Abstract
Liver X receptor α (LXRα) is a member of the ligand-activated transcription factor of nuclear hormonal receptor superfamily, whose activation leads to modulation in the expression of genes involved in cholesterol homeostasis including ATP-binding cassette transporter A1 (ABCA1), which plays a crucial role in plasma high-density lipoprotein cholesterol (HDL-C) remodeling. The purpose of this study was to investigate whether endurance training enhanced the expression level of liver LXRα gene. Twelve adult male Wistar rats (200–220 g) were divided into control and training groups. Training group received exercise on a motor-driven treadmill at 28 m/min (0 % grade) for 60 min/day, 5 days/week for 8 weeks. Twenty-four hours after the last exercise session, the rats were killed and blood was taken from the right ventricle of each rat. Plasma was collected for HDL-C, low-density lipoprotein cholesterol (LDL-C), TC and TG measurements. Furthermore, a portion of the liver of each rat was excised and washed in ice-cold saline and frozen in liquid nitrogen for assessment of LXRα and ABCA1 mRNA levels. Data indicated significant increase in both LXRα and ABCA1 mRNA levels in trained rats, compared to control rats. Plasma HDL-C concentration was significantly higher (P < 0.001) in trained rats at the end of treadmill exercise. However, there was a significant decrease in LDL-C (P < 0.003), TG, TC concentration, TC/HDL-C and LDL/HDL-C ratios in trained rats compared with those in the control group (P < 0.001). In conclusion, we found that endurance training induced significant elevation in LXRα gene expression, which correlated with enhanced levels of ABCA1 mRNA and plasma HDL-C concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is among one of the greatest causes of morbidity and mortality in most countries. The frequency of its incidence correlates well with increment in plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) concentrations. Moreover, population studies have shown a significant reverse relation between high-density lipoprotein cholesterol (HDL-C) level and atherosclerotic cardiovascular onset in humans (Nagasawa et al. 2012). The protective effect of HDL-C against atherosclerosis is well defined in reverse cholesterol transport (RCT) (Cooney et al. 2009). In the RCT pathway, HDL mediates the excess free cholesterol efflux from the peripheral cells to the liver for excretion into the bile (Baranowski 2008; Zhao and Dahlman-Wright 2010). Therefore, the liver acts as a lipid-regulating organ through numerous receptors. One of these receptors which plays a key role in cholesterol metabolism is the liver X receptor (LXR) that was identified in the rat liver in 1994 (Apfel et al. 1994). LXRs are the ligand-activated transcription factors of the nuclear receptor superfamily consisting of two isoforms α and β. Both are involved in regulation of the expression of genes in cholesterol homeostasis (Lehmann et al. 1997; Wójcicka et al. 2007; Beltowski and Semczuk 2010). LXRs exert their main function as intercellular sterol (especially cholesterol) sensors, which cause different adapting procedures in response to cholesterol overload. These mechanisms include: (a) the stimulation of the RCT pathway; (b) prevention of cholesterol synthesis; (c) inhibition of cholesterol absorption in the intestine; (d) stimulation of cholesterol efflux in the form of HDL and its transfer to the liver; (f) cholesterol conversion to biliary acids and its excretion (Lehmann et al. 1997; Wójcicka et al. 2007; Beltowski and Semczuk 2010). LXRs stimulate the RCT through mediating two different pathways: increasing ATP-binding cassette transporter A1 (ABCA1) gene expression and enhancing the availability of extracellular cholesterol acceptors including apoprotein E (Wouters et al. 2005; Sato et al. 2008). Several studies have shown that the natural and synthetic agonists of LXRs cause an increment in the gene expression of ABCA1 and excretion of cholesterol from the cells. Hence, they may play a potential therapeutic role for preventing atherosclerosis (Zhao et al. 2008). During the last few years, many studies have been conducted for understanding the effect of physical activities on the HDL level. It has been well accepted that regular endurance exercises could cause an increase in plasma HDL (Lespessailles et al. 2010; Dabidi 2011) and a decrease in plasma LDL and triglycerides (TG) levels (Zhao et al. 2011). However, the number of studies that have focused on the genetic mechanisms of HDL increase is very limited. Considering the importance of the LXRα gene and since there has not been any studies on the effect of physical exercises on the expression of this gene in the rat liver, this study was designed to show for the first time whether regular endurance exercises could cause any changes in the mRNA level of LXRα.
