpe for providing technical support.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David C. Poole.
Rights and permissions
About this article
Cite this article
Utomi, V., Oxborough, D., Ashley, E. et al. The impact of chronic endurance and resistance training upon the right ventricular phenotype in male athletes. Eur J Appl Physiol 115, 1673–1682 (2015). https://doi.org/10.1007/s00421-015-3147-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3147-3