Abstract
This study aimed at examined the effect of a 12-week whole body vibration (WBV) training program on leg blood flow and body composition in people with type 2 diabetes mellitus (T2DM). Forty participants were randomly assigned to either a WBV training group (WBV; n = 20) or usual-care control group (CON; n = 20). Body composition [waist circumference, waist to hip ratio (WHR), weight, height, percentage of body fat and fat-free mass], heart rate, and blood flow [femoral artery diameter, maximum systolic velocity, maximum diastolic velocity (DV), time averaged mean, pulsatility index and resistance index (RI), mean velocity (V med), and peak blood velocities (PBV)] were assessed at baseline and after 12 weeks. There were significant increases in the blood flow (p = 0.046), V med (p = 0.050), and DV (p = 0.037) after WBV compared with CON. Within-group analysis showed significant differences in V med, PBV, and DV in the WBV group. Significant decreases after the intervention in weight (p < 0.001), waist circumference (p < 0.001), WHR (p < 0.05), and body fat (p < 0.05) were also found, with significant between-groups decreases in all these outcomes in the WBV group. Significant correlations existed between changes in percent body fat and blood flow [blood flow (−0.761), V med (−0.607), PBV (−0.677), and RI (0.0510)]. WBV training can be considered an effective means to increase leg blood flow and to reduce adiposity in patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is increasing and is reaching epidemic proportions worldwide. Obesity, hypertension, insulin resistance, and hyperglycemia may contribute to the impaired arterial function in T2DM (Chudyk and Petrella 2011; Gerich 2007). Reduction in peripheral blood flow in T2DM is endothelial dependent (Lalande et al. 2008; Lohman et al. 2007). Arteries in lower extremities develop more severe dysfunction than those in upper extremities in middle-aged adults with T2DM (Silber et al. 2007).
Exercise therapy has been reported to reduce hyperglycemia, insulin resistance, dyslipidemia, and hypertension which lead to improved vascular function in T2DM (Sato et al. 2007) and, therefore, is recognized as a cornerstone of diabetes management (Chudyk and Petrella 2011). Evidence of vasodilation following aerobic and resistance exercise training in individuals with T2DM (Okada et al. 2010) suggests that exercise increases arterial diameter and skeletal muscle glucose uptake which contribute to favorable arterial and metabolic outcomes (Bloor 2005). Recent studies have shown that aerobic exercise training improves femoral artery blood flow (Mikus et al. 2011) and intra-abdominal adipose tissue in individuals with T2DM (Terada et al. 2013). Resistance training also has been shown to improve endothelial function in overweight women (Olson et al. 2006) and likewise to improve insulin sensitivity in elderly subjects with or without T2DM (Dela and Kjaer 2006). Unfortunately, patients suffering from T2DM are characterized by a compromised exercise tolerance (Regensteiner 2004) and not all patients are able to lift heavy loads (Egger et al. 2012).
Alternatively, whole body vibration (WBV), which is a novel training intervention, has been proven to be a safe and effective exercise modality for improving body composition and muscle strength in deconditioned individuals who cannot perform conventional exercise, even in those with T2DM (Behboudi et al. 2011). Some studies have suggested that WBV alone or in combination with other methods can decrease fat mass and increase muscle mass (Fjeldstad et al. 2009; Milanese et al. 2012; Machado et al. 2010; Bogaerts et al. 2007). However, the effect of WBV exercise on arterial function is poorly understood.
A single bout of WBV exercise was found to increase leg muscle blood flow (Kerschan-Schindl et al. 2001; Lythgo et al. 2009) and reduce leg arterial stiffness (Figueroa et al. 2012). Recent studies reported that repeated bouts of WBV (Maloney-Hinds et al. 2009; Devereux et al. 2010) upregulate the nitric oxide dilator system and improve endothelial function. However, while positive results in vascular function were reported after acute intermittent vibration protocols (Lohman et al. 2007; Kerschan-Schindl et al. 2001; Lythgo et al. 2009; Maloney-Hinds et al. 2009), others have not (Hazell et al. 2008; Button et al. 2007) and to date, no studies have explored the chronic effects of WBV exercise training on leg arterial blood flow. Evidence of reduced arterial stiffness and wave reflection in young overweight or obese non-diabetic women following WBV exercise training (Figueroa et al. 2012) suggests that WBV may be an effective training mode for improving leg artery blood flow.
