Abstract
Purpose
Decreased whole-body energy cost of running has been associated with an increased Achilles tendon stiffness. It is usually assumed that this lower energy cost can be attributed to less muscle fascicle shortening with a stiffer tendon. Increased fiber shortening is an important determinant of muscle energetics in vitro. However, other factors, like increased muscle activation may be important when considering whole muscle energetics in vivo.
Methods
To determine the effects of a small additional muscle shortening on skeletal muscle energy requirement, 19 subjects performed 30 plantarflexions on two separate occasions: isometric (ISO) and isokinetic (KIN, 6.98 rad s–1), each with a target of 50 % of maximum isometric torque. Medial gastrocnemius muscle fascicle length (FL) was measured by ultrasound and rate of oxyhemoglobin (HbO2) desaturation was measured during blood flow occlusion using near-infrared spectroscopy.
Results
KIN resulted in significantly greater muscle shortening (23.8 ± 1.3 mm) than ISO (18.3 ± 1.0 mm, p < 0.001, mean ± SEM), and greater shortening velocity (KIN = 2.5 ± 0.3 FL s–1, ISO = 1.1 ± 0.1 FL s–1, p < 0.001). Rate of HbO2 desaturation was 19 ± 7 %, greater in KIN than ISO (p < 0.01), despite 19 ± 2 % lower mean torque (p < 0.001) and 9.8 ± 1.6 Nm s lower mean impulse per contraction (p < 0.001) in KIN compared to ISO. Root mean square for EMG was significantly greater (p < 0.05) during KIN (73 ± 3 %) than during ISO (63 ± 2 %).
Conclusion
These results illustrate that muscle energy requirement is greater when muscle fascicle shortening and/or velocity of shortening is increased, and suggest that greater activation contributes to that increased energy requirement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The energy cost (EC) of exercise is primarily determined by the EC of muscle contraction yet little is known regarding the factors affecting the EC of generating muscular force and/or work in humans. The mechanical properties of the major force-generating muscles of the lower limbs have been well investigated, but interpretation of these muscle mechanics in terms of energetics relies on extrapolation from in vitro studies, often using amphibian muscle. It has been shown that a stiff Achilles tendon (AT) is associated with lower EC of running (Arampatzis et al. 2006; Fletcher et al. 2010). Furthermore, changes in AT stiffness have been shown to relate to changes in economy of running (Fletcher et al. 2010), confirming that this is likely a cause and effect relationship.
Ultimately, optimal AT stiffness is that which allows the muscles to operate relatively isometrically during contraction, while the length change of the entire muscle-tendon unit can be accommodated by the tendon alone. In keeping the muscle fascicles isometric, the force–length–velocity relationship of muscle is maximized (Askew and Marsh 1998). Considering that during running the triceps surae muscles do not undergo substantial stretch prior to shortening (Ishikawa et al. 2007a; Lichtwark et al. 2007), an optimally tuned AT would result in less fiber shortening to achieve active joint rotation. Furthermore, the elastic energy storage and release of the AT may also contribute to reducing the energy cost of running; however, this effect is small. Given the data reported by Fletcher et al. (2010), and assuming an AT elongation during running of 10 mm (Farris et al. 2011) and a hysteresis of 7 %, the elastic energy contribution of the AT is estimated to be between 5.4 and 5.7 % of the energy cost of each stride, assuming an energy cost of running between 4.40 and 4.64 kJ kg–1 km–1 and a stride length of 150 % of standing height.
It has typically been assumed that the lower EC of running associated with a stiff Achilles tendon is due to reduced shortening of the fibers of the triceps surae muscles (Alexander 1991; Arampatzis et al. 2006; Roberts et al. 1998); however, further explanation of this assertion is not given. Does this simply relate to the idea that shortening increases the energy cost of contraction as has been shown in maximally activated muscle (Fenn 1923; Hill 1938) or is something more involved?
It has been acknowledged for many years that the heat liberated (i.e., the energy) above that required for a purely isometric contraction, in vitro is proportional to the work done (Fenn 1923), which is to say any increase in muscle shortening with a given load would result in a higher EC of contraction. However, work accomplished during a contraction has a complicated relationship with total EC in whole muscle in situ (Stainsby 1982). Since much of what we know regarding the EC of muscle contraction has been performed in vitro at non-physiological temperatures, we wanted to investigate the relationship between muscle shortening and muscle group EC in humans at physiological temperatures.
It is important to consider that during running, we are dealing with a voluntary contraction, where the force is a consequence of the controlled motion of the leg. Comparing the same movement of the leg with a more compliant tendon (where additional shortening is permitted) should reveal additional potential factors that could affect the energetics of muscle contraction. A more compliant tendon will require not only greater muscle fiber shortening but also greater velocity of fiber shortening for a given load if the leg movement is not different. Greater velocity of shortening would most likely also require increased activation or recruitment of motor units to achieve the same force. This increased recruitment can be illustrated by consideration of the force–velocity relationship (see Fig. 1).
