Abstract
Purpose
Locomotor muscle fatigue impairs exercise performance during time to exhaustion tests. However, its effect on self-regulation of power output (pacing) is unknown. The primary aim of this study was to investigate the effects of locomotor muscle fatigue on pacing and time trial performance.
Methods
Ten healthy recreationally active men completed a 15-min time trial on a cycle ergometer 30 min after undergoing an eccentric fatiguing protocol designed to induce a substantial strength loss in the knee extensor muscles without inducing significant metabolic stress. This fatigue condition was compared with a control condition, using a randomly counterbalanced AB/BA crossover design.
Results
Total work completed during the 15-min cycling time trial was significantly reduced by 4.8 % in the fatigue condition compared with the control condition. This was caused by a significant reduction in power output. Rating of perceived exertion was significantly higher in the fatigue condition compared with the control condition only during the first 3 min of the time trial. Heart rate and vastus lateralis integrated electromyogram were not significantly different between the two conditions.
Conclusion
The results show that participants with fatigued locomotor muscles reduce their pace but do not change their pacing strategy. As a result, there was a significant reduction in time trial performance. As predicted by the psychobiological model of exercise performance, a slower pace may be a behavioral response to compensate for the significant increase in perception of effort induced by locomotor muscle fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacing refers to the self-regulation of speed/power output during endurance competitions and exercise performance tests in which the participant is free to vary the workload (time trials). Many physiological, psychological, and environmental factors are known to affect time trial performance and/or pacing (Tucker and Noakes 2009). These include hypoxia (Clark et al. 2007), environmental temperature (Peiffer and Abbiss 2011), hydration status (Dugas et al. 2009), caffeine ingestion (Wiles et al. 2006), motivation (Corbett et al. 2012), and music (Atkinson et al. 2004). The effects of locomotor muscle fatigue on pacing and time trial performance are, however, poorly understood. This is surprising if we consider that an exercise-induced reduction in muscle strength/power is considered by many as the ultimate factor limiting exercise performance (Allen et al. 2008; MacIntosh and Shahi 2011; Amann and Calbet 2008). Furthermore, there is evidence that significant locomotor muscle fatigue develops during time trials and endurance competitions (Froyd et al. 2013; Millet and Lepers 2004). Finally, although locomotor muscle fatigue is known to reduce time to exhaustion during high-intensity cycling at a fixed workload (Marcora et al. 2008), this exercise performance test does not provide any information about the effect of locomotor muscle fatigue on pacing. Therefore, it is important to experimentally investigate the effects of locomotor muscle fatigue during a time trial test.
In the only study on the effects of muscle fatigue on time trial performance published to date, Amann and Dempsey (2008) have investigated the effect of prior fatiguing exercise (high-intensity cycling to exhaustion) on pacing and performance during a subsequent 5-km time trial on a cycle ergometer. Time to complete 5 km was increased by 6.8 % (P < 0.05) after prior fatiguing exercise compared with a control condition (no prior fatiguing exercise). Because prior fatiguing exercise induced a significant reduction in locomotor muscle strength (−10.3 %), it could be argued that the observed impairment in time trial performance was caused by locomotor muscle fatigue. However, prior high-intensity cycling most likely caused significant accumulation of metabolites in the fatigued locomotor muscles as suggested by the high blood lactate concentration at exhaustion (13.5 ± 1.5 mmol l−1) (Amann and Dempsey 2008). Metabolites like lactic acid are known to stimulate Group III and IV muscle afferents (Rotto and Kaufman 1988), and the resultant afferent feedback has recently been shown to influence exercise performance in healthy participants (Amann et al. 2011) and patients with chronic obstructive pulmonary disease (Gagnon et al. 2012). The effect of afferent feedback from fatigued locomotor muscles on pacing is unclear because of poorly controlled placebo effect (Marcora 2010), but it may be significant (Amann et al. 2009). Furthermore, vigorous aerobic exercise can induce respiratory muscle fatigue with consequent negative effects on exercise performance (Romer and Polkey 2008). Because of all these uncontrolled confounding variables in the study by Amann and Dempsey (2008), it is impossible to draw any conclusions about the effects of reduced locomotor muscle strength on pacing and time trial performance.
The primary aim of the present experimental study was to isolate locomotor muscle fatigue to quantify its effects on pacing and time trial performance. To this aim, we used the 100 drop-jumps protocol, which is known to induce a significant reduction in locomotor muscle strength, without significant metabolic stress and consequent accumulation of muscle metabolites (Skurvydas et al. 2000, 2002; Nielsen et al. 2005; Marcora et al. 2008). Furthermore, this eccentric fatiguing protocol does not induce respiratory muscle fatigue because of its very low aerobic demands. In fact, unpublished data of 11 participants from our previous study on the effects of locomotor muscle fatigue on constant-workload cycling performance, using the same eccentric fatiguing protocol (Marcora et al. 2008) show that heart rate during the drop-jumps protocol was on average 112 ± 14 min−1 and maximal inspiratory pressure (MIP) measured shortly after the drop-jumps protocol was not significantly different from MIP before the drop-jump protocol (pre drop jumps: 110 ± 26 cmH2O; post drop jumps: 111 ± 27 cmH2O, P = 0.75). Moreover, there is evidence that eccentric exercise does not alter the afferent activity of muscle spindles and Golgi tendon organs (Gregory et al. 2002, 2004). Therefore, we can be confident that any effects of this eccentric fatiguing protocol on the subsequent time trial would be due to locomotor muscle fatigue rather than respiratory muscle fatigue or increased afferent feedback from the fatigued locomotor muscles. Moreover, by testing participants 30 min after this eccentric fatiguing protocol, we avoided the confounding effects of delayed onset muscle soreness (DOMS) on time trial performance (Marcora and Bosio 2007; Burt and Twist 2011; Twist and Eston 2009). In fact, DOMS does not occur until 3 h after eccentric exercise (Hamlin and Quigley 2001).