Therefore, the aim of the current investigation was first to measure the effect of a 12-week WBV training program on leg blood flow in individuals with T2DM and second, to determine whether changes in blood flow parameters after WBV training are associated with improvements in body composition. As WBV increases leg blood flow and reduces fat mass, we hypothesized that it would provide therapeutic benefits to people with diabetes characterized by impeded leg blood flow and abdominal obesity.
Methods
Participants
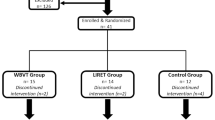
Fifty-seven volunteers completed detailed medical history questionnaires and underwent a medical examination to determine eligibility. Participants had T2DM confirmed by a primary care provider based on the American Diabetes Association diagnostic criteria. Exclusion criteria included history or evidence of advanced cardiovascular, renal or hepatic diseases, retinopathy, nephropathy, or neuropathy, insulin use, orthopedic, or other limitations that may interfere with their ability to exercise safely. Participants with HbA1c >10 % also were excluded. Moreover, participants receiving physical therapy were excluded to avoid possible interactions with the present intervention. Seven patients were excluded (cardiovascular diseases n = 2; musculoskeletal diseases n = 5) and 50 participants were randomly assigned using a computer-generated random number sequence to either a WBV group (WBV; n = 25) or usual-care control group (CON; n = 25). Randomization was undertaken by a member of the research team not directly involved in the recruitment or assessment of patients. The randomization sequence was not disclosed to the researcher responsible for the day-to-day running of the trial until patients had completed their baseline assessments. All subjects provided informed consent prior to their participation in this study, and the study was approved by the institutional ethics committee and conducted in accordance with the Declaration of Helsinki (2008).
Outcome measures
At baseline and after the 12-week intervention demographic information, clinical details including subject age, weight, height, waist and hip circumference, body composition, duration of T2DM, and vascular function outcomes were collected. Laboratory personnel were blinded to group allocation. Participants were asked not to alter their normal exercise and eating habits during the intervention period (simply instructed to “eat as usual”). In addition, participants were instructed to describe their dietary intake patterns during the previous 3 months.
Ultrasound measurements
Vascular function assessments were conducted in a quiet, temperature-controlled environment. Measurements were performed at the same time of day for individual subjects. Subjects fasted for 8 h, abstained from alcohol and caffeine for 12 h, and did not perform any exercise for 24 h before assessments. Prior to the session, subjects lay in a prone position for a period of 5 min. Following this period, the artery diameter and blood cell velocity outcomes of the common femoral artery of each subjects’ right leg (about 2 cm proximal to the bifurcation of the common femoral artery) were recorded by a pulsed color-coded Doppler ultrasound Scanner (Philips EnVisor; Philips Medical, Andover, MA, USA) with a 5–10 MHz broadband linear array transducer (Philips EnVisor C, L12-3, Andover, MA, USA). All measurements were taken by DG, and during the recording of these variables the investigator was holding the probe positioned parallel to the vessel with an insonation angle of 60° and used the screen of the ultrasound system as feedback to maintain the measure point in the middle of the vessel.
All data were calculated as the average of five consecutive measurements. Blood flow outcomes measured were maximum systolic velocity (SV), maximum diastolic velocity (DV), time averaged mean (TAM), heart rate (HR), pulsatility index (PI), which is the result of [systolic frequency (FS) − diastolic frequency (FD)]/TAM and resistance index (RI) which is the result of (FS − FD)/FS. For blood cell velocity measurements, intensity weighted mean velocity (V med), peak blood velocities (PBV), and acceleration time to peak flow (Tacel) were calculated. Finally, blood flow = V med π(diameter/2)2 × 60 was determined. In order to establish the reliability of the measurement protocol and baseline values, intraclass correlation coefficients (ICC3,1) were calculated for the common femoral artery in two separate visits. These values were found to demonstrate excellent reliability with all ICCs above 0.86 (0.86–0.99; p < 0.001).
Anthropometric and body composition measurements
The height and weight of the subjects were measured and body mass index (BMI) was then calculated by dividing weight (kg) by height squared (m2). Waist circumference was measured at the midpoint between the lower border of the ribs cage and the iliac crest using a non-elastic measuring tape to the nearest 0.1 cm. Bio-electrical impedance was used to determine body composition using Bodystat ©1500 (Bodystat Ltd, Douglas, Isle of Man, UK), which is a four terminal single frequency (500 μA at 50 kHz) analyzer. Resistance and reactance were measured between the right wrist and the right ankle and total body fat, fat-free mass, and their percentages were estimated from the manufacturers’ equations. The device was tested for validity and reliability with results showing good to excellent fat and fat-free mass test–retest reliability within-device and between-device when comparing the Bodystat and hydrodensitometry, dual energy X-ray absorptiometry, and air displacement plethysmography data (Meeuwsen et al. 2010).
