Abstract
Purpose
The purpose of this study was to investigate the effects of a fatigue-inducing bout of submaximal, intermittent isometric contractions on the electromechanical delay (EMD) of the leg extensors and flexors in young and old men.
Methods
Twenty young (mean ± SD: age = 25 ± 2.8 years) and sixteen old (age = 70.8 ± 3.8) recreationally active men performed maximal voluntary contractions (MVCs) followed by a fatigue-inducing protocol consisting of intermittent isometric contractions of the leg extensors or flexors using a 0.6 duty cycle (6 s contraction, 4 s relaxation) at 60 % of MVC until volitional fatigue. MVCs were again performed at 0, 7, 15, and 30 min post fatigue. A three-way mixed factorial ANOVA was used to analyze the EMD data.
Results
There was a two-way muscle × time interaction (P = 0.039) where the EMD of the leg flexors was greater (P = 0.001–0.034) compared with baseline at all post fatigue time periods, but was only greater at immediately post fatigue for the extensors (P = 0.001). A significant two-way interaction for muscle × age (P = 0.009) revealed that the EMD was greater (P = 0.003) for the extensors for the old compared with the young men, but not different for the flexors (P = 0.506).
Conclusions
These findings showed differential fatigue-induced EMD recovery patterns between the leg extensors and flexors with the flexors being slower to recover and also that age-related increases of EMD are muscle group specific. The sustained increased EMD of the flexors during recovery may have important injury and performance implications in a variety of populations and settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of muscle contraction involves an electromechanical delay (EMD) which has been defined as the time lapse that exists between the onset of electrical activity and the onset of tension or force production (Cavanagh and Komi 1979). EMD is a common neuromuscular assessment tool and has been used previously by researchers examining the effects of muscle fatigue (Mercer et al. 1998; Minshull et al. 2007, 2012a), muscle damage (Minshull et al. 2012b; Howatson 2010), training-induced changes (Reeves et al. 2003), musculotendinous stiffness (Badier et al. 1999), differences among muscle groups (Nilsson et al. 1977; Zhou et al. 1995b; Minshull et al. 2007), genders (Minshull et al. 2007), and age groups (Zhou et al. 1995b). The EMD has been suggested to primarily be influenced by the propagation of action potentials across the muscle membrane, the excitation–contraction coupling (EC-C) processes (Boncompagni et al. 2006; Cavanagh and Komi 1979), and the stretching of the series elastic component (SEC) (Herda et al. 2012; Cavanagh and Komi 1979; Winter and Brookes 1991), with the SEC representing the major portion of the measured time delay (Cavanagh and Komi 1979; Badier et al. 1999). For example, Sasaki et al. (2011) have suggested the amount of slack within the muscle–tendon unit may be a primary factor influencing EMD and Nordez et al. (2009) have reported during plantar flexion actions, the passive portion of the SEC attributes 47.5 % of the total EMD. Additionally, muscle fiber type has been reported to influence the EMD (Nilsson et al. 1977; Viitasalo and Komi 1981) with a shorter EMD being associated with higher proportions of type II muscle fibers (Viitasalo and Komi 1981; Zhou et al. 1995a). Since EMD has been reported to be related to higher muscle strength (Bell and Jacobs 1986), rate of force development (Zhou et al. 1995a), muscle fiber conduction velocity (Viitasalo and Komi 1981), and muscle stiffness (Zhou et al. 1995a), it has been suggested as being a potentially useful indicator of neuromuscular function (Zhou et al. 1995a).
Neuromuscular fatigue has been defined as an exercise-induced reduction in the ability to exert force or power, regardless of whether the task can be sustained (Bigland-Ritchie and Woods 1984). The implications of fatigue are far reaching, given its potential prevalence among a variety of populations, and in many performance-related tasks or activities of daily living. Thus, its measurement may provide important physiological and performance-based information regarding neuromuscular function in a variety of circumstances. For example, the effects of fatigue have been used to examine the influences and mechanisms of muscle specific responses (Minshull et al. 2012a; Zhou 1996; Howatson 2010), gender differences (Minshull et al. 2007), physiological mechanisms (Fitts 2006; Bigland-Ritchie and Woods 1984; Westerblad et al. 1991), and aging (Allman and Rice 2001). Given neuromuscular fatigue involves mechanisms associated with impairments in neuromuscular function, such as muscle membrane action potential propagation (Badier et al. 1999) and EC-C impairments (Boncompagni et al. 2006; Jones 1996), the EMD would appear to be consequently associated with these fatigue-inducing mechanisms. For instance, Zhou et al. (1996) reported an 18.7-ms increase in EMD (for the vastus lateralis) immediately following isometric maximum voluntary contractions (MVCs) of the leg extensors and that the EMD had recovered to baseline within 10 min following the fatigue task. Similarly, Minshull et al. (2012a) showed the leg flexors had increased EMD values (14.2 ms higher post fatigue) following MVCs and recovery was observed within 8 min following the fatigue task. An increased EMD resulting from fatiguing tasks may yield performance and injury risk consequences because of the elongated muscle excitation and contraction time responses. Specifically, individuals may be predisposed to increased injury risk due to the inability of the muscles to properly stabilize the knee joint during critical periods of mechanical loading (Blackburn et al. 2009; Twist et al. 2008), which may increase the likelihood for ligamentous injury (Mercer et al. 1998), specifically of the anterior cruciate ligament (ACL) (Hagood et al. 1990; Bennell et al. 1998). Although several studies have examined the influence of fatigue-induced EMD on the leg extensor muscle group, a paucity of data exists concerning these effects on the leg flexors. Because the leg flexors have been shown to be important for athletic-related tasks (Mero et al. 1992; Delecluse 1997; Thompson et al. 2012), fall risks (Bento et al. 2010) and injury prevention pertaining to the knee joint and ACL (Hagood et al. 1990; Bennell et al. 1998), impaired leg flexor muscle contraction times may be particularly detrimental to performance and injuries in a variety of populations and settings.