The effect of greater shortening velocity on muscle activation to achieve a target force. The force–velocity relationship, scaled to activation (Chow and Darling 1999). The short dashed and solid lines represent 50 and 85 % of maximal motor unit activation, respectively. The long dashed line represents maximal activation. When force can be generated isometrically, target force can be achieved with minimal motor unit activation, as shown by open square. When shortening is permitted, additional motor unit activation is required (filled square)
The force–length relationship could also play an important role here. If the more compliant tendon resulted in shortening of fibers on the descending limb of the force–length relationship, additional activation would be needed to reach the force needed for the required limb movement. By minimizing the magnitude of fiber shortening, a stiff Achilles tendon allows the muscles to operate near isometrically, and to remain near optimal length. In running, where the EC is determined mainly by the cost of producing force to support body weight (Kram and Taylor 1990; Taylor et al. 1980), operating at non-optimal muscle lengths requires a greater level of muscle activation to generate the required force, and thus would elevate the EC of running (Roberts et al. 1998). A reduction in muscle activation, if muscle can operate close to its optimal length, should contribute to minimize the EC of contraction (Bergstrom and Hultman 1988; Heglund et al. 1982; Hogan et al. 1998).
Near-infrared spectroscopy (NIRS) offers an affordable, portable solution to measuring muscle oxygen uptake. A number of thorough reviews have been dedicated to the use of NIRS during exercise (Ferrari et al. 2004; Hamaoka et al. 2007; McCully and Hamaoka 2000; Neary 2004). When blood flow is occluded to the exercising muscle, the relative rate of change in oxyhemoglobin (HbO2) to deoxyhemoglobin (HHbO2) signals is considered a reflection of the rate of muscle oxygen uptake (Ding et al. 2001; Im et al. 2001). Thus, NIRS appears to provide an effective tool in examining the link between the EC of muscle contraction and in vivo muscle shortening. Combined with ultrasound to simultaneously measure fascicle shortening and tendon mechanical properties, the effects of these properties on the EC of contraction can be investigated.
Despite a vast array of research examining the EC of running and/or the EC of muscle contraction in a wide range of conditions and species (Sih and Stuhmiller 2003; Taylor et al. 1970; Taylor and Heglund 1982), no studies to date have directly determined the effects of additional shortening on the EC of muscle contraction of human skeletal muscle at physiological temperatures. Therefore, the purpose of this study was to investigate the possible differences in the EC of contractions performed in vivo at physiological temperatures with minimal shortening, and for which extra shortening was allowed. It was hypothesized that when extra fiber shortening was permitted, a greater level of muscle activation would be required to achieve the target force and a greater EC of contraction would result. It seems logical to believe that if the hypothesis is supported, that the increased EC associated with greater activation and increased shortening can explain why optimally tuned AT stiffness is associated with a reduced EC of contraction.
Methods
Subjects
Characteristics of the 19 triathletes (9 males, 10 females) who participated in the study are shown in Table 1. These subjects were chosen because at the time of the study, all subjects were in the pre-competition phase of their run training for either the 10 km or half-marathon race distance. We also anticipated a wide range of AT stiffness and EC in this group. The subjects gave their informed written consent to the experimental procedures, which were approved by the University of Calgary Conjoint Health Research Ethics Board. None of the subjects had neuromuscular or musculoskeletal injuries at the time of the study. All subjects were familiar with the measurement of AT stiffness from previous experiments, but were further familiarized with each measurement prior to data collection. All tests were performed on the same day for each subject.
Tendon mechanical properties
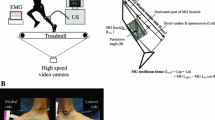
The experimental set-up is shown in Fig. 2. AT stiffness was determined according to Fletcher et al. (2010) and is briefly described here. Each subject performed ramp maximal voluntary isometric ankle plantarflexion contractions (MVC) on their right side. The subjects laid prone with their knee at 180° and their ankle at 90°. Before each MVC, the axis of rotation of the dynamometer (Biodex Medical Systems Inc., Shirley, NY, USA) was carefully aligned with the axis of rotation of the ankle joint. The shank and unshod foot were affixed to the dynamometer using Velcro straps. To further familiarize the subjects with the protocol and to locate at least one visually distinctive and persistent fascicle-aponeurosis cross-point, a warm-up consisting of 3–5 min of submaximal isometric plantarflexions was performed. Afterwards, the subjects performed three isometric ramp MVC plantarflexions, where they were instructed to gradually and continuously increase the measured torque until their voluntarily elicited maximum torque generation. The subjects then attempted to maintain this torque for 2–3 s, such that the entire ramp MVC took 5–7 s to complete. Torque during the MVC was sampled at 100 Hz. The trial eliciting the highest torque was used for analysis.