The secondary aim of the present study was to test the predictions of the psychobiological model of exercise performance based on Motivational Intensity Theory (Brehm and Self 1989; Wright 2008). According to this model, self-regulation of speed/power output during time trials is conscious and determined primarily by five psychological factors: (1) perception of effort; (2) potential motivation; (3) knowledge of the distance/duration to cover; (4) knowledge of the distance/duration covered/remaining, and (5) previous experience/memory of perceived exertion during exercise of varying intensity and duration (Marcora 2010). Because locomotor muscle fatigue is known to increase perception of effort (Marcora et al. 2008; de Morree et al. 2012), maintaining the same pace with fatigued locomotor muscles would result in premature exhaustion (Marcora et al. 2008). Because finishing the time trial is paramount, the psychobiological model of exercise performance predicts that fatigued participants decide to reduce their pace so that perception of effort does not reach its maximum before the end of the time trial despite the negative effect of locomotor muscle fatigue on perception of effort.
Methods
Participants
Ten male participants (age, 25 ± 6 years; height, 182 ± 6 cm; body mass, 82 ± 8 kg; peak oxygen uptake (VO2peak), 52 ± 5 ml kg−1 min−1) volunteered to take part in this study. The inclusion criteria were being free from injury and disease, and taking part in regular aerobic exercise at least twice a week. The experimental protocol was approved by the Ethics Committee of the School of Sport, Health and Exercise Sciences of Bangor University, and conformed to the standards set by the Declaration of Helsinki.
Procedures
All volunteers visited the laboratory on four occasions. A flowchart of the experiment is displayed in Fig. 1. The first visit was a preliminary visit. During this visit, participants signed an informed consent form, a medical and physical activity questionnaire was administered, and anthropometric measures were taken. An incremental exercise test (50 W increments every 2 min) was then performed until exhaustion (operationally defined as a cadence of <60 rpm for more than 5 s, despite strong verbal encouragement) on an electromagnetically braked cycle ergometer (Excalibur Sport; Lode, Groningen, The Netherlands) to measure VO2peak (highest 30 s average VO2 during the test) and peak power output (P peak). The latter was calculated following the equation by Kuipers and colleagues (P peak = P final + (t final/t stage) × P stage), where P final is the workload of the last completed stage of the incremental test in W, t final is the duration of the last (incomplete) stage in s, t stage is the duration of each stage in s, and P stage is the increment in workload of each stage in W (Kuipers et al. 1985). Throughout the test, VO2 was measured breath-by-breath using a computerized metabolic gas analysis system (MetaLyzer 3B; Cortex Biophysik, Leipzig, Germany). The cycle ergometer was set in hyperbolic mode, which allows the power output to be set independently of cadence over the range of 30–120 rpm. Before the incremental exercise test, the position on the cycle ergometer was adjusted for each participant, and settings were recorded and reproduced at each subsequent visit. Participants were given standard instructions on how to rate their perception of effort (i.e. the conscious sensation of how heavy, and strenuous a physical task is) on Borg’s 6–20 rating of perceived exertion (RPE) scale (Borg 1998). During the incremental exercise test, the low and high anchor points were established using standard procedures (Noble and Robertson 1996), and participants rated their effort at the end of every stage to familiarize with the scale.
The second visit was a time trial habituation, which took place minimally 48 h after the preliminary visit. Participants first warmed-up on the cycle ergometer at 30 % P peak for 3 min at a cadence between 30 and 100 rpm with the ergometer set in hyperbolic mode. Subsequently, the cycle ergometer was set in linear (cadence dependent) mode. The linear factor was calculated for each individual (power output = linear factor × cadence2) to reflect 75 % P peak at a cadence of 80 rpm. P peak during the preliminary test was 336 ± 33 W. As a result, the linear factor for the time trial was 0.039 ± 0.004. Participants were then asked to complete as much work as possible in 15 min. A stopwatch was placed in full sight of the participants and they were informed of how long they had left every 3 min and at the start of the last min. No feedback about performance was given to the participants during the time trial. Participants were asked to rate their effort on the RPE scale at the end of the first minute and at the end of every third minute thereafter.