Aging has been shown to adversely affect the neuromuscular system often leading to reduced functional performance capabilities and increased injury risks in older adults. It has also been shown that muscle fiber composition is altered with aging, where a greater proportion of Type I fibers is observed compared with Type II (Lexell 1995). The association between EMD and neuromuscular characteristics may further be supported in the observed age-related increases in EMD (Zhou et al. 1995b). Moreover, although the fatigue-induced effects on increased EMD have been demonstrated in young adults (Zhou 1996; Zhou et al. 1995a, 1996; Minshull et al. 2007, 2012a, b), we are aware of no studies that have examined these effects in older adults. Older adults have been reported to have impaired EC-C mechanisms (Boncompagni et al. 2006), which have been shown to be largely affected by fatigue (Fitts 2006; Green 1997; Miller et al. 1995), and because EMD is a function of EC-C, it is possible that aging may lead to differential effects of fatigue on EMD in old compared with young adults. Given that older adults are already often functionally impaired, or operating near the functional impairment threshold, even minor changes in performance capacities may yield large functional consequences. Further, previous authors have suggested that an increased EMD may reduce proprioceptive sensitivity, which may affect force generating capabilities and further exacerbate injury risk in an already vulnerable elderly population (Rack et al. 1983; Morse et al. 2005). These consequences have been acknowledged by Bento et al. (2010) who have stated, “…Declines in neuromuscular function of the lower limbs have been identified as a major risk factor for falls among elderly” (pg. 450).
Studies examining the fatigue-induced effects on EMD have primarily used intermittent isometric MVCs; however, it has been suggested that submaximal intermittent contractions may be more functionally relevant and typical of activities of daily living (ADLs) (Allman and Rice 2002). Thus, the purpose of the present investigation was to examine the effects of a fatigue-inducing bout of submaximal, intermittent isometric contractions on the EMD of the leg extensors and flexors in young and old men at immediately after and during a 30-min recovery period.
Methods
Participants
Twenty young (mean ± SD age = 25 ± 2.8 years; stature = 178.1 ± 6.5 cm; mass = 87.4 ± 22.3 kg) and sixteen old (age = 70.8 ± 3.8; stature = 177.5 ± 6.3; mass = 87.8 kg) men volunteered to participate in the study. This study was approved by the University Institutional Review Board and all participants completed and signed an informed consent document and health history questionnaire. None of the participants reported any current/ongoing neuromuscular diseases or musculoskeletal injuries of the knee or hip of their right leg within 1 year prior to testing.
Procedures
Participants visited the laboratory on three occasions with the first session being a familiarization trial where all participants practiced the maximal voluntary contractions (MVC’s) and the fatigue protocol of both the leg extensor and flexor muscle groups and the next two visits were the experimental trials. Within 2–4 days following the familiarization trial, participants reported back to the laboratory for the first experimental trial (day 2) and then again 7 (±1) days later for the second experimental trial (day 3). The two experimental trials involved testing of either the leg extensor or flexor muscle group only, on each respective test day. The order of testing of the muscle groups was randomized. For both experimental trials, participants were instructed to refrain from any caffeine consumption within 12 h of testing and any vigorous physical activity or exercise within 48 h of all testing.