During each MVC, a 12.5-MHz linear array ultrasound probe (50 mm, Philips Envisor, Philips Healthcare, Eindhoven, Netherlands) was used to visualize the deep aponeurosis of the medial gastrocnemius (MG). The ultrasound probe was placed on the MG muscle belly and secured using a custom-built apparatus. Ultrasound scans were captured at 49 Hz. To determine if the probe moved during the contraction, a point on the ultrasound images where a muscle fascicle attaches to the deep aponeurosis was identified both before and after a test contraction for each subject. This point was always in the same position following the test contraction. An external function generator (B-K Precision 3010, Dynascan Corp., Chicago, IL, USA) was manually started at the initiation of the contraction and acted as a time-stamp for synchronization between image, NIRS and moment data collection. Ultrasound images were recorded and a clear echo point where a fascicle inserts into the deep aponeurosis was followed throughout the contraction and its displacement was measured using publicly available image analysis software (ImageJ, NIH, Baltimore MD, USA). This displacement of a fascicle-aponeurosis junction was interpreted as tendon elongation during these MVCs.
Correction for joint rotation
The amount of joint rotation during the MVC was measured according to Fletcher et al. (2010). This inevitable joint rotation would result in a lower resultant torque and would contribute erroneously to the apparent tendon elongation measured during the contraction (Muramatsu et al. 2001; Spoor et al. 1990). The resultant moment and apparent tendon elongation were corrected according to Fletcher et al. (2010). Ankle joint motion during the contraction was imaged at 24 Hz using a portable video camera (Canon GL1, Canon Inc., Tokyo, Japan). Joint angle change was determined by drawing two to four small dots on the medial aspect of the unshod right foot. From this, ankle joint angle could be calculated throughout the contraction using ImageJ. We assumed the moment about the ankle resulted in a force perpendicular to the foot. Any change in angle of the foot relative to the biodex lever will result in an underestimation of the ankle joint moment. To estimate this error, we measured the change in angle of the foot relative to the biodex lever, and the corrected moments were calculated as:
where M C and M M are the corrected and measured moments, respectively, and θ is the angle of the foot at peak moment. The corrected moments were used for further calculation of plantarflexion force.
The moment arm of the AT was estimated using the tendon travel method (An et al. 1984) under in vivo conditions (Ito et al. 2000; Maganaris 2000). The displacement of a fascicle-aponeurosis cross-point (d L, mm) caused by rotating the ankle from 5° of dorsiflexion to 5° of plantarflexion (d θ, rad) was calculated from the passive rotation. The AT moment arm was calculated as the ratio d L/d θ (mm rad–1). Triceps surae force was calculated by dividing the ankle joint moment by the estimated AT moment arm.
The measured force–elongation data were fitted to a quadratic equation:
AT stiffness was defined as the force–elongation slope from 50–100 % of maximal isometric plantarflexion force, calculated from the quadratic force–elongation relationship (Eq. 2).
Measurement of EC of contraction
To evaluate the effects of muscle fascicle shortening, during brief contractions with specific target force, on MG EC, the subjects laid prone on the dynamometer in the same position as for the testing of AT mechanical properties. The ankle was affixed to the dynamometer and the ultrasound probe placed on the MG and the subject’s MVC was determined as described previously.
Testing protocol
Following the MVC trials, the subjects performed 30 plantarflexions at a frequency of 1 Hz, attempting to reach 50 % of maximum torque with each brief contraction under two conditions (see below). This load was chosen as it is similar to the force exerted on the Achilles tendon during running at 3 m s–1 (Kyröläinen et al. 2003), equivalent to approximately 84 % of the speed associated with the lactate threshold for our subjects. Contractions were performed on an isokinetic dynamometer under two conditions, in random order: isometric (ISO) and isokinetic (KIN). Plantarflexion angular velocity was set at 6.98 rad s–1 during KIN. Throughout the contractions, torque, angular velocity, and position angle signals were collected from the dynamometer at 100 Hz using data acquisition software (WinDaq Pro+, DataQ Instruments Inc., Akron, OH, USA). The subjects received feedback on the magnitude of contractile torque from a monitor displaying the torque signal as % MVC in front of them.
The maximal joint rotation allowed during KIN was set prior to the contractions based on an estimated additional AT elongation (d L) of 15 mm. The estimated additional joint rotation (d θ, rad) required for this elongation during KIN was estimated from the previously determined calculation of Achilles tendon moment arm (MA, mm rad–1):
This magnitude of elongation was chosen because based on pilot testing, this magnitude of AT elongation represented an increase of 50 % of the magnitude of the non-corrected AT elongation during an isometric MVC. Based on a maximum plantarflexion force of 3,000 N, this increased magnitude of elongation was estimated to represent an apparent increase in AT compliance of 40 %. This increase in AT compliance is consistent with a 2.7 % increase in the EC of running (Fletcher et al. 2010) and represents an increase of approximately 2 kcal km–1 for the runners in this study.
During both experimental conditions, the rate of hemoglobin (HbO2) desaturation was measured during blood flow occlusion using spatially resolved near-infrared spectroscopy (NIRS, PortaMon, Artinis, Zetten, The Netherlands) collected at 10 Hz. Blood flow occlusion was achieved by rapidly inflating a blood pressure cuff placed around the subject’s thigh. Cuff pressure was maintained at 240 mmHg for the duration of the contractions. Blood flow occlusion was confirmed by examining the change of HbO2 saturation and desaturation signals throughout the contraction protocol. In all cases, a symmetrical change for HbO2 saturation and desaturation existed, suggesting no change in total Hb implying that no additional saturated blood had entered the area during the contractions (Ryan et al. 2012).