Materials and methods
Animals
All experiments with animals were carried out according to the policy of the Ethics Committee of the University of Isfahan. Twelve Wistar male rats with an estimated weight of 200–220 g under normal light conditions (12 h light–dark cycle), temperature of 23 ± 1 °C and moisture of 50 ± 3 % were kept in special cages. Animals were fed with pellet rodent diet ad libitum and had free access to the water. The whole study period was carried out by one person. After 2 weeks of work in the laboratory and human intervention, rats were randomly divided to training (n = 6) and control (n = 6) groups.
Exercise training protocol
The training program began with adaptation of rats to the apparatus for 7 days by placing them on the motor-driven treadmill (School of Medicine, Isfahan University of Medical Sciences). The training protocol was as follows: first, rats were exercised on the treadmill at 16 m/min for 15 min. One week after starting the experiment, the time and speed of running were increased steadily to 60 min/day at 20 m/min. After this step, the experiment group received a progressive exercise. They were again made to run on a treadmill for 60 min/day, 5 days a week. During the first week of exercises, the speed was set to 20 m/min, while for the second, third and fourth weeks it was adjusted to 23, 25 and 28 m/min, respectively.
Running exercises were continued for the next 4 weeks with a speed of 28 m/min, 60 min in each session and five times per week. The angle of inclination was 0 % gradient during the whole period of the study. This condition corresponded to a moderate intensity of about 65 % of maximal oxygen consumption (Powers et al. 1993; Vincent et al. 2000).
Liver biopsy and blood sample
Twenty-four hours after the last exercise session (eighth week), rats were anesthetized intraperitoneally with a mixture of ketamine (30–50 mg/kg of body weight) and xylazine (3–5 mg/kg of body weight). Three ml of blood was taken from the right ventricle of each rat and immediately transferred to a test tube. The blood samples were centrifuged for 15 min at 4,000 rpm to separate the serum. Obtained sera were inserted into the test tubes and kept in a deep freezer (−80 °C) for future measurements.
After collecting the samples, abdominal part of rats were opened and a portion of the liver was excised and washed in ice-cold saline. Then they were immediately frozen in liquid nitrogen for extraction of LXRα mRNA. The frozen liver tissues were kept in −80 °C for further experiments.
Measurements of lipids and lipoproteins
To determine the concentration of TC, TG and HDL-C, enzymatic methods were used in a calibrated biochemical analyzer (Hitachi 902 Automatic analyzer, Roche Diagnostics, USA) as follows. TC and TG were measured by assessment of the produced H2O2 (Parakh and Gank 1982). For measurement of the HDL-C content, chemical precipitation of lipoproteins containing apoprotein B was performed using dextran sulfate-Mg2+. Then HDL-C was measured by coupling the product of cholesterol oxidase reaction to an indicator reaction as described (Warnick et al. 1982). The amount of LDL-C was calculated with respect to the values for TC, TG and HDL-C (Demacker et al. 1984).
mRNA level assessment of LXRα and ABCA1
To extract RNA, 50 mg of the frozen liver tissue was homogenized. Total RNA was isolated by the RNA-Plus kit (CinnaGen, Iran) according to the manufacturer’s instruction. Then, the RNA solution was extracted and decontaminated from any DNA and destructive RNA enzymes using RNase free DNaseI (Fermentas, Germany). Two μg of RNA from each sample was used for synthesizing the first cDNA using the cDNA synthesis kit (Fermentas, Germany) utilizing the oligo dT primer. RT-PCR was performed using 2 μl cDNA and 5 pmol of each primer (Table 1) in a total volume of 20 μl PCR reaction mixture. Real-time (SYBRGreen) PCR was carried out in a thermal cycler (Biorad, USA) as suggested by the protocol (TaKaRa). The PCR mixture contained 10 μl Rotor-Gene SYBR Green PCR Master Mix (TaKaRa), 3 pmol of each primer and 25 ng cDNA for each reaction in a final volume of 20 μl.