Whole body vibration program
All subjects underwent three supervised training sessions per week separated by at least 48 h for 12 weeks. The WBV training included leg exercises standing on a WBV platform (Physio Wave 700, Globus, Italy). A training session consisted of eight different dynamic and static exercises (lunge, step up and down, squat, calf raises, left and right pivot, shoulder abduction with elastic bands, shoulder abduction with elastic bands while squatting, arm swinging with elastic bands; ESM Appendix). During the isometric squat exercise the participants were instructed to stand with bent knees and hips on the platform with a 100° knee flexion (considering 180° as full knee extension). The dynamic exercises were performed with slow movements at a rate of 2 s for both concentric and eccentric phases. The vibration intensity was progressed by increasing the frequency (12–16 Hz) and keeping a peak-to-peak displacement of 4 mm. The duration of the sets was progressively increased (30–60 s) with rest periods of 30 s. The total duration of the training session ranged from 12 to 20 min depending on the phase of periodization of the training program.
Data analysis
The normality of the data was evaluated by the Kolmogorov–Smirnov test, whereas non-parametric distribution, differences between groups at baseline, and after treatment were tested using Mann–Whitney’s. 1,000 bootstrap technique (1,000 replicates) was used for non-parametric analysis. For intra-group comparisons (baseline and after 12 weeks), the Wilcoxon test was used. Whereas parametric distribution and differences between groups at baseline were tested using t test for independent measures, the time effects and the interaction effect (time × group) were tested using repeated measures ANOVA followed by Bonferroni’s post hoc test adjusted for age. Chi-squared analysis was used for categorical variables in any case. Spearman Rho correlations were used to find the relationship between changes in two variables. Values are shown as mean ± SD and statistical significance was set at p ≤ 0.05. All statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). A previous study was used to estimate that ten subjects would enable 80 % power to detect a 13 % increase in V med (Kerschan-Schindl et al. 2001).
Results
The total number of patients who completed the trial was 40 (WBV group n = 20 and CON group n = 20). Five patients in the WBV did not complete the study. One participant dropped out because of work incompatibilities, another four could not participate due to personal reasons. In the CON group, five participants were lost to follow-up. Attendance at the exercise session was at least 80 %. Characteristics of study participants are reported in Table 1.
Table 2 shows the effects of the training program on vascular function in both groups. There were no significant differences in all measurements between CON and WBV groups at baseline. Significant group interactions were found for blood flow (p = 0.026), V med (p = 0.027) and DV (p = 0.017) after the 12-week intervention. Intra-group analysis showed significant differences in V med (p = 0.048), PBV (p = 0.041) and DV (p = 0.045) in the WBV group.
The effect of the exercise program on body composition is reported in Table 3. No significant inter-group differences before the intervention were found. Significant group-by-time interactions in weight (p < 0.001), BMI (p = 0.013), waist to hip ratio (WHR) (p < 0.05), waist circumference (p < 0.001), and percentage of body fat (p < 0.05) were found. Inter-group differences in weight (p < 0.001), WHR (p = 0.052), waist circumference (p < 0.001), and body fat (p = 0.047) were found in the WBV group. Dietary intake and physical activity (self-reported) were not modified during the study. As illustrated in Table 4, Spearman’s correlation coefficients between changes in blood flow and body composition after the 12-week intervention were significant for blood flow, V med, PBV, and PI when correlated with the percentage of body fat and the percentage of fat-free mass. There was no significant association between changes in arterial diameter, SV, DV, or Tacel with changes in the body composition outcomes. When the data were adjusted for age, additional correlation between the changes in RI and changes in both body fat (r 2 = −0.555, p < 0.05; Fig. 1) were found.
Discussion
To our knowledge, this study is the first to determine the impact of WBV training on femoral artery blood flow and body composition in T2DM patients. The primary finding of this study was that a 12-week WBV program can significantly increase leg blood flow velocity and reduce DV and PI in T2DM patients. Moreover, the WBV intervention was effective in reducing weight, waist circumference, WHR and the percentage of body fat. Finally, this study provides evidence that the changes obtained in body composition after the low-frequency vibration program are positively associated with the changes in V med, PBV, PI, or RI, mainly when data were adjusted for age.