After a minimum of 48 h, participants reported to the laboratory for the third time. They were randomly assigned to first undergo either the fatigue or the control condition with a one-to-one allocation ratio. DOMS was assessed using a seven-point Likert scale (Vickers 2001). After a 3-min warm-up on the cycle ergometer at 30 % P peak, baseline knee extensor strength was assessed by measuring force during isometric maximal voluntary contraction (MVC) of the right knee extensor muscles. Participants were seated in a rigid, straight-backed isometric chair with a 90° knee and hip angle. A harness with shoulder and waist straps was used to prevent changes in hip angle during the trials. After three submaximal warm-up and familiarization trials (25, 50, 75 % of maximal effort), participants were asked three times to push maximally for five s against pads placed just proximal to their ankle joints. Between all six trials, 2-min rest periods were observed. During the maximal trials, strong verbal encouragement was given.
The fatigue condition consisted of an eccentric fatiguing protocol, developed by Skurvydas et al. (2000), which is known to induce a ~20 % strength loss without accumulation of muscle metabolites or changes in muscle receptor sensitivity (Nielsen et al. 2005; Skurvydas et al. 2002; Marcora et al. 2008). Participants dropped 100 times from a 40-cm-high platform down to 90° knee angle before jumping upward as high as possible. Subsequent jumps were separated by 20 s rest. This eccentric fatiguing protocol took 33 min in total. The control condition, therefore, consisted of resting comfortably for 33 min. Two minutes after completing the assigned treatment, knee extensor strength was assessed again with three maximal trials (pre-cycling test). After this second strength test, a 30-min rest period was prescribed to allow for further recovery of the 100 drop jumps.
After the rest period, participants took place on the cycle ergometer. Just before the warm-up started, a 5 μl sample of whole fresh blood was taken from the left earlobe to measure lactate concentration using a portable analyzer (Lactate Pro LT-1710; Arkray Inc., Shiga, Japan). The protocol for the warm-up and subsequent time trial was the same as during the habituation trial. Participants were reminded to cycle as fast as they could throughout the entire time trial and, as an extra motivation, a monetary incentive was promised for the best performance. Heart rate was measured throughout the test with a telemetric monitor (Polar S610i; Polar Electro, Kempele, Finland), and values were recorded at the end of the first minute and at the end of every third minute together with RPE. EMG of the vastus lateralis was measured throughout the time trial (see section on “EMG recording”). At the end of the time trial, the amount of work (in kJ) completed during the 15-min time trial was recorded and used as a measure of performance. Immediately after the time trial, another blood lactate sample was taken and participants moved to the isometric chair to assess knee extensor strength within 2 min of time trial termination. In this post-cycling strength test, participants were asked to perform three maximal trials.
After a period of 10–17 days to washout the detrimental effects of muscle damage induced by the 100 drop-jumps protocol, participants reported for the fourth time to the laboratory. During this visit, the procedures of the third visit were repeated except for the experimental treatment, which was the opposite of the third visit (randomized counterbalanced AB/BA crossover design).
Environmental conditions in the laboratory were 17–21 °C for temperature and 41–71 % for humidity. A fan was placed in front of the participants during the cycling exercise and the setting was kept the same between visits. All participants were instructed to avoid intense exercise and alcohol consumption to maintain their usual diet and to drink 40 ml of water per kg body weight during the 24-h preceding each visit. Moreover, they were asked to have a good night’s sleep (at least 7 h) prior to the test and were instructed to avoid smoking and caffeine consumption at least 3 h before each test and have a light meal about 3 h before each test.
EMG recording
Throughout the time trial and during the MVCs, bipolar, single differential surface EMG signals were recorded from the vastus lateralis of the right leg. Electrodes were placed at the beginning of the third and fourth visit. Electrode position was estimated using the SENIAM recommendations: 2/3 of the line from the anterior spina iliaca superior to the lateral side of the patella (Hermens et al. 1999). Subsequently, the distal innervation zone was identified using a 16-channel silver bar array with a 5-mm interelectrode distance (OT Bioelettronica, Torino, Italy) to make sure that electrodes were not placed in proximity of the innervation zone. Before the electrodes were placed, the skin was shaved, cleaned with alcohol swabs, and abraded with abrasive paste. The skin was dried before the adhesive, pre-gelled, Ag/AgCl electrodes (Neuroline 720-00-S, Ambu Inc., Olstykke, Denmark; recording area: ø 11 mm) were placed with a 20-mm interelectrode distance in the direction of the muscle fibers. Electrode positions were marked with indelible ink to ensure equal electrode placement between sessions. A ground strap was placed around the wrist. The EMG signals were amplified by a multichannel EMG amplifier (EMG 16, OT Bioelettronica, Torino, Italy; bandwidth: 10–500 Hz, 4th order Bessel bandpass filter), fed into a 12-bit acquisition board (DAQCard-6024E, National Instruments Corporation, Austin, TX) at a sampling rate of 2,048 Hz, displayed on a PC, and recorded for offline analysis. During the MVCs, force was measured using a load cell (model 615; Tedea Huntleigh, Vishay, CA) connected to a biomechanical signal amplifier (MISO II, OT Bioelettronica, Torino, Italy; bandwidth: 0–60 Hz, 5th order Bessel lowpass filter) that was connected to the auxiliary channel of the EMG amplifier.
Data analysis
The EMG and force data were analyzed using Matlab 7.1 (The Mathworks Inc., Natick, MA). The force data were zero-lag lowpass filtered with a 2nd order Butterworth filter (cutoff frequency 5 Hz) and the EMG data were zero-lag bandpass filtered with a 4th order Butterworth filter (cutoff frequencies 20 and 400 Hz). For each maximal trial, a 0.5 s average was calculated at the peak plateau of the force signal and the highest of the three trials was recorded as the MVC. Integrated EMG (iEMG) was calculated over this same period.