Relative mRNA concentrations were calculated from takeoff point of reactions using the software provided by the manufacturer and normalized to β-actin expression level in the same samples. All measurements were done in duplicate and data were assessed and reported according to the ∆∆Ct method.
Statistics
All results were expressed as mean ± SD (standard deviations). All variables were compared by independent t test. Correlation was calculated using the Pearson product moment correlation. All statistical analysis was performed using SPSS (Version 13). P values <0.05 were considered to be significant.
Results
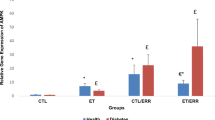
Data revealed that liver LXRα expression was significantly (P < 0.001) higher in trained rats when compared with control rats (Fig. 1). A similar result was obtained for assessment of hepatic ABCA1 expression level which was significantly (P < 0.05) higher in trained rats (Fig. 2). Plasma HDL-C was also significantly (P < 0.001) higher in trained rats (Table 2). However, plasma LDL-C was significantly (P < 0.003) decreased in trained rats (Table 2). Furthermore, plasma TC, TG concentrations, TC/HDL-C and LDL/HDL-C ratios were significantly (P < 0.001) decreased in trained rats following 8 weeks of treadmill running programs (Table 2) consistent with previous studies (Petridou et al. 2005; Karanth and Jeevaratnam 2009).
There were positive and significant correlations between liver LXRα mRNA expression and plasma HDL-C (r = 0.82, P < 0.001) concentrations, whereas a reverse significant correlation between LXRα mRNA expression and LDL-C (r = −0.66, P < 0.01), TG (r = −0.78, P < 0.002) and TC (r = −0.79, P < 0.002) was observed.
Discussion
Physical exercise is a well-recognized activity that modulates HDL-C and affects the RCT process (Gupta et al. 1993; Leaf 2003; Olchawa et al. 2004). A recent study by Hong and coworkers has elucidated that LXRα plays an important role in the whole-body sterol homeostasis, mainly through the upregulation of genes involved in the reverse RCT process (Hong et al. 2012). Furthermore, the activation of LXRα increased fatty acid utilization during exercise and prevented the fatigue caused by glucose insufficiency (Baranowski et al. 2011). It has been already indicated that low-intensity exercises caused an enhancement in LXRα gene expression in human leukocytes (Butcher et al. 2008). However the effect of physical activity, especially endurance exercise, on LXRα expression level in the liver has remained obscure. Thus, the present study was designed to address whether endurance training modulated LXRα expression level in the liver. Our data revealed a significant increase in LXRα expression level in the liver, resulting from performing endurance training. Furthermore, we found a positive significant correlation between liver LXRα mRNA expression and plasma HDL-C. The possible cause of plasma HDL-C increment following physical activities is that several modulations happen with respect to exercise such as an enhancement in the activity of several enzymes including lipoprotein lipase, lecithin:cholesterol acyltransferase and hepatic lipase, as well as in the content of phospholipid transport protein, cholesterol esteryl transport protein and ATP-binding cassette transporters family (Olchawa et al. 2004; Roth et al. 2011). Particularly in the latter group, activities of ABCA1 and ABCG1 are regulated by LXRα (Tang et al. 2012). Studies have shown that regular endurance exercises may also cause an increase in hepatic ABCA1 gene expression (Khabazian et al. 2008; Ghanbari-Niaki et al. 2007). Numerous studies have shown that LXRα may activate ABCA1 gene expression (Brunham et al. 2006; Fukumoto et al. 2002; Zhou et al. 2010). Here, we have shown that endurance exercise enhanced hepatic ABCA1 mRNA level, implicating an increase in LXRα content and its activity may cause such enhancement. These modulations could affect an alteration in HDL content and therefore speed up the process of RCT. Very recently, in contrast to our results, Cote et al. (2013) have indicated that exercise did not increase hepatic LXRα transcripts. This discrepancy may be reflected by the effect of different intensities and various modes of exercise. The condition of exercise in the present study corresponded to a moderate intensity of about 65 % of maximal oxygen consumption versus 75 % of VO2 max, which was reported by Cote et al. However, more investigations are required to clarify the effect of different conditions of exercise on the contents of hepatic LXRα transcripts.