The present study investigated the influence of a 3-month vibration-exercise program on parameters of blood flow in T2DM patients. A major finding of the present study is that vibration training lead to an increment in the blood flow of the common femoral artery. To the best of our knowledge, the current study is the first to demonstrate the chronic effect of WBV on blood flow in a muscular artery. In support to our result, Kerschan-Schindl et al. (2001) reported that acute WBV (26 Hz–3 mm) increased blood flow of the popliteal artery. Furthermore, Lythgo et al. (2009) found that the same type of vibration (20–30 Hz) also increased femoral artery blood flow. However, when higher frequencies are assessed (45–60 Hz) no increases in femoral artery blood flow were reported in healthy people (Hazell et al. 2008; Button et al. 2007). One possible explanation to these discrepancies was that participants were seated with knees and ankles at 90°, which suggests that vibration transmission may have been damped, attenuating the effect of WBV on blood flow. Previous studies have shown that passive vibration can acutely increase skin blood flow in the exposed limbs (Kerschan-Schindl et al. 2001; Maloney-Hinds et al. 2009), suggesting that part of the effect of WBV exercise on blood flow is due to vibration. Therefore, the possible mechanisms underlying the vasodilation effects of WBV exercise may be related to an acute increase in the production of vasodilatory substances including metabolites (Herrero et al. 2011a) and nitric oxide (Maloney-Hinds et al. 2009). Another possible explanation was suggested by Lohman et al. (2007) who reported that skin blood flow might also increase due to friction forces applied by the mechanical vibration on the endothelial cells at the cellular level.
Despite this, the evidence in the literature regarding WBV on blood flow is from acute studies and to our knowledge no study has been conducted to analyze the impact of WBV training on arterial function. Only Figueroa et al. (2012) assessed the effectiveness of a 6-week WBV training program on arterial function in young overweight/obese women and showed significant decreases in systemic arterial stiffness (brachial to ankle pulse wave velocity), wave reflection, and systolic blood pressure. Increased pulse wave velocity was positively associated with popliteal artery RI, indicating increased leg artery resistance to blood flow in T2DM patients (Suzuki et al. 2001). In the current study, pulse wave velocity and the augmentation index were not assessed. However, significant improvements in V med and PBV were found in the WBV group. These findings are in accordance with previous studies indicating that V med is increased after acute WBV in different populations (Herrero et al. 2011a, b). Kerschan-Schindl et al. (2001) reported statistically significant increments of the mean speed of blood flow in the popliteal artery in healthy individuals while Herrero et al. (2011b) reported that leg blood flow velocity was increased during the application of WBV in Friedreich’s ataxia patients. The same research group also showed an increase in femoral artery blood flow after three 3-min sets of vibration in patients with paraplegia (Herrero et al. 2011a). The magnitude of blood flow response observed in our study is lower than that in previous studies (Kerschan-Schindl et al. 2001; Lythgo et al. 2009). These greater responses in comparison with our results could be due to the fact that the frequency employed in the current study (12–16 Hz) was lower than the one used previously. In this sense, Lythgo et al. (2009) aimed to investigate the effect of WBV on leg blood flow velocity when delivered at different dosage levels (frequency and amplitude) by a oscillating platform and found an increment close to 80 % in femoral artery mean blood flow velocity (1 min, 30 Hz–4 mm). Moreover, Kerschan-Schindl et al. (2001) found a 46 % increase in mean blood flow velocity in the popliteal artery (9 min, 26 Hz–3 mm). These inconsistencies may be attributable to differences in the populations studied, interventions employed, methods used to measure blood flow, and/or the duration of the intervention. More importantly, as our measurements were recorded at least 24 h after the last bout of WBV, the improved blood flow in the femoral artery may be primarily attributed to endothelial factors and not to contraction-related vasodilatory metabolites.