HbO2 desaturation was assumed to be proportional to energy use, the rate of which was expressed as AU s–1. The NIRS device was positioned medially relative to the position of the ultrasound probe. HbO2 desaturation was calculated as the first derivative of the HbO2 desaturation signal using Matlab (ver. R2010a, Mathworks, Natick, MA, USA).
Fascicle lengths were measured using ImageJ at rest and throughout the 10th, 15th, 20th, 25th, and 30th contractions. Wherever possible, the same fascicle was measured throughout the contractions. Where this was not possible, a visually distinctive fascicle near the vicinity of the originally measured fascicle was used. In a small number of cases, a complete MG fascicle could not be seen on the ultrasound image. In those cases, fascicle length was measured using linear extrapolation (Finni et al. 2001) by measuring the distance between superficial and deep aponeuroses and dividing this distance by the sine of resting pennation angle (Austin et al. 2010).
Internal muscle work (J) was calculated as the integral:
from rest to peak force generation, where dL is the change in MG fascicle length, and F is the mean plantarflexion force over the time-course of dL. It was assumed that dL was the same for all triceps surae muscles during the contractions. This was considered a better assumption than estimating the MG muscle contribution to plantarflexor force based on the physiological cross-section of individual triceps surae muscles. Power (W) was calculated as muscle work divided by duration of positive work.
The level of muscle activation was assessed using surface electromyography (EMG) throughout the contractions. Prior to the contractions, a 4 cm × 8 cm area on the skin over the muscle belly of the lateral gastrocnemius (LG) and soleus (SOL) muscles, as well as over the head of the fibula were shaved and cleaned with alcohol. Two EMG electrodes (Norotrode 20 bipolar Ag–AgCl electrodes, Myotronics Inc, Kent, WA, USA, inter-electrode distance: 22 ± 1 mm), were affixed longitudinally to the shaved area over each muscle oriented along the direction of the muscle fibers, as confirmed for each muscle by ultrasound. A single electrode over the head of the fibula served as a ground. EMG of the MG was not possible due to space limitations on the muscle as a result of the ultrasound probe and NIRS device. EMG data were recorded at 2,048 Hz using the NeXus-10 Biotrace+ (version 1.16) Wireless Biofeedback System (Mindmedia, Roermond-Herten, The Netherlands). To reduce noise and signal artifact, the signal was filtered through a 5th order Butterworth filter (high and low-pass filter of 20 and 500 Hz, respectively). EMG amplitude was calculated as the root mean square (RMS) of the raw EMG signal. This RMS was interpreted as the level of muscle activation: an accumulation of recruitment and rate coding. EMG RMS of the LG and SOL were evaluated on the same contractions as the fascicle measurements for each experimental condition. In a subset of subjects on a separate day (n = 4, 26 ± 3 years 1.65 ± 0.04 m, 61.3 ± 12 kg), the EMG amplitudes of the MG and LG were evaluated, without the use of NIRS or ultrasound, to determine whether the EMG amplitude of LG provided an appropriate estimate of MG activation during the experimental trials where the NIRS and ultrasound probe were placed on the MG. EMG amplitude was measured and calculated as described above, during a ramp MVC, and during similar ISO and KIN trials. To confirm whether EMG amplitude increased equally as a function of load (expressed as % MVC), the EMG amplitude was also collected while the subjects attempted to maintain a constant isometric torque at 30, 40, and 50 % of MVC.
Statistics
Data are presented as mean ± standard error of the mean (SEM) and were analyzed using SPSS analysis software (SPSS Inc. v15.0, Chicago, IL, USA). A two-way repeated measures ANOVA was performed to examine the condition × contraction number for the following: torque, impulse, MG shortening length, MG shortening velocity and for EMG RMS. No significant interactions were found, so statistical comparison of these variables refers to main effects. Paired t tests were used to test for differences between conditions for HbO2 desaturation, Pearson product–moment correlations were used to identify relationships between HbO2 desaturation. The a priori level of statistical significance was set at alpha <0.05.
Results
AT stiffness was 151 ± 66 N mm–1, dLROM was 15.5 ± 2.2 mm. The calculated ankle range of motion during KIN was 23.4° ± 3.1°. Average torque during ISO was 56.2 ± 5.1 Nm (52.6 ± 2.1 % MVC). Average torque during KIN was 34.5 ± 2.2 Nm (33.4 ± 1.4 % MVC), significantly lower than during ISO (p < 0.001) and substantially less than the target. Mean impulse was also significantly greater for ISO (19.6 ± 1.9 Nm s) compared to KIN (9.8 ± 0.8 Nm s, p < 0.001).
Mean MG fascicle length (L f) measured prior to the contractions was 55 ± 2 mm. L f at peak torque during the MVC trial was 32 ± 2 mm. Mean L f at peak torque was significantly greater (p < 0.01) for ISO (38 ± 1 mm) compared to KIN (32 ± 2 mm). The ISO contractions were of significantly longer duration (0.33 ± 0.03 s) compared to KIN (0.19 ± 0.01 s). Mean shortening velocity for ISO was 1.13 ± 0.13 L f s–1 and for KIN was 2.48 ± 0.31 L f s–1. This difference was significant (p < 0.001).