Isometric strength assessments
All MVC testing procedures have been described previously (Thompson et al. 2013a); however, these procedures will be discussed briefly below. MVCs were performed with the right leg using a calibrated Biodex System 4 isokinetic dynamometer (Biodex Medical Systems, Inc. Shirley, NY, USA). For all strength assessments, participants were seated with restraining straps placed over the trunk, pelvis, and thigh. The input axis of the dynamometer was aligned with the axis of rotation of the knee. All MVCs were performed at leg angles of 60° and 30° below the horizontal plane for the leg extensors and flexors, respectively. Prior to the maximal strength testing, participants performed a 5-min warm-up on a cycle ergometer (Monark Exercise 828E, Vansbro, Sweden) at a self-selected low-intensity workload, followed by three submaximal isokinetic leg extension and leg flexion muscle actions at 60° s−1 at approximately 75 % of their perceived maximal effort. Depending on the randomized testing order, and prior to the experimental protocol, the participant performed two–three MVC’s with either the leg extensors or flexors with 1 min of recovery between each contraction. During all MVC measurements the participants were verbally instructed for the leg flexion and extension MVCs to “pull” or “push”, “as hard and fast as possible” for a total of 3–4 s (Thompson et al. 2012).
Experimental fatigue protocol and recovery
The highest torque value (peak torque; PT) recorded from the baseline isometric MVCs (Pre) was used to determine the target torque level for the subsequent experimental fatigue protocol. The target torque level was set at 60 % of PT, which has been used effectively in previous investigations (Vollestad 1997; Allman and Rice 2001) to elicit a neuromuscular fatigue response during intermittent submaximal contractions. Five minutes following the Pre MVCs, participants performed the fatigue protocol, which consisted of cyclical intermittent contractions using a 0.6-duty cycle, involving a 6-s contraction followed by a 4-s relaxation phase (Vollestad 1997; Bigland-Ritchie et al. 1986). During the fatigue protocol, participants were required to track their torque production by tracing a horizontal line set at the target torque level, on a computer monitor placed directly in front of them which displayed the real time torque signal. The fatigue test was terminated when participants were no longer able to reach their target torque level, despite giving a maximal effort, which was visually assessed by the researcher similar to procedures as described by (Bilodeau et al. 2001; Vollestad 1997). Upon termination of the fatigue protocol, 2–3 MVCs were then recorded immediately (Post), 7 (Recov7), 15 (Recov15) and 30 (Recov30) minutes following the fatigue task. Strong verbal encouragement was provided throughout the duration of the fatigue protocol, and during all MVCs.
Electromyography measurements
Surface EMG recordings were examined from the vastus lateralis (VL) and biceps femoris (BF) (Mitchell et al. 2011) muscles as to be representative of the leg extensor and flexor muscle groups, respectively. Prior to the electrode placements, the skin at the specified location of the vastus lateralis and biceps femoris was shaved, abraded, and cleaned with isopropyl alcohol, to reduce inter-electrode impedance and increase the signal-to-noise ratio. Pre-gelled bipolar surface electrodes (EL502, Biopac Systems Inc, Goleta, CA, USA) with an inter-electrode distance of 25 mm were then placed over the VL and BF muscles of the right thigh. For the VL, the electrode was placed at 66 % of the distance between the anterior superior iliac spine and the lateral superior border of the patella, and for the BF the electrode was placed at 50 % of the distance between the ischial tuberosity and the lateral tibial condyle. The electrode placements were in accordance with the recommendations of the SENIAM project (Hermens et al. 1999). A single, pre-gelled surface electrode was placed on the tibial tuberosity to serve as a reference electrode.
Signal processing
The torque (Nm) and EMG (μV) signals were sampled simultaneously at 2 kHz with a Biopac data acquisition system (MP150WSW, Biopac Systems, Inc.; Santa Barbara, CA, USA), stored on a personal computer (Dell Inspiron 8200, Dell Inc., Round Rock, TX, USA), and processed off-line with custom-written software (Labview 8.5, National Instruments, Austin, TX, USA). The scaled torque signal was filtered using a fourth-order, zero-phase shift, low-pass Butterworth filter with a 10-Hz cutoff frequency. The passive baseline torque value was considered the limb weight and subtracted from the signal so that the new baseline value was set at 0 Nm. All subsequent analyses were performed on the scaled, filtered, and gravity-corrected torque signal. Isometric MVC PT was determined as the highest 0.5 s epoch during the entire 3–4 s MVC plateau. The raw EMG signals (μV) were digitally bandpass filtered at 20–400 Hz using a zero-phase fourth-order butterworth filter and subsequently rectified.
The EMD was identified as the time (ms) difference between the EMG and torque onsets. The EMG onset was determined manually, using custom-written software which provided an interactive graph from which a high-resolution window was subsequently scaled (y axis = ~30 μV and x axis = ~100 ms) to establish an optimal view for cursor placement and consequently onset detection. A horizontal line was displayed on the graph that represented 3 SDs above baseline (Barry et al. 2005) and provided a visual guide to aid the researcher in determining the EMG onset (Fig. 1). Torque onset determination was automated and was initiated when the torque signal reached 7.5 and 4 Nm for the leg extensors and flexors, respectively (Thorlund et al. 2008; Thompson et al. 2013a).