Another notable finding in our study is the change in adiposity observed after WBV training. The effects of WBV on percent body fat and fat-free mass has not been well documented in the literature, and no-study has been performed in patients with T2DM even though T2DM patients are frequently obese and have abnormal amounts of abdominal fat which may increase the risk of developing metabolic and cardiovascular diseases (Iannucci et al. 2007). In the current study, we found a significant decrease (7.2 %, p < 0.01) in body fat, which is in agreement with a 3.2 % decrease in body fat reported by Fjeldstad et al. (2009) in sedentary post-menopausal women who performed WBV (30/40 Hz–3 mm) combined with resistance training (3 sets 10 repetitions 80 % strength) for 8 months (3 days/week) while Vissers et al. (2010) also demonstrated that adding WBV to a hypocaloric diet lad to a significant decrease in the percentage of body fat after 3 (4.8 %), 6 (7.0 %) and 12 (5.5 %) weeks of WBV training. Recently, Milanese et al. (2012) concluded that an 8-week WBV training is effective in inducing positive body composition changes (fat mass decreased) in women. Finally, we reported that the WBV intervention was effective in reducing waist circumference by 6 cm. This is in agreement with the decrease in 2 cm reported by Song et al. (2011) after 8-week WBV training. This is an important finding as central body fat was reported to the development of insulin resistance and the metabolic syndrome (Piche et al. 2008).
Finally, a major finding of the present study was that changes in blood flow were significantly associated with changes in fat mass. While previous studies indicated that visceral fat is associated with arterial stiffness (Maple-Brown et al. 2005; Snijder et al. 2004), this is the first study to show significant associations between improvements in blood flow and percent fat mass after WBV training. Besides the metabolic action, insulin increases peripheral blood flow through stimulation of nitric oxide production (Steinberg et al. 1994). It is possible that abdominal fat loss may contribute to the improved blood flow by enhancing insulin-mediated vasodilation. Thus, abdominal fat reduction may be the main determinant for the improved insulin sensitivity associated with exercise training in T2DM patients (Bacchi et al. 2012). The findings of the present study may be of clinical importance as increased femoral artery blood flow improves insulin-mediated glucose uptake in the skeletal muscle (Baron et al. 1995), which may explain the beneficial effect of WBV training on glucose control in men with T2DM (Behboudi et al. 2011).
Based on these results, it may be concluded that WBV is an effective method to increase leg blood flow and to improve adiposity in patients with T2DM. In addition, there were several associations between the changes in both outcomes; therefore, WBV training would be an alternative exercise modality for individuals with T2DM.
References
Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, Zoppini G, Cevese A, Bonadonna RC, Schena F, Bonora E, Lanza M, Moghetti P (2012) Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care 35:676–682
Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G (1995) Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Investig 96:786–792
Behboudi L, Azarbayjani MA, Aghaalinejad H, Salavati M (2011) Effects of aerobic exercise and whole body vibration on glycaemia control in type 2 diabetic males. Asian J Sports Med 2:83–90
Bloor CM (2005) Angiogenesis during exercise and training. Angiogenesis 8:263–271
Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM (2007) Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci 62:630–635
Button C, Anderson N, Bradford C, Cotter JD, Ainslie PN (2007) The effect of multidirectional mechanical vibration on peripheral circulation of humans. Clin Physiol Funct Imaging 27:211–216
Chudyk A, Petrella RJ (2011) Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care 34:1228–1237
Dela F, Kjaer M (2006) Resistance training, insulin sensitivity and muscle function in the elderly. Essays Biochem 42:75–88
Devereux GR, Wiles JD, Swaine IL (2010) Reductions in resting blood pressure after 4 weeks of isometric exercise training. Eur J Appl Physiol 109:601–606
Egger A, Niederseer D, Diem G, Finkenzeller T, Ledl-Kurkowski E, Forstner R, Pirich C, Patsch W, Weitgasser R, Niebauer J (2012) Different types of resistance training in patients with type 2 diabetes mellitus: effects on glycemic control, muscle mass and strength. Eur J Prev Cardiol. [Epub ahead of print]
Figueroa A, Gil R, Wong A, Hooshmand S, Park SY, Vicil F, Sanchez-Gonzalez MA (2012) Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res 35:667–672
Fjeldstad C, Palmer IJ, Bemben MG, Bemben DA (2009) Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas 63:79–83
Gerich JE (2007) Type 2 diabetes mellitus is associated with multiple cardiometabolic risk factors. Clin Cornerstone 8:53–68