Conclusion
Data indicated that treadmill running induced elevation in hepatic LXRα expression levels, which correlated well with an enhancement in hepatic ABCA1 expression level and plasma HDL-C and decreased levels of plasma LDL-C, TG and TC.
Abbreviations
- ABCA1:
-
ATP-binding cassette transporter A1
- CAD:
-
Coronary artery disease
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LXR:
-
Liver X receptor
- RCT:
-
Reverse cholesterol transport
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
References
Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M (1994) A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14:7025–7035
Baranowski M (2008) Biological role of liver X receptors. J Physiol Pharmacol 59:31–55
Baranowski M, Zabielski P, Błachnio-Zabielska AU, Harasiuk D, Go′rski J (2011) LXR activation prevents exhaustive exercise-induced hypoglycaemia and spares muscle glycogen but does not enhance running endurance in untrained rats. Acta Physiol 201:373–379
Beltowski J, Semczuk A (2010) Liver X receptor (LXR) and the reproductive system—a potential novel target for therapeutic intervention. Pharmacol Rep 62:15–27
Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR (2006) Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res 99(7):672–674
Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K (2008) Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc 40:1263–1270
Cooney MT, Dudina A, De Bacquer D, Wilhelmsen L, Sans S, Menotti A, De Backer G, Jousilahti P, Keil U, Thomsen T, Whincup P, Graham IM (2009) HDL cholesterol protects against cardiovascular disease in both genders, at all ages and at all levels of risk. Atherosclerosis 206(2):611–616
Cote I, Ngo Sock ET, Lévy E, Lavoie JM (2013) An atherogenic diet decreases liver FXR gene expression and causes severe hepatic steatosis and hepatic cholesterol accumulation: effect of endurance training. Eur J Nutr (in press) (Epub ahead of print). doi:10.1007/s00394-012-0459-5
Dabidi RV (2011) Effect of training on high sensitive C-reactive protein and blood lipids responses in rats. Middle East J Sci Res 1:115–122
Demacker PN, Hijmans AG, Brenninkmeijer BJ, Jansen AP, van ‘t Laar A (1984) Five methods for determining low density lipoprotein cholesterol compared. Clin Chem 30(11):1797–1800
Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW (2002) Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Abeta levels. J Biol Chem 277(50):48508–48513
Ghanbari-Niaki A, Khabazian B, Hissainin-Kakhak A, Rahbarizadeh F, Hedayat M (2007) Treadmill exercise enhances ABCA1 in rat liver. Biochem Biophys Res Commun 361:841–846
Gupta AK, Ross EA, Myers JN, Kashyap ML (1993) Increased reverse cholesterol transport in athletes. Metabolism 42(6):684–690
Hong C, Bradley MN, Rong X, Wang X, Wagner A, Grijalva V, Castellani LW, Salazar J, Realegeno S, Boyadjian R, Fogelman AM, Van Lenten BJ, Reddy ST, Lusis AJ, Tangirala RK, Tontonoz P (2012) LXRα is uniquely required for maximal reverse cholesterol transport and atheroprotection in ApoE-deficient mice. J Lipid Res 53(6):1126–1133
Karanth J, Jeevaratnam K (2009) Effect of dietary lipid, carnitine and exercise on lipid profile in rat blood, liver and muscle. Indian J Exp Biol 47:748–753
Khabazian BM, Ghanbari-Niaki A, Rahbarizadeh F, Hosseini Kakhak SA, Jabari Kouchabi M (2008) The effect of 6 weeks endurance training of hepatic ABCA1 in male Wistar rats. Sport Sci 6:101–114
Leaf DA (2003) The effect of physical exercise on reverse cholesterol transport. Metabolism 52(8):950–957