Combining the results of fascicle shortening and force to estimate internal muscle work between conditions, ISO resulted in significantly more work compared to KIN (ISO = 32.2 ± 5.3 J contraction–1, KIN = 19.9 ± 1.8 J contraction–1, p < 0.05); however, the rate of performing that work (i.e., power) was not different (p > 0.05) between conditions (ISO = 98 ± 13 W contraction–1, KIN = 117 ± 14 W contraction–1).
Despite a lower mean torque and impulse in KIN, the mean rate of HbO2 desaturation was significantly greater in KIN (p < 0.01). KIN resulted in an 18.6 ± 6.5 % greater HbO2 desaturation (p < 0.01) and required a shorter period of time to reach the maximum rate of HbO2 desaturation (Fig. 3). The contraction number at which maximum rate of HbO2 desaturation occurred was, on average, the 12th contraction (range 6th to 18th contraction) for ISO and the 9th contraction (range 6th to 13th) for KIN. Results of the paired t test revealed this contraction number to be significantly fewer for KIN than ISO (p = 0.03). Taken together, these results suggest a greater rate of energy use and thus a greater EC of contraction in KIN. Results for rate of HbO2 desaturation and mean torque are shown in Fig. 4. Combining these results, the energy required to maintain a given torque (HbO2 desaturation impulse–1) was greater in KIN (6.2 ± 0.6 AU Nm–1 s–1) compared to ISO (2.4 ± 0.3 AU Nm–1 s–1). This represents a difference in EC approaching 160 %. HbO2 desaturation was also significantly related to the average amount of shortening during ISO and KIN conditions (Fig. 5) and the average velocity of shortening (Fig. 6).
The relationship between the rate of energy use to maintain a given torque (HbO2 impulse––1, AU Nm s–1) and magnitude of MG muscle fascicle shortening (cm). The open diamonds represent measurements made during the ISO condition. The filled squares represent those measurements made during the KIN condition. When combined together, the relationship was significant (r 2 = 0.21, p = 0.004). Because of similar values between subjects, some data points are over-lapped
The relationship between the rate of energy use to maintain a given torque (HbO2 impulse–1, AU Nm–1 s–1) and shortening velocity (L f s–1). The open diamonds represent measurements made during the ISO condition. The filled squares represent those measurements made during the KIN condition. When combined together, the relationship was significant (r 2 = 0.38, p < 0.001). Because of similar values between subjects, some data points are over-lapped
In a separate series of measurements, EMG of MG and LG was measured to determine if LG EMG changed in a similar way as EMG of MG. On average, the EMG amplitude of MG was twofold larger than that of LG and the relationship between EMG amplitudes of the MG and LG during the submaximal steady-state and maximal contractions was significant (r 2 = 0.752, p < 0.0001). Furthermore, the change in EMG amplitude from 30 to 100 % MVC (as evaluated from the slopes of the EMG amplitude–%MVC relationships) was not different between MG and LG (p = 0.478). This confirms that changes in EMG amplitude in LG during the trials could be interpreted to represent changes in EMG amplitude of MG. Mean RMS amplitude during the MVC for LG and SOL was 0.570 and 0.907 V, respectively. Figure 7 shows EMG RMS data, presented relative to the EMG RMS amplitude measured during the isometric MVC. Two-way repeated measures ANOVA revealed no significant effect of contraction number on EMG RMS amplitude; however, there was a significant main effect of experimental condition on EMG amplitude. EMG amplitude in KIN was significantly higher than ISO (p < 0.05).
EMG amplitude for LG (top) and SOL (bottom) for both experimental conditions expressed relative to EMG amplitude measured during the isometric MVC (100 % MVC). One-way repeated measures ANOVA revealed a significant effect of experimental condition on EMG amplitude, with KIN resulting in a greater EMG amplitude compared to ISO
Discussion
The purpose of this study was to investigate the effects of additional MG fascicle shortening on the EC of muscle contraction. The main finding in this study was that when greater MG fascicle shortening was imposed, the rate of muscle oxygen uptake increased. We assume this measured oxygen uptake is proportional to the total EC of the muscle contractions; that is, any anaerobic energy utilization would increase in proportion with the increases in oxygen uptake. The additional shortening and EC during KIN may help to explain the reported benefit of a stiff Achilles tendon in reducing the whole-body EC of running (Albracht and Arampatzis 2006; Arampatzis et al. 2006; Fletcher et al. 2010). Given the current data, we propose that the explanation for the increased EC in vivo at physiological temperatures is more complex than simply explaining EC on the basis of extra shortening.
