Abstract
Loading using variable resistance devices, where the external resistance changes in line with the force:angle relationship, has been shown to cause greater acute neuromuscular fatigue and larger serum hormone responses. This may indicate a greater potential for adaptation during long-term training. Twelve (constant resistance group) and 11 (variable resistance group) men completed 20 weeks of resistance training with 10 men as non-training controls. Training-induced adaptations were assessed by bilateral leg press one repetition maximum, a repetition to failure test using 75 % 1RM, lower limb lean mass and vastus lateralis cross-sectional area. Only the variable resistance training group improved the total number of repetitions (41 ± 46 %) and volume load (52 ± 37 %) during the repetition to failure test (P < 0.05). Similar improvements in maximum strength and hypertrophy of the lower limbs were observed in both training groups. Also, constant and variable resistance 5 × 10RM leg press loadings were performed before and after training in a crossover design. Acute loading-induced responses were assessed by concentric and isometric force, serum hormone concentrations and phosphorylation of intramuscular signalling proteins (0–30 min post-loading). Greater acute decreases in force (P < 0.05–0.01), and greater increases in serum testosterone and cortisol concentration (P < 0.05) and ERK 1/2 phosphorylation (P < 0.05) were observed following variable resistance loadings before and after training. Greater training-induced improvements in fatigue resistance occurred in the variable resistance training group, which may be due to greater acute fatigue and physiological responses during variable versus constant resistance loadings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recreational resistance-trained athletes regularly use resistance machines, which provide either constant or variable external resistance. Devices providing constant resistance maintain the lever arm distance throughout the range of motion, while those providing variable resistance alter the lever arm distance to provide greater resistance during certain phases of the range of motion (Frost et al. 2010). During a multi-joint leg press action, a cam system installed on the device increases the resistance at more extended knee angles (e.g. 120°–180°, Walker et al. 2011a) as maximum leg extension force is greater at these knee angles, as shown by Eloranta and Komi (1980). Previous studies in our laboratory have shown that, during loading, the use of variable resistance produces greater acute neuromuscular fatigue (Häkkinen et al. 1988; Walker et al. 2011b) and serum total testosterone, growth hormone and cortisol responses (Walker et al. 2011b) compared to constant resistance. These results may be due to greater muscle activity at the knee joint angles where greater resistance is produced by the device (Walker et al. 2011a).
It is thought that training adaptations are specific to the causes of fatigue within the neuromuscular system (Campos et al. 2002; Mohr et al. 2007) and are perhaps relative to the magnitude of the acute responses. It may be assumed that variable resistance devices have the potential to provide a greater training stimulus. However, there is currently no consensus regarding the magnitude of training adaptations to constant versus variable resistance training, as both greater (e.g. Anderson et al. 2008) and similar (e.g. Manning et al. 1990) improvements in maximum strength have been observed. One possible reason for the similar training-induced improvements in some studies (e.g. Pipes 1978; Manning et al. 1990) may be that the magnitude of acute fatigue/responses did not differ significantly between the training protocols in these studies. Typical acute responses to medium intensity, high volume resistance loading (referred to as “hypertrophic loading” hereafter) include elevated blood lactate (Dudley 1988) and serum hormone concentrations (Kraemer et al. 1990; Häkkinen and Pakarinen 1993) and activation of intramuscular protein signalling pathways (Coffey et al. 2006; Hulmi et al. 2012).
Serum hormones and intramuscular signalling pathways have been shown to interact (Rommel et al. 2001) and work independently (Hornberger et al. 2004) to cause muscle remodelling and growth. Evaluation of the acute responses of these molecules may give insight into the effectiveness of the resistance loading protocol to induce adaptation, as positive relationships have been observed between training-induced muscle hypertrophy and acute hormone (McCall et al. 1999) and intramuscular signalling (Baar and Esser 1999; Terzis et al. 2008) response. Therefore, it is important to establish whether loading using variable resistance may induce greater acute responses in intramuscular signalling, along with serum hormone concentrations, and consequently whether these greater acute responses accompany greater increases in neuromuscular performance and muscle hypertrophy during training.
The present study is the first to investigate long-term adaptations to constant versus variable resistance through several performance measures and possible mechanisms underpinning these adaptations. The present study aims to: (1) determine whether greater acute neuromuscular fatigue, as well as serum hormone and intramuscular signalling responses occur during variable versus constant resistance hypertrophic loading both before and after a 20-week training period, (2) determine whether a different magnitude of neuromuscular performance and hypertrophy occurs following 20 weeks of variable versus constant resistance hypertrophic training in the lower limbs and (3) whether differences in acute responses may help to explain potential differences in training adaptation.