Statistical analyses
A three-way mixed factorial ANOVA (muscle [leg extensors vs. leg flexors] × age [young vs. old men] × time phase [Pre vs. Post vs. Recov7 vs. Recov15 vs. Recov30]) was used to analyze the EMD data. When appropriate, follow-up analyses included one-way repeated measures ANOVAs and independent samples t tests with Bonferroni-corrections on either the simple main effects (when a significant interaction was present) or main effects collapsed across the opposing variable (when no significant interaction was present). PASW software version 20.0 (SPSS Inc, Chicago, IL, USA) was used for all statistical analyses. An alpha value of P ≤ 0.05 was considered statistically significant for all comparisons.
Results
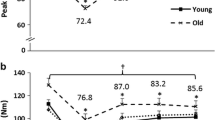
Means and SD values for EMD are presented in Table 1 for all time phases for the leg extensors and flexors for the young and old men. There was no significant three-way interaction (muscle × age × time phase, P = 0.755) but there were two-way interactions for muscle × time (P = 0.039) and muscle × age (P = 0.009). For muscle × time, the EMD was greater for the leg flexors at all post fatigue time phases (i.e., Post, Recov7, Recov15, Recov30; (P = 0.009–0.034) but was only greater at immediate Post for the leg extensors (P = 0.001) compared with Pre fatigue (Fig. 2). For muscle × age, the EMD was greater for the leg extensors for the old compared with the young men (P = 0.003), but no differences were observed for the leg flexors (P = 0.506) (Fig. 3).
Electromechanical delay (EMD) values of the knee extensors and flexors collapsed across age groups for all time phases. Dagger indicates an interaction (muscle × time phase) showing the leg extensors had a differential recovery pattern compared with the flexors (*significantly different than Pre). Values are mean ± SEM
Electromechanical delay (EMD) values of the leg extensors and flexors collapsed across all time phases for the young and old age groups. Dagger Indicates an interaction (muscle × age group) showing the old had greater EMD compared with the young for the leg extensors but not for the leg flexors. Values are mean ± SEM
Discussion
The primary findings of the present study revealed that a fatigue-inducing bout of intermittent isometric contractions elicited increased EMD at Post compared with Pre fatigue values for the leg extensors and flexors in the young and old men. However, differential recovery patterns for the EMD were observed between the leg extensors and flexors such that the leg extensors exhibited a more rapid recovery (no differences between Pre vs. Recov7, Recov15 and Recov30) compared with the leg flexors in which no recovery was present up to 30 min post fatigue (i.e., greater EMD at all recovery periods compared to Pre values) (Fig. 2). Additionally, the EMD of the leg extensors was greater for the old compared with the young men, but no age-related differences were observed for the leg flexors (Fig. 3).
The present study showed that EMD increased as a result of neuromuscular fatigue for the leg extensors and flexors in both the young and old men. These findings are similar to previous studies which have reported fatigue-induced increases in the EMD for the leg extensors (Zhou 1996; Zhou et al. 1995a, b; Häkkinen and Komi 1983), leg flexors (Minshull et al. 2007, 2012a; Mercer et al. 1998), adductor pollicis (Duchateau and Hainaut 1985), and biceps brachii (Howatson 2010) muscles. Additionally, there were no age-related differences regarding the effects of fatigue on EMD increases at Post fatigue; which suggests that aging does not appear to be associated with impairments in the physiological responses influencing a longer EMD induced by neuromuscular fatigue, in a population of recreationally active men. Because EMD is a function of both the structural components (i.e. SEC, tendon properties) (Minshull et al. 2012b; Zhou 1996; Cavanagh and Komi 1979) and the muscle membrane action potential propagation and EC-C processes (Zhou 1996; Fitts 2006; Green 1997), the observed increases in EMD resulting from neuromuscular fatigue may be a consequence of alterations or impairments in these physiological mechanisms. For example, Minshull et al. (2007) suggests cyclical loading of mechanical stress on the muscle–tendon unit, which would occur during the completion of a muscle fatiguing task, may result in elongation of the connective tissues and muscle–tendon unit over time due to the viscoelastic properties of the tissue, which may affect compliance characteristics. It is also plausible that the mechanical loading from repeated contractions produced alterations in tendon properties possibly leading to tendon creep (Maganaris 2002) and potentially influencing the EMD response. Given the SEC is a large contributor to the EMD, an increase in compliance characteristics and laxity of the tissues may increase the time it takes to transmit intrinsic muscular forces to the external load, which would theoretically lead to an increase in the EMD. Additionally, fatigue-induced elongation of the EMD may be influenced by muscle membrane action potential propagation and EC-C processes which have been suggested to be impaired as a result of processes associated with fatigue (Green 1997; Fitts 2006). Previous authors (Zhou 1996; Fitts 2006) have suggested that the slowing of the sarcolemma and t-tubule conduction processes may contribute to the prolongation of EMD with fatigue. Sjøgaard (1990) further suggests that repeated isometric contractions may elicit an excessive accumulation of potassium and lower levels of sodium ions in the extracellular fluids, which would likely result in impaired EC-C processes, and potentially result in an increased EMD.