Lehmann JM, Kliewer SA, Moore LB (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272:3137–3140
Lespessailles E, Jaffre C, Rochefort GY, Dolle E, Benhamou CL, Courteix D (2010) Exercise and zoledronic acid on lipid profile and bone remodeling in ovariectomized rats: a paradoxical negative association? Lipids 45:337–344
Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H (2012) Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65,594 individuals from 10 cohort studies in Japan. J Am Heart Assoc 1(5):e001974
Olchawa B, Kingwell BA, Hoang A, Schneider L, Miyazaki O, Nestel P, Sviridov D (2004) Physical fitness and reverse cholesterol transport. Arterioscler Thromb Vasc Biol 24:1087–1091
Parakh AC, Gank DH (1982) Free and total cholesterol. In: Bauer JD (ed) Clinical laboratory methods. Mosssby Company, Toronto, pp 546–549
Petridou A, Nikolaidis MG, Matsakas A, Schulz TH, Michna H, Mougios V (2005) Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue. Eur J Appl Physiol 94:84–92
Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA (1993) Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol 265:2094–2098
Roth SM, Rankinen T, Hagberg JM, Loos RJ, Pérusse L, Sarzynski MA, Wolfarth B, Bouchard C (2012) Advances in exercise, fitness, and performance genomics in 2011. Med Sci Sports Exerc 44(5):809–817
Sato M, Kawata Y, Erami K, Ikeda I, Imaizumi K (2008) LXR agonist increases the lymph HDL transport in rats by promoting reciprocally intestinal ABCA1 and apo A-I mRNA levels. Lipids 43:5–131
Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, Fu YC, Tang CK (2012) PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis 222(2):344–354
Vincent HK, Powers SK, Stewart DJ, Demirel HA, Shanely RA, Naito H (2000) Short term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol Occup Physiol 81:67–74
Warnick GR, Benderson J, Albers J (1982) Dextran sulfate-Mg2+ precipitation procedure for quantitation of high density lipoprotein cholesterol. Clin Chem 28:1378–1388
Wójcicka G, Jamroz-Wiśniewska A, Horoszewicz K, Bełtowski J (2007) Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw 61:736–759
Wouters K, Shiri-Sverdlov R, van Gorp PJ, van Bilsen M, Hofker MH (2005) Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoE and ldlr mice. Clin Chem Lab Med 43:470–479
Zhao C, Dahlman-Wright K (2010) Liver X receptor in cholesterol metabolism. J Endocrinol 204:233–240
Zhao SP, Yu BL, Xie XZ, Dong SZ, Dong J (2008) Dual effects of oxidized low-density lipoprotein on LXR–ABCA1–apoA-I pathway in 3T3-L1 cells. Int J Cardiol 8:42–47
Zhao J, Tian Y, Xu J, Liu D, Wang X, Zhao B (2011) Endurance exercise is a leptin signaling mimetic in hypothalamus of Wistar rats. Lipids Health Dis 10:225–231
Zhou X, Yin Z, Guo X, Hajjar DP, Han J (2010) Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J Biol Chem 285(9):6316–6326
Acknowledgments
This study was funded by a grant in aid of research from the Chancellorship Office for Research and Technology of the University of Isfahan (Grant No. 90/97891) awarded to Jamal Moshtaghian, Ph.D., which was used to support Fatemeh Kazeminasab for obtaining her M.Sc. degree from the University of Isfahan.
Conflict of interest
None of the authors has any conflicts of interest to disclose and all authors support submission to this journal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Kazeminasab, F., Marandi, M., Ghaedi, K. et al. Endurance training enhances LXRα gene expression in Wistar male rats. Eur J Appl Physiol 113, 2285–2290 (2013). https://doi.org/10.1007/s00421-013-2658-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2658-z