Hazell TJ, Thomas GW, Deguire JR, Lemon PW (2008) Vertical whole-body vibration does not increase cardiovascular stress to static semi-squat exercise. Eur J Appl Physiol 104:903–908
Herrero AJ, Martín J, Martín T, García-López D, Garatachea N, Jiménez B, Marín PJ (2011a) Whole-body vibration alters blood flow velocity and neuromuscular activity in Friedreich’s ataxia. Clin Physiol Funct Imaging 31:139–144
Herrero AJ, Menendez H, Gil L, Martin J, Martin T, Garcia- Lopez D, Gil-Agudo A, Marín PJ (2011b) Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 49:554–559
Iannucci CV, Capoccia D, Calabria M, Leonetti F (2007) Metabolic syndrome and adipose tissue: new clinical aspects and therapeutic targets. Curr Pharm Des 13:2148–2168
Kerschan-Schindl K, Grampp S, Henk C, Resch H, Preisinger E, Fialka-Moser V, Imhof H (2001) Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol 21:377–382
Lalande S, Gusso S, Hofman PL, Baldi JC (2008) Reduced leg blood flow during submaximal exercise in type 2 diabetes. Med Sci Sports Exerc 40:612–617
Lohman EB 3rd, Petrofsky JS, Maloney-Hinds C, Betts-Schwab H, Thorpe D (2007) The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit 13:CR71–CR76
Lythgo N, Eser P, de Groot P, Galea M (2009) Whole-body vibration dosage alters leg blood flow. Clin Physiol Funct Imaging 29:53–59
Machado A, Garcia-Lopez D, Gonzalez-Gallego J, Garatachea N (2010) Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports 20:200–207
Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA (2009) The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther 11:39–43
Maple-Brown LJ, Piers LS, O’Rourke MF, Celermajer DS, O’Dea K (2005) Central obesity is associated with reduced peripheral wave reflection in Indigenous Australians irrespective of diabetes status. J Hypertens 23:1403–1407
Meeuwsen S, Horgan GW, Elia M (2010) The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin Nutr 29:560–566
Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, Uptergrove GM, Deo SH, Kim A, Kanaley JA, Fadel PJ, Thyfault JP (2011) Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol 111:657–664
Milanese C, Piscitelli F, Simoni C, Pugliarello R, Zancanaro C (2012) Effects of whole-body vibration with or without localized radiofrequency on anthropometry, body composition, and motor performance in young nonobese women. J Altern Complement Med 18:69–75
Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Goto Y, Yoshimasa Y, Miyamoto Y (2010) Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb 17:828–833
Olson TP, Dengel DR, Leon AS, Schmitz KH (2006) Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc 38:1558–1564
Piche ME, Lapointe A, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J, Lemieux S (2008) Regional body fat distribution and metabolic profile in postmenopausal women. Metabolism 57:1101–1107
Regensteiner JG (2004) Type 2 diabetes mellitus and cardiovascular exercise performance. Rev Endocr Metab Disord 5:269–276
Sato Y, Nagasaki M, Kubota M, Uno T, Nakai N (2007) Clinical aspects of physical exercise for diabetes/metabolic syndrome. Diabetes Res Clin Pract 77(Suppl 1):S87–S91
Silber HA, Lima JA, Bluemke DA, Astor BC, Gupta SN, Foo TK, Ouyang P (2007) Arterial reactivity in lower extremities is progressively reduced as cardiovascular risk factors increase: comparison with upper extremities using magnetic resonance imaging. J Am Coll Cardiol 49:939–945
Snijder MB, Henry RM, Visser M, Dekker JM, Seidell JC, Ferreira I, Bouter LM, Yudkin JS, Westerhof N, Stehouwer CD (2004) Regional body composition as a determinant of arterial stiffness in the elderly: the Hoorn Study. J Hypertens 22:2339–2347
Song GE, Kim K, Lee DJ, Joo NS (2011) Whole body vibration effects on body composition in the postmenopausal Korean obese women: pilot study. Korean J Fam Med 32:399–405
Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD (1994) Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Investig 94:1172–1179
Suzuki E, Kashiwagi A, Nishio Y, Egawa K, Shimizu S, Maegawa H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R (2001) Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 24:2107–2114
Terada T, Friesen A, Chahal BS, Bell GJ, McCargar LJ, Boulé NG (2013) Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract 99(2):120–129
Vissers D, Verrijken A, Mertens I, Van Gils C, Van de Sompel A, Truijen S, Van Gaal L (2010) Effect of long-term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts 3:93–100
Acknowledgments
This study was supported by University of Seville.
Conflict if interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Guido Ferretti.
Trial Number: ACTRN12613000021774.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sañudo, B., Alfonso-Rosa, R., del Pozo-Cruz, B. et al. Whole body vibration training improves leg blood flow and adiposity in patients with type 2 diabetes mellitus. Eur J Appl Physiol 113, 2245–2252 (2013). https://doi.org/10.1007/s00421-013-2654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2654-3