Early reports by Fenn (1923, 1924) would suggest the EC of maximally activated muscle is proportional to the amount of work done; however, in this situation the load was constant and work was proportional to shortening. Given that the EC of achieving a target force is greater than that of maintaining it (Foley and Meyer 2005; Russ et al. 2002), we speculated that it was the additional muscle shortening in KIN which contributed to, but is not the sole factor in the elevated EC. Here, we now demonstrate that it is not the amount of work performed per se which dictates the EC of muscle contraction, since the EC during KIN was significantly higher than during ISO, despite more work performed in the latter condition. Rather, the EC of voluntary muscle contraction performed in vivo is determined by a combination of muscle shortening, shortening velocity and level of motor unit recruitment.
The amount and velocity of shortening are dictated by the mechanics of joint movement and the mechanical properties of the tendon. However, the muscle’s in vivo force–length and force–velocity relationships dictate the magnitude of activation required to achieve a given shortening (Praagman et al. 2006). The force–velocity relationship dictates that force production for a given level of activation is maximal when that force can be developed isometrically (Biewener 1998; Gabaldon et al. 2008; Roberts et al. 1997) and decreases as shortening velocity increases.
It has been suggested that the EC of contraction in vivo should be related not only to the amount of fiber shortening and the shortening velocity but also the level of motor unit activation (Stainsby and Lambert 1979). In fact, Stainsby and Lambert (1979) suggest that the major determinant of metabolic cost of contraction in voluntary movement should be motor unit recruitment. This notion is consistent with the observed (RMS) EMG during cycling, which has a minimum at a unique cadence associated with a given power (MacIntosh et al. 2000), and this cadence is closely related to the optimal cadence for best efficiency (Coast and Welch 1985). Load, shortening and velocity of shortening have less impact on the magnitude of energy requirement (Stainsby and Lambert 1979). For submaximal contractions like those imposed during the present study, the level of activation (as measured by EMG) needed to generate a given (target) force can be minimized when the fascicles are allowed to develop force isometrically. This is illustrated in Fig. 1. In our data (Fig. 7), 50 % of MVC was achieved in ISO with just 50 % of maximal (RMS) EMG but for KIN, the required level of activation increased to above 80 %. It is presumed that the EC of contraction during KIN was greater compared to ISO as a result of the increased rate and amount of MG activation required to achieve the target force in the face of increased fascicle shortening and shortening velocity during the KIN contractions.
The additional fascicle shortening during KIN also impacts force because the muscle is operating at a different place on its force–length relationship (Gordon et al. 1966; Maganaris 2003). For a given muscle force required to perform the task (e.g., running a particular speed and supporting body weight), the level of activation can be minimized if the muscle is operating near optimal length. In keeping the level of activation to a minimum, active muscle volume to generate the required force is minimized and so is the considerable cost of muscle activation associated with ion pumping (Heglund et al. 1982; Hogan et al. 1998). The EC of running is determined primarily by the force of supporting the athlete’s body weight and the time-course of generating this force (Kram and Taylor 1990). When the speed of running is increased, the EC is elevated because the required force is developed more rapidly (Roberts et al. 1998).
It has previously been shown that in maximally activated voluntary isometric contraction, the MG muscle is on the ascending limb of the force–length relationship at anatomical ankle joint angles (Maganaris 2003), with the highest force at +20° dorsiflexion. Assuming this joint configuration corresponds with optimal sarcomere length, sarcomere length during an MVC at a neutral ankle angle of 90° can be estimated as approximately 83 % of optimal gastrocnemius sarcomere length during MVC (Maganaris 2003). The current study measured fascicle length for maximal (31.7 mm) and submaximal (37.8 mm) contractions at an ankle angle of 90°. Assuming an optimal sarcomere length during maximal activation of 2.6 μm at the short side of the plateau (Herzog and ter Keurs 1988), it is estimated that the sarcomere lengths were 2.57 μm for ISO and 2.19 μm for KIN. The EC of the force impulse is increased at short muscle length (de Haan et al. 1986). This increase occurs because the energy for activation (ion pumping) is independent of length (Homsher et al. 1972; Woledge et al. 1985), and energy for force development is proportional to force. However, the small differences we observed in estimated sarcomere length would indicate that this effect was minor.
Despite greater muscle work performed during the ISO contractions, KIN resulted in a significantly greater power output compared to ISO. However, the EC of contraction was directly related to the amount and rate of MG fascicle shortening. The rate of energy use has been shown previously to relate to the rate of muscle shortening (Fenn 1924; He et al. 2000; Hill 1938). However, it is clear from the above data that this is not the only factor which dictates the EC of in vivo voluntary muscle contraction.
In spite of greater muscle work in ISO, the EC was significantly reduced in this condition compared to KIN. Despite greater force per contraction in ISO, muscle shortening under this condition was less than that in KIN. Heglund et al. (1982) demonstrated that the energetic cost of locomotion is related to the rate at which muscles are turned on and off, such that a faster rate of activation is associated with an elevated metabolic cost. These results suggest that short-duration contractions (such as those seen in KIN) may require a higher amount of total energy as a result of ion pumping associated with each activation cycle. The present results support this notion, since more EMG was observed, indicating more motor unit activation. This probably relates to differences in sarcomere length and the impact of the force–velocity relationship.