For the time trial, to identify the bursts in the EMG signal, the signal was full-wave rectified and zero-lag lowpass filtered with a 2nd order Butterworth filter (cutoff frequency 5 Hz) to obtain the envelope of the signal. The envelope was then normalized to its peak value and the bursts were identified as the portions of the envelope exceeding 5 % of the peak value. The first and last bursts were excluded from the EMG analysis. All bursts were visually inspected to verify the burst detection procedure. The iEMG of the bandpass (20–400 Hz) filtered EMG data was calculated for each burst. Subsequently, the iEMG values were averaged over 1-min periods and were normalized to the iEMG during the baseline MVC. The number of bursts in each minute of cycling was recorded as the cadence. From the cadence and the linear factor, the power output was calculated for each minute (power output = linear factor × cadence2).
Statistical analysis
Unless otherwise noted, data are shown as means ± standard deviations. The effects of condition (fatigue vs. control) and test stage on blood lactate concentration (pre-cycling vs. post-cycling) and on knee extensor strength (baseline, pre-cycling and post-cycling), and the effects of condition (fatigue vs. control) and time (minutes 1, 3, 6, 9, 12, and 15) on RPE, heart rate, power output, cadence, and vastus lateralis iEMG were tested using fully repeated measures analysis of variance (ANOVA). If a significant condition × test stage or condition × time interaction was revealed, the main effect of condition was not considered and tests of simple main effects of condition were conducted using the Holm-Bonferroni method (Holm 1979). The Greenhouse-Geisser correction was applied when the assumption of sphericity was violated. In those cases, the uncorrected degrees of freedom, epsilon values, and corrected probability levels are reported. When comparing two means, paired t tests were used. Effect sizes are reported as partial eta squared (η 2p ). Significance was set at P < 0.05 (two-tailed) for all analyses, which were conducted using the Statistical Package for the Social Sciences version 14 (SPSS Inc., Chicago, IL).
Results
Eccentric fatiguing protocol validation
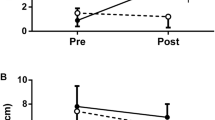
There were no significant baseline differences in DOMS between conditions (median/interquartile range: fatigue 0.00/0.00, control 0.00/0.00). Furthermore, follow-up tests of the significant condition × test stage interaction (F (2,18) = 17.13, P < 0.001, η 2p = 0.66) for knee extensor strength revealed no significant baseline difference in this parameter (fatigue 520 ± 80 N, control 509 ± 109 N; t (9) = 0.68, P = 0.515, η 2p = 0.05) (Fig. 2), but pre-cycling knee extensor strength was significantly lower in the fatigue condition (421 ± 58 N) compared with control (491 ± 98 N) (t (9) = −4.31, P = 0.002, η 2p = 0.67). Post-cycling knee extensor strength was not significantly different between the fatigue and control conditions (fatigue, 418 ± 62 N, control, 440 ± 87 N; t (9) = −1.46, P = 0.178, η 2p = 0.19). Follow-up tests of the significant condition × test stage interaction for blood lactate concentration (F (1,9) = 13.27, P = 0.005, η 2p = 0.60) revealed no pre-cycling difference in blood lactate concentration between the two conditions (fatigue, 1.2 ± 0.4 mmol l−1, control, 1.1 ± 0.2 mmol l−1; t (9) = 1.33, P = 0.217, η 2p = 0.16). However, at the end of the time trial, blood lactate concentration was significantly higher in the control condition than in the fatigue condition (fatigue, 10.4 ± 2.0 mmol l−1, control, 11.7 ± 2.2 mmol l−1; t (9) = −3.02, P = 0.014, η 2p = 0.50). None of the participants reported muscle pain after the 100 drop jumps.
Effects of experimental treatment and time trial cycling on knee extensor strength (n = 10). The fatigue condition consisted of 100 drop jumps over 33 min. The control condition consisted of 33 min of rest. #Significant main effect of test stage (P < 0.05). §Significant condition × test stage interaction (P < 0.05). †Significant simple main effect of condition. Data are presented as mean ± SEM
Time trial performance
All but one participant showed a reduction in time trial performance in the fatigue condition compared with control. On average, work produced was 219 ± 29 kJ in the fatigue condition and 230 ± 32 kJ in the control condition (t (9) = −3.01, P = 0.015, η 2p = 0.50). There was no significant order effect on performance between the first and the second time trial regardless of condition (t (9) = −0.60, P = 0.563, η 2p = 0.04).