Methods
Subjects
The present study consisted of two parts: a training study and an acute loading study. Thirty-three young men completed all requirements for the training study, having been randomly selected to one of three groups (constant group: n = 12, age 29 ± 5 years, height 181 ± 6 cm, body mass 79 ± 11 kg; variable group: n = 11, age 27 ± 5 years, height 177 ± 6 cm, body mass 71 ± 8 kg; control group: n = 10, age 30 ± 4 years, height 180 ± 6 cm, body mass 84 ± 14 kg). It was estimated that ten subjects in each training group would provide at least 80 % power compared to control with a significance level of 0.05. None of the subjects had previously taken part in systematic strength training at a frequency greater than once per week, but they were physically active and regularly took part in recreational physical activity (endurance or ball games activity took place no more than three times per week).
From the total group of subjects, a subgroup of volunteers from the variable (n = 7) and constant (n = 7) training groups performed acute loadings before and after the training intervention (age 28 ± 5 years, height 179 ± 6 cm, body mass 76 ± 11 kg). Each subject was carefully informed of all potential risks and discomforts and, thereafter, signed an informed consent document. The study was conducted according to the Declaration of Helsinki, and ethical approval was granted by the ethics committees of the University of Jyväskylä and the Central Hospital of Jyväskylä, Finland.
Training study: resistance training programme
The variable resistance group (n = 11) performed training using cam-based devices for the lower limbs (leg press, knee extension, knee flexion). The constant resistance group (n = 12) performed training with the same devices (David M16, D200 and D300, respectively; David Health Solutions Ltd, Helsinki, Finland), which were modified by replacing the cam with a wheel. Briefly, the cam system increased the resistance, in line with the force:angle relationship, by ~70 % at 120°–180° knee angles in the leg press and by ~30 % at 100°–140° at knee angles in the knee extension exercise compared to the constant resistance device at those knee angles (P < 0.01). Both groups trained the upper limbs and torso with constant resistance devices for the following exercises: bench press, shoulder press, lat pulldown, seated row, bicep curl, triceps pushdown, abdominal crunches and back raises. Training was performed twice per week, with all major muscle groups trained in one session using a combination of eight to nine exercises per session. Lower limb exercises such as leg press, knee extension and knee flexion, were performed before exercises of other muscle groups, and all limb exercises were performed bilaterally. Furthermore, the three lower limb exercises were performed in every training session.
Training was split into two identical 10-week periods to determine whether variable resistance training would provide a greater stimulus for continued adaptation during the period when constant resistance training would likely lead to a plateau in performance development. The subjects performed medium intensity, high volume training consisting of two to three sets and 12–14 repetitions (60–70 % 1RM) per exercise (weeks 1–4), then two to three sets and 10–12 repetitions (70–80 % 1RM) per exercise (weeks 5–7) and three to four sets per exercise and 8–10 repetitions (75–85 % 1RM) per exercise (weeks 8–10). This programme was then repeated during the second 10-week period. One min rest was given between sets during weeks 1–4, and then 2 min rest was given between sets during the remaining weeks 5–10. One set was performed to failure during each training session. Training group subjects were given nutritional counselling during the study, for example to consume ~20 g of protein within 1 h of training and in total ~1.5–1.8 g of protein per kilogram body mass per day, to optimize the muscle hypertrophy response. The control group subjects were instructed to maintain their normal physical activity levels and refrain from resistance training throughout the intervention.
Performance and hypertrophy measurements
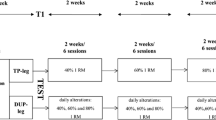
Performance and hypertrophy measurements were performed before, after 10 weeks (“Mid”), and after 20 weeks of training (Fig. 1a). These tests were performed 4–5 days after the last training session. Strength tests included maximum concentric leg press one repetition maximum (1RM) and a repetition to failure test using 75 % 1RM load. These tests were performed using a device (David 210, David Health Solutions Ltd, Helsinki, Finland) that increased resistance by ~6 % between 120° and 180° knee angle, and so more closely matched the force–angle curve of the constant resistance device used in training. Furthermore, each subject’s 10RM was monitored throughout training using the group’s training-specific device.