A key finding of the present investigation was that the EMD of the leg flexors did not recover to baseline values during all phases of the recovery period up to 30 min following the fatigue task, while the leg extensors had recovered to baseline values by minute 7 of recovery (Recov7). In agreement with these findings, previous authors have also shown that an elongated EMD of the leg extensors following fatigue had recovered back to baseline within 3–10 min following the fatigue task (Häkkinen and Komi 1983; Zhou 1996). Although Minshull et al. (2012a) reported the EMD of the leg flexors was greater following a fatigue task compared with baseline, their findings revealed the EMD had returned to baseline at 8 min of recovery, showing a more rapid recovery compared with the present study. These discrepancies may be attributed to differences in fatigue protocols, as Minshull et al. (2012a) used a maximal intermittent protocol, in comparison with the submaximal intermittent protocol used in the present investigation. The observed differential recovery patterns between the leg extensors and flexors may be attributed to differences in physiological and activity pattern characteristics between muscle groups. For example, in addition to the leg flexors being smaller muscles than the leg extensors (Wickiewicz et al. 1983), it has been suggested that they may have higher proportions of type II muscle fibers (Garrett et al. 1984), longer muscle fibers (Wickiewicz et al. 1983), and differences in tendon properties (Noyes et al. 1984) and muscle architecture including smaller pennation angles (Chen et al. 2011) compared with the leg extensors. It has further been suggested that the leg flexors may not contribute largely to many normal activities of daily living (Chen et al. 2011; Jamurtas et al. 2005), particularly where low levels of locomotor exertion may be involved. Thus, the leg flexors may not be as accustomed to intense fatiguing contractions as other locomotor muscle groups (i.e. leg extensors), which may result in greater physiological disruptions from fatiguing contractions, and consequently delayed recovery processes. Collectively, these findings demonstrate that neuromuscular fatigue of the leg flexors may lead to increased EMD for an extended period of time following the completion of a fatigue task, which has been suggested as being a factor that may negatively affect the functional performance of the involved muscles (Cavanagh and Komi 1979; Minshull et al. 2012b). The increased EMD of the leg flexors may be particularly detrimental to performance because the leg flexors have been shown to be important contributors to performance-related tasks (Thompson et al. 2012, 2013b; Delecluse 1997). The present study showed a 22 % increase in EMD Post fatigue for the leg flexors and maintained a 9–11 % increased EMD during recovery, which may lead to an elevated incapacity to effectively stabilize the knee during critical periods of mechanical loading (Twist et al. 2008) for an extended time period following the onset of fatigue. An increased EMD could slow the initial contraction time of the leg flexor muscle group which may increase ACL injury risk, given the important contribution of these muscles in stabilizing the knee joint (Hagood et al. 1990). It has been proposed that with fatigue in older individuals, an increased tendon laxity and EMD may reduce the proprioceptive sensitivity, which can increase the time needed to generate force rapidly for postural readjustments (Morse et al. 2005; Rack et al. 1983) to recover from a loss of balance and prevent falls (Bento et al. 2010; Pijnappels et al. 2008). Although no differences were observed between the young and old men regarding the EMD recovery between muscle groups, it is possible the delayed recovery of the EMD for the leg flexors may have greater detrimental effects for the elderly, given they are nearer their functional daily living thresholds.