Cadence and power output
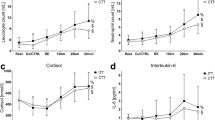
Self-selected cadence during the time trials increased significantly during the time trial (main effect of time, F (5,45) = 5.71, ε = 0.33, P = 0.018, η 2p = 0.39) and self-selected cadence was significantly lower during the fatigue condition compared with control (average fatigue, 78 ± 5 rpm, control 80 ± 5 rpm; main effect of condition, F (1,9) = 5.19, P = 0.049, η 2p = 0.37). There was no condition × time interaction (F (5,45) = 1.31, ε = 0.41, P = 0.30, η 2p = 0.13). As a result of the changes in cadence, power output increased significantly during the time trial (main effect of time, F (5,45) = 5.34, ε = 0.35, P = 0.02, η 2p = 0.37) and was significantly lower throughout the entire time trial in the fatigue condition compared with control (average fatigue, 240 ± 39 W, control, 253 ± 41 W; main effect of condition, F (1,9) = 6.03, P = 0.036, η 2p = 0.40) (Fig. 3a). There was no significant condition × time interaction for power output (F (5,45) = 1.83, ε = 0.48, P = 0.18, η 2p = 0.17).
Effects of muscle fatigue on a power output, b rating of perceived exertion, c heart rate, and d vastus lateralis integrated electromyogram (iEMG) during time trial cycling (n = 10). The fatigue condition consisted of 100 drop jumps over 33 min. The control condition consisted of 33 min rest. #Significant main effect of time (P < 0.05). *Significant main effect of condition (P < 0.05). §Significant condition × time interaction (P < 0.05). †Significant simple main effect of condition. Data are presented as mean ± SEM
Rating of perceived exertion, heart rate, and vastus lateralis EMG
As was expected, RPE increased significantly during the time trial (main effect of time, F (5,45) = 365.94, ε = 0.49, P < 0.001, η 2p = 0.98). A significant condition × time interaction was found for RPE (F (5,45) = 4.25, ε = 0.55, P = 0.017, η 2p = 0.32). Tests of simple main effects revealed that RPE was significantly higher in the fatigue condition than in the control condition at minutes 1 and 3 (minute 1, t (9) = 4.73, P = 0.001, η 2p = 0.71, minute 3, t (9) = 4.30, P = 0.002, η 2p = 0.67) (Fig. 3b). Heart rate also increased significantly during the time trial (main effect of time, F (5,45) = 49.19, ε = 0.36, P < 0.001, η 2p = 0.85) (Fig. 3c), but there was no significant main effect of condition (F (1,9) = 0.22, P = 0.653, η 2p = 0.023), nor was there a significant condition × time interaction (F (5,45) = 1.70, ε = 0.49, P = 0.203, η 2p = 0.16). Vastus lateralis iEMG increased significantly during the time trial (main effect of time, F (5,45) = 23.05, ε = 0.34, P < 0.001, η 2p = 0.72), and although there was a significant condition × time interaction (F (5,45) = 6.27, P < 0.001, η 2p = 0.41), tests of simple main effects revealed no significant differences between fatigue and control at any time point for vastus lateralis iEMG (Fig. 3d).
Discussion
We have previously shown that locomotor muscle fatigue, defined as an exercise-induced reduction in locomotor muscle strength, has a negative impact on exercise performance (Marcora et al. 2008). However, in our previous study, a time to exhaustion test was used to measure exercise performance. Albeit sensitive to changes in exercise performance (Amann et al. 2008), a time to exhaustion test does not provide any information about pacing because power output is fixed. The primary aim of the current study was to investigate the effect of locomotor muscle fatigue on pacing using a test in which the participant is free to vary cadence and thereby power output. Based on the psychobiological model of exercise performance (Brehm and Self 1989; Wright 2008), we hypothesized a reduction in cadence and power output with similar RPE, causing a reduction in time trial performance. The results of the present study broadly confirm these hypotheses. Furthermore, we have shown that locomotor muscle fatigue does not affect pacing strategy.
We did not find any baseline differences in DOMS or knee extensor strength. These markers of exercise-induced muscle damage confirm that the 10–17 days of washout period between the third and the fourth visit was sufficient. In combination with the knowledge that the DOMS induced by the eccentric fatiguing protocol did not occur until several hours after completion of the test, this shows that our results were not confounded by the effect of DOMS on time trial performance (Marcora and Bosio 2007; Twist and Eston 2009; Burt and Twist 2011). In the present study, the eccentric fatiguing protocol induced a significant 19 % reduction in knee extensor strength. This degree of locomotor muscle fatigue is similar to that reported in previous studies using the same eccentric fatiguing protocol (Nielsen et al. 2005; Skurvydas et al. 2002; de Morree and Marcora 2010; Marcora et al. 2008). Furthermore, the eccentric fatiguing protocol induced a degree of locomotor muscle fatigue similar to that observed after the cycling time trial in the control condition (Fig. 2), which is consistent with other studies showing a strength loss during time trials (Froyd et al. 2013; Millet and Lepers 2004).
In contrast with the study by Amann and Dempsey (2008), we found no difference in blood lactate concentration between the conditions prior to the time trial. This suggests that our eccentric fatiguing protocol did not induce significant metabolic stress and consequent accumulation of metabolites in the fatigued locomotor muscles, as previously suggested by muscle biopsy studies (Nielsen et al. 2005). Actually, the blood lactate concentration at the end of the time trial was significantly lower in the fatigue condition than in the control condition. This is an expected consequence of the lower total work performed during the time trial in the fatigue condition. Contrary to the high-intensity cycling to exhaustion protocol used by Amann and Dempsey (2008) to pre-fatigue their participants, the eccentric fatiguing protocol used in our study does not induce respiratory muscle fatigue because it has very low aerobic demands (see “Introduction”), and eccentric exercise does not affect afferent activity from muscle spindles and Golgi tendon organs (Gregory et al. 2002, 2004). Altogether, we are confident that any effects of our eccentric fatiguing protocol during the subsequent time trial were due to locomotor muscle fatigue rather than DOMS, increased afferent feedback from locomotor muscles, or respiratory muscle fatigue.