Body composition was measured by dual-energy X-ray absorptiometry (DXA) (LUNAR Prodigy Advance, GE Medical Systems, Madison, USA) from which the lean mass of the upper and lower limbs are isolated from the trunk and estimated using the software-generated regions (enCORE 2005, version 9.3). Cross-sectional area of the vastus lateralis was assessed by B-mode axial-plane ultrasound using a 10-MHz linear-array probe (60-mm width) with the extended field of view mode (model SSD-α10, Aloka Co Ltd, Japan) at 50 % of femur length (see Ahtiainen et al. 2010 for protocol details). Six images were taken and measured in total, and an average of the four most similar cross-sectional area values was used in further analysis.
Acute loading study: acute loading procedures
Subjects reported to the laboratory at their allocated test time, which was maintained for all loadings to ensure that the time of day did not influence the results (test times were 10 a.m.–4 p.m.). Hypertrophic loadings (5 × 10RM) were performed on two testing days. The subjects from the variable group (n = 7) performed variable resistance loading and then constant resistance loading following 7 days of rest (Fig. 1a). The subjects from the constant group (n = 7) performed loadings in the reverse order. It was estimated that nine subjects would provide at least 80 % power to detect differences between loadings with a significance level of 0.05. This order was maintained after the training intervention. The subjects did not exercise for 48 h before each loading test. To control for the effects of pre-loading nutrition (Hulmi et al. 2005, 2009) and hydration status (Judelson et al. 2007, 2008), subjects fasted for 3 h and consumed 0.5 l of water 1 h before loading. Additionally, dietary intake was recorded in diaries for 1 day before and on the day of the first loading. The subjects were given a photocopy of this record and instructed to replicate their diet as closely as possible before the subsequent loading days.
A specifically tailored David M16 leg press device (David Health Solutions Ltd, Helsinki, Finland) was used during loadings, as well as training (see Walker et al. 2011a for force–angle curves of the constant and “exponential” variable resistance settings). The starting (flexed knee) position was 58° ± 2.3° knee angle (approximate hip angle of 70°). Subjects completed five sets of ten repetitions to failure beginning with 80 % 1RM. If the subjects could complete ten repetitions without the need for assistance, then the load was increased for the next set. If the subjects could not voluntarily complete ten repetitions, some assistance was provided and the load was reduced for the next set. Two minutes rest was given between sets. Subjects were verbally encouraged throughout the loadings and were instructed to maintain a lifting tempo of 2 s concentric and 2 s eccentric contractions with the aid of a metronome. This protocol has been widely used in the literature and can differentiate between loading protocols (Hulmi et al. 2012) and device settings (Walker et al. 2011b). Concentric force was recorded during loading by a force plate installed onto the leg press (Department of Biology of Physical Activity, University of Jyväskylä, Finland).
Isometric leg press tests
Immediately pre-loading (Fig. 1b), subjects performed three maximal bilateral isometric leg extension contractions with the knee flexed to 107° (straight leg = 180°) and a hip angle of 110° using a custom built electromechanical device (Department of Biology of Physical Activity, University of Jyväskylä, Finland). The subjects were instructed to push “as fast and as hard as possible” and maintain their maximum force for approximately 3 s. Post-loading, two maximal isometric leg extension contractions were performed immediately. Isometric leg extension contractions were sampled at 2,000 Hz and filtered by a 10 Hz low-pass filter. A customized script (Signal 4.04, CED, UK) was used to analyse force data from a time window of 500–1,500 ms.
Surface electromyography recording and analysis
Bipolar Ag/AgCl electrodes (10-mm diameter, 20-mm inter-electrode distance, common mode rejection ratio >100 dB, input impedance >100 MΩ, baseline noise <1 μV rms; Department of Biology of Physical Activity, University of Jyväskylä, Finland) were positioned, following shaving and abrasion, on the vastus lateralis (VL), vastus medialis (VM) and biceps femoris (BF) of the right leg according to SENIAM guidelines. Raw signals were passed from a transportable pack to the receiving box (Telemyo 2400R, Noraxon, Scottsdale, USA) and were then relayed to an AD converter (Micro1401, Cambridge Electronic Design, UK) and recorded by Signal 4.04 software (Cambridge Electronic Design, UK). After testing, EMG signals were band-pass filtered (20–350 Hz). Concentric EMG data (rms and median frequency) were assessed between ~60° and 120°. During isometric actions, EMG median frequency data were analysed over the force plateau’s most stable 1 s time window by fast Fourier transformation (Hamming, 2,048 data points).