The contractions by the MG fascicles during running are nearly isometric (Ishikawa et al. 2007b) thus, the results of the current study may be relevant to running, and may explain why a stiffer tendon helps to minimize the EC of whole-body locomotion. As indicated by several authors, a more compliant Achilles tendon would require a greater amount of fascicle shortening (Albracht and Arampatzis 2006; Arampatzis et al. 2006; Fletcher et al. 2010) However, it should be acknowledged that additional related factors contribute to the increased energy cost. This includes increased velocity of shortening for a given joint movement and increased activation of the involved muscles. We observed that with additional shortening and a similar target force, there was increased velocity of shortening and a greater level of motor unit recruitment. This increased recruitment would contribute to the elevated EC of contraction.
In conclusion, the results of the current investigation confirm previous reports that the EC of muscle contraction is related to the amount and rate of muscle shortening. Furthermore, these results may explain why the EC of running is elevated when Achilles tendon compliance is increased, since a greater amount and rate of shortening are required for force transmission under these conditions. According to the in vivo force–length and force–velocity relationships of skeletal muscle, this shortening and velocity will impact the EC, not simply because of the greater shortening, but because increased muscle activation is required to permit similar force development when shortening velocity is greater.
Abbreviations
- AT:
-
Achilles tendon
- d L :
-
Fascicle-aponeurosis displacement
- d ϴ :
-
Ankle joint displacement
- EC:
-
Energy cost
- EMG:
-
Electromyography
- F:
-
Force
- FL:
-
Fascicle length
- HHbO2 :
-
Deoxyhemoglobin
- HbO2 :
-
Oxyhemoglobin
- ISO:
-
Isometric
- KIN:
-
Isokinetic
- LG:
-
Lateral gastrocnemius
- M C :
-
Corrected moment
- M M :
-
Measured moment
- MA:
-
Moment arm
- MG:
-
Medial gastrocnemius
- MVC:
-
Maximal voluntary contraction
- NIRS:
-
Near-infrared spectroscopy
- RMS:
-
Root mean square
- SOL:
-
Soleus
References
Albracht K, Arampatzis A (2006) Influence of the mechanical properties of the muscle-tendon unit on force generation in runners with different running economy. Biol Cybern 95:87–96
Alexander RM (1991) Energy-saving mechanisms in walking and running. J Exp Biol 160:55–69
An KN, Takahashi K, Harrigan TP, Chao EY (1984) Determination of muscle orientations and moment arms. J Biomech Eng 106:280–282
Arampatzis A, De Monte G, Karamanidis K, Morey-Klapsing G, Stafilidis S, Bruggemann GP (2006) Influence of the muscle-tendon unit’s mechanical and morphological properties on running economy. J Exp Biol 209:3345–3357
Askew GN, Marsh RL (1998) Optimal shortening velocity (V/Vmax) of skeletal muscle during cyclical contractions: length-force effects and velocity-dependent activation and deactivation. J Exp Biol 201:1527–1540
Austin N, Nilwik R, Herzog W (2010) In vivo operational fascicle lengths of vastus lateralis during sub-maximal and maximal cycling. J Biomech 43:2394–2399
Bergstrom M, Hultman E (1988) Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol 65:1500–1505
Biewener AA (1998) Muscle function in vivo: a comparison of muscles used for elastic energy savings versus muscles used to generate mechanical power1. Integr Comp Biol 38:703
Chow JW, Darling WG (1999) The maximum shortening velocity of muscle should be scaled with activation. J Appl Physiol 86:1025–1031
Coast JR, Welch HG (1985) Linear increase in optimal pedal rate with increased power output in cycle ergometry. Eur J Appl Physiol Occup Physiol 53:339–342
de Haan A, Jong J, Doorn J, Huijing P, Woittiez R, Westra H (1986) Muscle economy of isometric contractions as a function of stimulation time and relative muscle length. Pflügers Archiv Eur J Physiol 407:445–450
Ding H, Wang G, Lei W et al (2001) Non-invasive quantitative assessment of oxidative metabolism in quadriceps muscles by near infrared spectroscopy. Br J Sports Med 35:441–444
Farris DJ, Trewartha G, McGuigan MP (2011) The effects of a 30-min run on the mechanics of the human Achilles tendon. Eur J Appl Physiol 112(2):653–660
Fenn WO (1923) A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol 58:175–203
Fenn WO (1924) The relation between the work performed and the energy liberated in muscular contraction. J Physiol 58:373–395
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Finni T, Ikegaw S, Lepola V, Komi P (2001) In vivo behavior of vastus lateralis muscle during dynamic performances. Eur J Sport Sci 1:1–13
Fletcher JR, Esau SP, Macintosh BR (2010) Changes in tendon stiffness and running economy in highly trained distance runners. Eur J Appl Physiol 110:1037–1046
Foley JM, Meyer RA (2005) Energy cost of twitch and tetanic contractions of rat muscle estimated in situ by gated 31P NMR. NMR Biomed 6:32–38
Gabaldon AM, Nelson FE, Roberts TJ (2008) Relative shortening velocity in locomotor muscles: turkey ankle extensors operate at low V/V(max). Am J Physiol Regul Integr Comp Physiol 294:R200–R210