In the present study, locomotor muscle fatigue induced by the eccentric fatiguing protocol caused a 4.8 % reduction in the amount of work completed during the 15-min time trial. This reduction in time trial performance is reflected in the average power output, which was 4.7 % lower in the fatigue condition compared with control. This is consistent with reductions found during a 5-km cycling time trial (Amann and Dempsey 2008), a 15-min cycling time trial (Burt and Twist 2011), a 5-min cycling time trial (Twist and Eston 2009), and a 30-min running time trial (Marcora and Bosio 2007) performed after various fatiguing/muscle damaging exercise protocols. However, in these studies, the effects of the exercise-induced reduction in locomotor muscle strength on time trial performance were confounded by increased afferent feedback from fatigued locomotor muscles, respiratory muscle fatigue, and/or DOMS. We are the first to demonstrate that isolated locomotor muscle fatigue causes a significant reduction in time trial performance. This is in agreement with our previous study where isolated locomotor muscle fatigue induced by the eccentric fatiguing protocol reduced performance during a subsequent time to exhaustion test of similar duration (10–15 min) to the time trial used in the present study (Marcora et al. 2008).
In the fatigue condition, RPE during the time trial was similar or even higher than that in the control condition despite a significantly lower power output. These results are consistent with our previous findings that locomotor muscle fatigue increases RPE during high-intensity cycling at a fixed power output (Marcora et al. 2008). Because of this negative effect of locomotor muscle fatigue on perception of effort, maintaining the same pace with fatigued locomotor muscles would have resulted in higher RPE and premature exhaustion (Marcora et al. 2008). Because finishing the time trial is paramount, the psychobiological model of exercise performance (Brehm and Self 1989; Wright 2008) predicts that fatigued participants decide to reduce their pace so that RPE does not reach its maximum before the end of the time trial, despite the negative effect of locomotor muscle fatigue on perception of effort. The results of the present study are consistent with this prediction. In fact, RPE reached its maximum at the end of the time trial, and it was similar between conditions. Furthermore, there was a significant reduction in cadence and power output during the time trial. Our finding that RPE was slightly higher in the fatigue condition for the first 3 min of the time trial suggests that participants did not realize immediately that the power output at which they chose to start was too high and that keeping that pace could cause premature exhaustion. Participants in the study by Amann and Dempsey (2008) were made to start off at the same power output after the pre-fatiguing trial as during the control trial, but they also soon reduced the power output to a more sustainable one.
In addition to perception of effort (Factor 1), the psychobiological model postulates that another four factors affect pacing and time trial performance: (2) potential motivation; (3) knowledge of the distance/duration to cover; (4) knowledge of the distance/duration covered/remaining; and (5) previous experience/memory of perceived exertion during exercise of varying intensity and duration (Marcora 2010). In both the fatigue and control condition, participants were instructed to complete as much work as possible in 15 min, and they were informed of the duration covered/remaining via a stopwatch. It is not biologically plausible that muscle fatigue would affect memory or the ability to understand the concept of time. Therefore, we assume that locomotor muscle fatigue did not affect factors 3–5. Potential motivation (the highest effort a participant is willing to exert in order to succeed in a task) is also unlikely to be affected by muscle fatigue, as it is mainly associated with the available incentive (Wright 2008). In the present study, the available incentive (monetary reward for the best performance) was the same in both conditions. Furthermore, the highest effort exerted by the participants was similar between conditions as shown by similar RPE values at the end of both time trials. This finding suggests that potential motivation was similar in the fatigue and control condition. Therefore, the most likely factor determining the negative effect of locomotor muscle fatigue on time trial performance was the higher-than-normal effort perceived by fatigued participants.
Importantly, the results of our study provide evidence against the validity of exercise performance models based on afferent feedback related to whole-body homeostasis [central governor model (St Clair Gibson et al. 2006)] or metabolite accumulation in fatigued locomotor muscles [afferent feedback model (Amann and Dempsey 2008)]. These theoretical models predict that an experimental treatment that does not significantly alter afferent feedback should not have any significant effect on pacing and time trial performance. Our findings clearly show that these predictions are not valid and that the reduction in locomotor muscle strength induced by exercise can, on its own, have a significant effect on performance.
Interestingly, locomotor muscle fatigue did not affect pacing strategy during the time trial. In fact, in both conditions participants adopted a negative pacing strategy with power output increasing over time, particularly during the last 3 min of the time trial. A possible explanation for this pacing strategy is that it is very difficult to consciously anticipate perception of effort and power output at the end of the time trial (Marcora 2010; de Koning et al. 2011). Therefore, because finishing the race is paramount, participants usually choose a slightly conservative pace for most of the time trial. Near the end of the time trial, when the information provided by the conscious sensation of effort is more reliable and the risk of not finishing the race is negligible, most participants significantly increase power output in the so-called end spurt (Marcora 2010) which occurred in both conditions. An end spurt was also seen in the study by Amann and Dempsey (2008).