The present findings showed revealed the EMD of the leg extensors was greater for the old compared with the young men, but no age-related differences were observed for the leg flexors. These findings are similar to those of previous studies that have reported longer EMD in old compared with young individuals for the leg extensors (Zhou et al. 1995b; Clarkson and Kroll 1978) and plantar flexors (Morse et al. 2005). Zhou et al. (1995b) reported that the EMD of the vastus lateralis of the older men (56–78 years) was longer than that of young college-aged men (18–24 years). Although the absolute EMD values between their study and the present investigation vary in magnitude with Zhou et al. (1995b) reporting lower EMD values compared with the present study (33.9–40.4 and 97.66–110.8 ms, respectively), similar relative age-related increases in EMD were observed between the studies (17.5 and 13 % greater EMD for old vs. young for Zhou et al. and the present findings, respectively). The differences in the absolute EMD values between Zhou et al. (1995b), and the present study may be due to the differences in knee joint angles tested (90° vs. 120°), testing devices (load cell transducer vs. dynamometer), electrode placement, the method of calculating EMD, and/or the differences in age characteristics of the old men between studies. For example, in their study, EMD was calculated using automated force and EMG onset detection thresholds of 3.6 N and 15 μV, respectively, in which force was derived from a customized load cell testing apparatus, whereas the present study calculated the EMD using an automated torque threshold of 7.5 Nm and a manual detection method for the EMG set at ~3.0 SD above baseline (Barry et al. 2005). The mechanisms responsible for age-related increases in EMD may be linked to morphological and structural changes in the muscle–tendon unit across the life span. For example, it has been demonstrated that aging results in morphological changes in muscle composition, such that older adults exhibit a selective loss of type II muscle fibers, yielding relatively higher type I to type II fiber areas (Lexell 1995). Changes in muscle fiber type would theoretically support the observed age-related increased EMD, as previous authors have suggested that EMD may be related to muscle fiber type composition, (Zhou et al. 1995b; Viitasalo and Komi 1981; Nilsson et al. 1977), where type II fibers and motor units have been reported to be associated with shorter EMD times (Zhou et al. 1995b; Viitasalo and Komi 1981). Since previous studies have shown that SEC compliance is lower (Valour and Pousson 2003) and musculotendinous stiffness is greater (Valour and Pousson 2003; Ochala et al. 2005) in older compared with young adults, the longer EMD in old age is likely not explained by these structural changes, as a stiffer SEC and muscle–tendon unit would in theory result in a shorter EMD (Valour and Pousson 2003). Thus, a reasonable explanation for the observed increased EMD in older adults may be a result of impairments of the EC-C processes. Findings from Boncompagni et al. (2006) support this theory as they have reported the vastus lateralis of old men had lower calcium ion release units (CRUs) compared with younger men, resulting in age-related impaired EC-C mechanisms. The impaired ability of calcium ions to cross the transverse tubules and sarcolemma may inhibit muscular activation/contraction processes (Hill 1950; Kahya et al. 2010), by increasing the response time of EC-C mechanisms, which may lead to a longer EMD. Interestingly, the leg flexors did not show any age-related increases in EMD, which to our knowledge have not previously been examined in old compared with young populations. Thus, the effects of aging on increases in EMD appear to be muscle group-specific, which may be a function of the previously mentioned (see above) differences between the leg extensors and flexors; however, future investigations are needed to further examine the influence of age and EMD among these and other muscle groups. Ultimately, an age-related increase in EMD may yield functional consequences among older individuals due to the longer response times required to yield force output (Morse et al. 2005).
In conclusion, these findings demonstrated fatigue-induced increases in EMD following a fatigue task for both the leg extensors and flexors in young and old men with differential recovery patterns observed between the two muscle groups, where EMD recovered slower for the leg flexors than the leg extensors. There were no age-related differences for the effects of fatigue and recovery on EMD, suggesting mechanisms associated with fatigue-induced EMD increases may be similar in old compared with young healthy men. Last, EMD of the leg extensors was greater for the old compared with the young men, but no age-related differences were observed for the leg flexors. Taken together, the increased EMD immediately post fatigue for the leg extensors and flexors, delayed recovery times for the leg flexors, and longer EMD in older men for the extensors suggest that factors involving fatigue status, age, and muscle group may yield impaired physiological and potentially functional characteristics. These impairments may result in elongated neuromuscular response times, as measured by EMD. Such effects may lead to functional performance-based deficiencies and increased risk for injury among a variety of populations where fatigue may be present, or in older adults where EMD may be elongated. Coaches, practitioners, and researchers may choose to use these findings and perhaps exercise caution when working with clients that exhibit characteristics of neuromuscular fatigue, or old age, regarding activities (such as rapid or explosive type movements) where neuromuscular delays may potentially lead to decreased functional performance abilities and increased injury risks.