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12:062105
He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C (2000) ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79:945–961
Heglund NC, Fedak MA, Taylor CR, Cavagna GA (1982) Energetics and mechanics of terrestrial locomotion. IV. Total mechanical energy changes as a function of speed and body size in birds and mammals. J Exp Biol 97:57–66
Herzog W, ter Keurs HEDJ (1988) Force–length relation of in vivo human rectus femoris muscles. Pflügers Archiv Eur J Physiol 411:642–647
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc Royal Soc Lond Series B Biol Sci 126:136–195
Hogan MC, Ingham E, Kurdak SS (1998) Contraction duration affects metabolic energy cost and fatigue in skeletal muscle. Am J Physiol Endocrinol Metab 274:E397–E402
Homsher E, Mommaerts W, Ricchiuti N, Wallner A (1972) Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol (Lond) 220:601–625
Im J, Nioka S, Chance B, Rundell KW (2001) Muscle oxygen desaturation is related to whole body VO2 during cross-country ski skating. Int J Sports Med 22:356–360
Ishikawa M, Pakaslahti J, Komi P (2007a) Medial gastrocnemius muscle behavior during human running and walking. Gait Posture 25:380–384
Ishikawa M, Pakaslahti J, Komi PV (2007b) Medial gastrocnemius muscle behavior during human running and walking. Gait Posture 25:380–384
Ito M, Akima H, Fukunaga T (2000) In vivo moment arm determination using B-mode ultrasonography. J Biomech 33:215–218
Kram R, Taylor CR (1990) Energetics of running: a new perspective. Nature 346:265–267
Kyröläinen H, Finni T, Avela J, Komi P (2003) Neuromuscular behaviour of the triceps surae muscle-tendon complex during running and jumping. Int J Sports Med 24:153–155
Lichtwark GA, Bougoulias K, Wilson AM (2007) Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech 40:157–164
MacIntosh BR, Neptune RR, Horton JF (2000) Cadence, power, and muscle activation in cycle ergometry. Med Sci Sports Exerc 32:1281
Maganaris CN (2000) In vivo measurement-based estimations of the moment arm in the human tibialis anterior muscle-tendon unit. J Biomech 33:375–379
Maganaris CN (2003) Force–length characteristics of the in vivo human gastrocnemius muscle. Clin Anat 16:215–223
McCully KK, Hamaoka T (2000) Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28:123–127
Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T (2001) Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol 90:1671–1678
Neary JP (2004) Application of near infrared spectroscopy to exercise sports science. Can J Appl Physiol 29:488–503
Praagman M, Chadwick EK, van der Helm FC, Veeger HE (2006) The relationship between two different mechanical cost functions and muscle oxygen consumption. J Biomech 39:758–765
Roberts TJ, Marsh RL, Weyand PG, Taylor CR (1997) Muscular force in running turkeys: the economy of minimizing work. Science 275:1113–1115
Roberts TJ, Kram R, Weyand PG, Taylor CR (1998) Energetics of bipedal running. J Exp Biol 201:2745–2751
Russ DW, Elliott MA, Vandenborne K, Walter GA, Binder-Macleod SA (2002) Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am J Physiol Endocrinol Metab 282:E448–E457
Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK (2012) Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol 113:175–183
Sih BL, Stuhmiller JH (2003) The metabolic cost of force generation. Med Sci Sports Exerc 35:623–629
Spoor CW, van Leeuwen JL, Meskers CG, Titulaer AF, Huson A (1990) Estimation of instantaneous moment arms of lower-leg muscles. J Biomech 23:1247–1259
Stainsby WN (1982) Energetic patterns of normally circulated mammalian muscle in situ. Fed Proc 41:185–188
Stainsby WN, Lambert CR (1979) Determination of oxygen uptake in skeletal muscle. Exerc Sport Sci Rev 7:125–151
Taylor CR, Heglund NC (1982) Energetics and mechanics of terrestrial locomotion. Annu Rev Physiol 44:97–107
Taylor CR, Schmidt-Nielsen K, Raab JL (1970) Scaling of energetic cost of running to body size in mammals. Am J Physiol 219:1104–1107
Taylor CR, Heglund NC, McMahon TA, Looney TR (1980) Energetic cost of generating muscular force during running: a comparison of large and small animals. J Exp Biol 86:9–18
Woledge RC, Curtin NA, Homsher E (1985) Energetic aspects of muscle contraction. Monogr Physiol Soc 41:1–357
Acknowledgments
This study was supported by the Natural Sciences and Engineering Research Council of Canada.
Conflict of interest
The authors report no commercial involvement which may bias the process of data collection, reporting and/or interpretation.
Ethical standard
The authors declare that the experiments comply with current Canadian laws and all experimental procedures were approved by the University of Calgary Conjoint Health Research Ethics Board.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guido Ferretti.
Rights and permissions
About this article
Cite this article
Fletcher, J.R., Groves, E.M., Pfister, T.R. et al. Can muscle shortening alone, explain the energy cost of muscle contraction in vivo?. Eur J Appl Physiol 113, 2313–2322 (2013). https://doi.org/10.1007/s00421-013-2665-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2665-0