We have found no effect of locomotor muscle fatigue on heart rate and vastus lateralis EMG during the time trial. These physiological parameters have been used as markers of central motor command (Norton et al. 1999; Smith et al. 2003; Amann and Dempsey 2008; St Clair Gibson and Noakes 2004; Amann et al. 2011). Therefore, these results suggest that central motor command was similar between conditions. This is not surprising if we consider that participants down-regulated their power output to maintain a similar perception of effort throughout most of the time trial. In fact, we have recently shown a significant positive correlation between RPE and the magnitude of central motor command, which is in agreement with the hypothesis that perception of effort arises from corollary discharges of the central motor command sent to the active muscles (de Morree et al. 2012).
Conclusion
The results of this study show for the first time that participants with fatigued locomotor muscles reduce their pace but do not change pacing strategy during a time trial. The result is a significant reduction in time trial performance. As predicted by the psychobiological model of exercise performance, a slower pace may be a behavioral response to compensate for the significant increase in perception of effort induced by locomotor muscle fatigue. These results suggest that time trial performance can be improved in two very different ways. The first, most obvious, way is to reduce the degree of locomotor muscle fatigue experienced during endurance competitions. In fact, this is one of the main aims of any endurance training program. The second way is to compensate the negative effect of locomotor muscle fatigue on perception of effort using nutritional or cognitive strategies known to reduce perception of effort.
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88(1):287–332. doi:10.1152/physrev.00015.2007
Amann M, Calbet JA (2008) Convective oxygen transport and fatigue. J Appl Physiol 104(3):861–870. doi:10.1152/japplphysiol.01008.2007
Amann M, Dempsey JA (2008) Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586(1):161–173. doi:10.1113/jphysiol.2007.141838
Amann M, Hopkins WG, Marcora SM (2008) Similar sensitivity of time to exhaustion and time-trial time to changes in endurance. Med Sci Sports Exerc 40(3):574–578. doi:10.1249/MSS.0b013e31815e728f
Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2009) Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587(Pt 1):271–283. doi:10.1113/jphysiol.2008.163303
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2011) Implications of group III and IV muscle afferents for high intensity endurance exercise performance in humans. J Physiol 589(21):5299–5309. doi:10.1113/jphysiol.2011.213769
Atkinson G, Wilson D, Eubank M (2004) Effects of music on work-rate distribution during a cycling time trial. Int J Sports Med 25(8):611–615. doi:10.1055/s-2004-815715
Borg GA (1998) Borg’s perceived exertion and pain scales. Human Kinetics, Champaign
Brehm JW, Self EA (1989) The intensity of motivation. Annu Rev Psychol 40:109–131. doi:10.1146/annurev.ps.40.020189.000545
Burt DG, Twist C (2011) The effects of exercise-induced muscle damage on cycling time-trial performance. J Strength Cond Res 25(8):2185–2192. doi:10.1519/JSC.0b013e3181e86148
Clark SA, Bourdon PC, Schmidt W, Singh B, Cable G, Onus KJ, Woolford SM, Stanef T, Gore CJ, Aughey RJ (2007) The effect of acute simulated moderate altitude on power, performance and pacing strategies in well-trained cyclists. Eur J Appl Physiol 102(1):45–55. doi:10.1007/s00421-007-0554-0
Corbett J, Barwood MJ, Ouzounoglou A, Thelwell R, Dicks M (2012) Influence of competition on performance and pacing during cycling exercise. Med Sci Sports Exerc 44(3):509–515. doi:10.1249/MSS.0b013e31823378b1
de Koning JJ, Foster C, Bakkum A, Kloppenburg S, Thiel C, Joseph T, Cohen J, Porcari JP (2011) Regulation of pacing strategy during athletic competition. PLoS One 6(1):e15863. doi:10.1371/journal.pone.0015863
de Morree HM, Marcora SM (2010) The face of effort: frowning muscle activity reflects effort during a physical task. Biol Psychol 85(3):377–382. doi:10.1016/j.biopsycho.2010.08.009
de Morree HM, Klein C, Marcora SM (2012) Perception of effort reflects central motor command during movement execution. Psychophysiology 49(9):1242–1253. doi:10.1111/j.1469-8986.2012.01399.x
Dugas JP, Oosthuizen U, Tucker R, Noakes TD (2009) Rates of fluid ingestion alter pacing but not thermoregulatory responses during prolonged exercise in hot and humid conditions with appropriate convective cooling. Eur J Appl Physiol 105(1):69–80. doi:10.1007/s00421-008-0876-6
Froyd C, Millet GY, Noakes TD (2013) The development of peripheral fatigue and short-term recovery during self-paced high-intensity exercise. J Physiol 591(Pt 5):1339–1346. doi:10.1113/jphysiol.2012.245316
Gagnon P, Bussieres JS, Ribeiro F, Gagnon SL, Saey D, Gagne N, Provencher S, Maltais F (2012) Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186(7):606–615. doi:10.1164/rccm.201203-0404OC