References
Allman BL, Rice CL (2001) Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve 24(9):1156–1167
Allman BL, Rice CL (2002) Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve 25(6):785–796
Badier M, Guillot C, Danger C, Tagliarini F, Jammes Y (1999) M-wave changes after high- and low-frequency electrically induced fatigue in different muscles. Muscle Nerve 22(4):488–496
Barry BK, Warman GE, Carson RG (2005) Age-related differences in rapid muscle activation after rate of force development training of the elbow flexors. Exp Brain Res 162(1):122–132
Bell DG, Jacobs I (1986) Electro-mechanical response times and rate of force development in males and females. Med Sci Sports Exerc 18(1):31–36
Bennell K, Wajswelner H, Lew P, Schall-Riaucour A, Leslie S, Plant D, Cirone J (1998) Isokinetic strength testing does not predict hamstring injury in Australian Rules footballers. Br J Sports Med 32(4):309–314
Bento PCB, Pereira G, Ugrinowitsch C, Rodacki ALF (2010) Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech 25(5):450–454
Bigland-Ritchie B, Woods JJ (1984) Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7(9):691–699
Bigland-Ritchie B, Cafarelli E, Vollestad NK (1986) Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556:137–148
Bilodeau M, Henderson TK, Nolta BE, Pursley PJ, Sandfort GL (2001) Effect of aging on fatigue characteristics of elbow flexor muscles during sustained submaximal contraction. J Appl Physiol (Bethesda, Md: 1985) 91(6):2654–2664
Blackburn JT, Bell DR, Norcross MF, Hudson JD, Engstrom LA (2009) Comparison of hamstring neuromechanical properties between healthy males and females and the influence of musculotendinous stiffness. J Electromyogr Kinesiol 19(5):e362
Boncompagni S, d’Amelio L, Fulle S, Fanò G, Protasi F (2006) Progressive disorganization of the excitation–contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: a possible role in the decline of muscle performance. J Gerontol Ser A Biol Sci Med Sci 61(10):995–1008
Cavanagh PR, Komi PV (1979) Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol 42(3):159–163
Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K (2011) Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol 111(2):211–223
Clarkson PM, Kroll W (1978) Practice effects on fractionated response time related to age and activity level. J Mot Behav 10(4):275–286
Delecluse C (1997) Influence of strength training on sprint running performance. Current findings and implications for training. Sports Med 24(3):147–156
Duchateau J, Hainaut K (1985) Electrical and mechanical failures during sustained and intermittent contractions in humans. J Appl Physiol 58(3):942–947
Fitts RH (2006) The muscular system: fatigue processes. ACSM’s advanced exercise physiology. Lippincott Williams & Wilkins, Philadelphia
Garrett WE, Califf JC, Bassett FH (1984) Histochemical correlates of hamstring injuries. Am J Sports Med 12(2):98–103
Green HJ (1997) Mechanisms of muscle fatigue in intense exercise. J Sports Sci 15(3):247–256
Hagood S, Solomonow M, Baratta R, Zhou BH, D’Ambrosia R (1990) The effect of joint velocity on the contribution of the antagonist musculature to knee stiffness and laxity. Am J Sports Med 18(2):182–187
Häkkinen K, Komi PV (1983) Electromyographic and mechanical characteristics of human skeletal muscle during fatigue under voluntary and reflex conditions. Electroencephalogr Clin Neurophysiol 55(4):436–444
Herda TJ, Walter AA, Costa PB, Cramer JT (2012) The effects of a doublet stimulus and pre-tension force level on the electromechanical delay. J Strength Cond Res [Epub ahead of print]
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G (1999) SENIAM European recommendations for surface electromyography: results of the SENIAM Project. Roessingh Research and Development, Enschede
Hill AV (1950) The series elastic component of muscle. Proc R Soc Lond B Biol Sci 137(887):273–280
Howatson G (2010) The impact of damaging exercise on electromechanical delay in biceps brachii. J Electromyogr Kinesiol 20(3):477–481
Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, Paschalis V, Koutedakis Y, Nosaka K (2005) Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol 95(2–3):179–185
Jones DA (1996) High-and low-frequency fatigue revisited. Acta Physiol Scand 156(3):265–270
Kahya MC, Yavuz SU, Turker KS (2010) Cutaneous silent period in human FDI motor units. Exp Brain Res 205(4):455–463
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol Ser A Biol Sci Med Sci 50(Spec No):11–16
Maganaris CN (2002) Tensile properties of in vivo human tendinous tissue. J Biomech 35(8):1019–1027
Mercer TH, Gleeson NP, Claridge S, Clement S (1998) Prolonged intermittent high intensity exercise impairs neuromuscular performance of the knee flexors. Eur J Appl Physiol 77(6):560–562
Mero A, Komi PV, Gregor RJ (1992) Biomechanics of sprint running. A review. Sports Med 13(6):376–392
Miller RG, Kent-Braun JA, Sharma KR, Weiner MW (1995) Mechanisms of human muscle fatigue. Quantitating the contribution of metabolic factors and activation impairment. Adv Exp Med Biol 384:195–210