Gregory JE, Brockett CL, Morgan DL, Whitehead NP, Proske U (2002) Effect of eccentric muscle contractions on Golgi tendon organ responses to passive and active tension in the cat. J Physiol 538(Pt 1):209–218. doi:10.1013/jphysiol.2001.012785
Gregory JE, Morgan DL, Proske U (2004) Responses of muscle spindles following a series of eccentric contractions. Exp Brain Res 157(2):234–240. doi:10.1007/s00221-004-1838-9
Hamlin MJ, Quigley BM (2001) Quadriceps concentric and eccentric exercise 1: changes in contractile and electrical activity following eccentric and concentric exercise. J Sci Med Sport 4(1):88–103
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G (1999) European recommendations for surface electromyography, 2nd edn. Roessingh Research and Development B.V., Enschede
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G (1985) Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med 6(4):197–201. doi:10.1055/s-2008-1025839
MacIntosh BR, Shahi MR (2011) A peripheral governor regulates muscle contraction. Appl Physiol Nutr Metab 36(1):1–11. doi:10.1139/H10-073
Marcora S (2010) Counterpoint: Afferent feedback from fatigued locomotor muscles is not an important determinant of endurance exercise performance. J Appl Physiol 108(2):454–456. doi:10.1152/japplphysiol.00976.2009a (discussion 456–457)
Marcora SM, Bosio A (2007) Effect of exercise-induced muscle damage on endurance running performance in humans. Scand J Med Sci Sports 17(6):662–671. doi:10.1111/j.1600-0838.2006.00627.x
Marcora SM, Bosio A, de Morree HM (2008) Locomotor muscle fatigue increases cardiorespiratory responses and reduces performance during intense cycling exercise independently from metabolic stress. Am J Physiol Regul Integr Comp Physiol 294(3):R874–R883. doi:10.1152/ajpregu.00678.2007
Millet GY, Lepers R (2004) Alterations of neuromuscular function after prolonged running, cycling and skiing exercises. Sports Med 34(2):105–116
Nielsen JS, Madsen K, Jorgensen LV, Sahlin K (2005) Effects of lengthening contraction on calcium kinetics and skeletal muscle contractility in humans. Acta Physiol Scand 184(3):203–214. doi:10.1111/j.1365-201X.2005.01449.x
Noble B, Robertson R (1996) Perceived exertion. Human Kinetics, Champaign
Norton KH, Gallagher KM, Smith SA, Querry RG, Welch-O’Connor RM, Raven PB (1999) Carotid baroreflex function during prolonged exercise. J Appl Physiol 87(1):339–347
Peiffer JJ, Abbiss CR (2011) Influence of environmental temperature on 40 km cycling time-trial performance. Int J Sports Physiol Perform 6(2):208–220
Romer LM, Polkey MI (2008) Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol 104(3):879–888. doi:10.1152/japplphysiol.01157.2007
Rotto DM, Kaufman MP (1988) Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64(6):2306–2313
Skurvydas A, Jascaninas J, Zachovajevas P (2000) Changes in height of jump, maximal voluntary contraction force and low-frequency fatigue after 100 intermittent or continuous jumps with maximal intensity. Acta Physiol Scand 169(1):55–62. doi:10.1046/j.1365-201x.2000.00692.x
Skurvydas A, Dudoniene V, Kalvenas A, Zuoza A (2002) Skeletal muscle fatigue in long-distance runners, sprinters and untrained men after repeated drop jumps performed at maximal intensity. Scand J Med Sci Sports 12(1):34–39
Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH (2003) Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551(Pt 3):1013–1021
St Clair Gibson A, Noakes TD (2004) Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sports Med 38(6):797–806. doi:10.1136/bjsm.2003.009852
St Clair Gibson A, Lambert EV, Rauch LH, Tucker R, Baden DA, Foster C, Noakes TD (2006) The role of information processing between the brain and peripheral physiological systems in pacing and perception of effort. Sports Med 36(8):705–722
Tucker R, Noakes TD (2009) The physiological regulation of pacing strategy during exercise: a critical review. Br J Sports Med 43(6):e1. doi:10.1136/bjsm.2009.057562
Twist C, Eston RG (2009) The effect of exercise-induced muscle damage on perceived exertion and cycling endurance performance. Eur J Appl Physiol 105(4):559–567. doi:10.1007/s00421-008-0935-z
Vickers AJ (2001) Time course of muscle soreness following different types of exercise. BMC Musculoskelet Disord 2:5
Wiles JD, Coleman D, Tegerdine M, Swaine IL (2006) The effects of caffeine ingestion on performance time, speed and power during a laboratory-based 1 km cycling time-trial. J Sports Sci 24(11):1165–1171. doi:10.1080/02640410500457687
Wright RA (2008) Refining the prediction of effort: Brehm’s distinction between potential motivation and motivation intensity. Soc Pers Psychol Compass 2(2):682–701. doi:10.1111/j.1751-9004.2008.00093.x
Acknowledgments
The authors thank Athanasios Milousis for his help with participant recruitment and data collection and Enrico Merlo for his support during the data analysis. No funding was received for this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Toshio Moritani.
Rights and permissions
About this article
Cite this article
de Morree, H.M., Marcora, S.M. Effects of isolated locomotor muscle fatigue on pacing and time trial performance. Eur J Appl Physiol 113, 2371–2380 (2013). https://doi.org/10.1007/s00421-013-2673-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2673-0