Minshull C, Gleeson N, Walters-Edwards M, Eston R, Rees D (2007) Effects of acute fatigue on the volitional and magnetically-evoked electromechanical delay of the knee flexors in males and females. Eur J Appl Physiol 100(4):469–478
Minshull C, Eston R, Bailey A, Rees D, Gleeson N (2012a) Repeated exercise stress impairs volitional but not magnetically evoked electromechanical delay of the knee flexors. J Sports Sci 30(2):217–225
Minshull C, Eston R, Rees D, Gleeson N (2012b) Knee joint neuromuscular activation performance during muscle damage and superimposed fatigue. J Sports Sci 30(10):1015–1024
Mitchell C, Cohen R, Dotan R, Gabriel D, Klentrou P, Falk B (2011) Rate of muscle activation in power-and endurance-trained boys. Int J Sports Physiol Perform 6(1):94
Morse CI, Thom JM, Birch KM, Narici MV (2005) Tendon elongation influences the amplitude of interpolated doublets in the assessment of activation in elderly men. J Appl Physiol 98(1):221–226
Nilsson J, Tesch P, Thorstensson A (1977) Fatigue and EMG of repeated fast voluntary contractions in man. Acta Physiol Scand 101(2):194–198
Nordez A, Gallot T, Catheline S, Guevel A, Cornu C, Hug F (2009) Electromechanical delay revisited using very high frame rate ultrasound. J Appl Physiol 106(6):1970–1975
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Jt Surg Am 66(3):344–352
Ochala J, Lambertz D, Van Hoecke J, Pousson M (2005) Effect of strength training on musculotendinous stiffness in elderly individuals. Eur J Appl Physiol 94(1–2):126–133
Pijnappels M, van der Burg PJ, Reeves ND, van Dieën JH (2008) Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol 102(5):585–592
Rack PM, Ross HF, Thilmann AF, Walters DK (1983) Reflex responses at the human ankle: the importance of tendon compliance. J Physiol 344:503–524
Reeves ND, Maganaris CN, Narici MV (2003) Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548(Pt 3):971–981
Sasaki K, Sasaki T, Ishii N (2011) Acceleration and force reveal different mechanisms of electromechanical delay. Med Sci Sports Exerc 43(7):1200–1206
Sjøgaard G (1990) Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol Scand Suppl 593:1–63
Thompson BJ, Ryan ED, Sobolewski EJ, Smith DB, Conchola EC, Akehi K, Buckminster T (2012) Can maximal and rapid isometric torque characteristics predict playing level in division I American collegiate football players? J Strength Cond Res 27(3):655–661
Thompson BJ, Ryan ED, Sobolewski EJ, Conchola EC, Cramer JT (2013a) Age related differences in maximal and rapid torque characteristics of the leg extensors and flexors in young, middle-aged and old men. Exp Gerontol 48(2):277–282
Thompson BJ, Ryan ED, Sobolewski EJ, Smith DB, Akehi K, Conchola EC, Buckminster T (2013b) Relationships between rapid isometric torque characteristics and vertical jump performance in Division I collegiate American football players: Influence of body mass normalization. J Strength Cond Res [Epub ahead of print]
Thorlund JB, Michalsik LB, Madsen K, Aagaard P (2008) Acute fatigue-induced changes in muscle mechanical properties and neuromuscular activity in elite handball players following a handball match. Scand J Med Sci Sports 18(4):462–472
Twist C, Gleeson N, Eston R (2008) The effects of plyometric exercise on unilateral balance performance. J Sports Sci 26(10):1073–1080
Valour D, Pousson M (2003) Compliance changes of the series elastic component of elbow flexor muscles with age in humans. Pflugers Arch Eur J Physiol 445(6):721–727
Viitasalo JT, Komi PV (1981) Interrelationships between electromyographic, mechanical, muscle structure and reflex time measurements in man. Acta Physiol Scand 111(1):97–103
Vollestad NK (1997) Measurement of human muscle fatigue. J Neurosci Methods 74(2):219–227
Westerblad H, Lee JA, Lännergren J, Allen DG (1991) Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol 261(2 Pt 1):C195–C209
Wickiewicz TL, Roy RR, Powell PL, Edgerton VR (1983) Muscle architecture of the human lower limb. Clin Orthop Relat Res 179:275–283
Winter EM, Brookes FB (1991) Electromechanical response times and muscle elasticity in men and women. Eur J Appl Physiol 63(2):124–128
Zhou S (1996) Acute effect of repeated maximal isometric contraction on electromechanical delay of knee extensor muscle. J Electromyogr Kinesiol 6(2):117–127
Zhou S, Lawson DL, Morrison WE, Fairweather I (1995a) Electromechanical delay in isometric muscle contractions evoked by voluntary, reflex and electrical stimulation. Eur J Appl Physiol 70(2):138–145
Zhou S, Lawson DL, Morrison WE, Fairweather I (1995b) Electromechanical delay of knee extensors: the normal range and the effects of age and gender. J Hum Mov Stud 28(3):127–146
Zhou S, McKenna MJ, Lawson DL, Morrison WE, Fairweather I (1996) Effects of fatigue and sprint training on electromechanical delay of knee extensor muscles. Eur J Appl Physiol 72(5–6):410–416
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Toshio Moritani.
Rights and permissions
About this article
Cite this article
Conchola, E.C., Thompson, B.J. & Smith, D.B. Effects of neuromuscular fatigue on the electromechanical delay of the leg extensors and flexors in young and old men. Eur J Appl Physiol 113, 2391–2399 (2013). https://doi.org/10.1007/s00421-013-2675-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2675-y