Muscle biopsies
Muscle biopsies were obtained 30 min pre- and 30 min post-loading (Fig. 1b), previously shown to observe differences in phosphorylation of proteins between loadings (Hulmi et al. 2012). Biopsies were taken from the VL with a 5-mm Bergström biopsy needle together with suction, midway between the patella and greater trochanter. Muscle depth was kept constant through markings on the needle. The pre-loading biopsy was always from the right leg and post-loading biopsy from the left leg. To avoid any residual effects of the previous biopsies, the biopsies during subsequent loadings were obtained ~2 cm from the previous biopsy location. These procedures have been shown not to affect phosphorylation levels in control settings (Hulmi et al. 2009). The muscle sample was cleaned of any visible connective and adipose tissue as well as blood and frozen immediately in liquid nitrogen (−180 °C) and stored at −80 °C. See Hulmi et al. (2009) for tissue processing procedures.
Western immunoblot analyses
Aliquots of muscle lysate, containing 30 μg of total protein, were solubilized in Laemmli sample buffer and heated at 95 °C for 10 min and then separated by sodium dodecyl sulphate polyacrylamide (SDS-PAGE) for 60–90 min at 200 V using 4–20 % gradient gels on Criterion electrophoresis cell (Bio-Rad Laboratories, Richmond, CA, USA). All samples from each subject were run on the same 18-sample gel. Proteins were transferred to microporous polyvinylidene difluoride (PVDF) membranes at 350 mA constant current for 3 h on ice at 4 °C. Membranes were blocked in Tris-buffered saline (TBS) with 0.1 % Tween 20 (TBS-T) containing 5 % non-fat dry milk for 1 h and then incubated overnight at 4 °C with rabbit polyclonal primary antibodies. Antibodies recognized phosphorylated eukaryotic elongation factor 2 (eEF2) at Thr56, protein kinase B (Akt) at Ser473, mechanistic target of rapamycin (mTOR) at Ser2481, 70-kDa ribosomal protein S6 kinase (p70S6K) at Thr389, p38 mitogen-activated protein kinase (MAPK) at Thr180/Tyr182, extracellular signal-regulated kinase (ERK1/2) (p44/p42) at Thr202/Tyr204, mitogen-activated protein kinase activated protein kinase 2 (MAPKAPK-2) at Thr334, and ribosomal protein S6 (rpS6) at both Ser235/236 and at Ser240/244 (Cell Signaling Technology, Beverly, MA, USA). Additionally, total proteins of mTOR, Akt, p70S6K (Santa Cruz Biotechnology, USA), ERK1/2 and rpS6 (Cell Signaling Technology) with respective antibodies were analysed to verify that the total protein content of part of the phosphospecific proteins analysed of signalling proteins does not significantly change during loading or due to training. The uniformity of protein loading was confirmed by staining the membrane with Ponceau S and by re-probing the membrane with an antibody against α-actin (Sigma, St Louis, MO, USA).
All the primary antibodies were diluted 1:2 000 in TBS-T containing 2.5 % non-fat dry milk except against α-actin which was diluted 1:20,000. Membranes were then washed (5 × 5 min) in TBS-T, incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit IgG; Cell Signaling Technology, USA), diluted 1:25,000 in TBS-T with 2.5 % milk for 1 h, followed by washing in TBS-T. Phosphorylated proteins were visualized by ECL according to the manufacturer’s protocol (SuperSignal west femto maximum sensitivity substrate, Pierce Biotechnology, Rockford, USA) and quantified (band intensity × volume) using a ChemiDoc XRS in combination with Quantity One software (version 4.6.3. Bio-Rad Laboratories, USA). Quantification of p38 was based on the average of two visible bands at 42 and 44 kDa, purportedly α/β and γ, respectively, and the 47-kDa band was used to quantify MAPKAPK-2 (Ronkina et al. 2007).
Blood analysis procedures
Venous blood samples (5 ml blood into Venosafe serum tubes; Terumo Medical Co, Leuven, Belgium) were collected pre-, post-, 15 min post-, and 30 min post-loadings (Fig. 1b). Samples were centrifuged for 10 min at 3,500 rpm (Magefuge 1.0R, Heraeus, Germany) to separate the serum. Serum samples were analysed for total testosterone (TT), 22-kDa growth hormone and cortisol by immunometric chemiluminescence techniques using the Immulite 1000 and hormone-specific immunoassay kits (Immulite, Siemens, IL, USA) (see Hulmi et al. 2005 for analytical sensitivity and reliability values). Data presented are uncorrected for plasma volume changes as there were no differences in loading-induced changes between any loadings. Fingertip blood lactate samples (20 μl) were collected immediately pre-, post- and 15 min post-loadings into capillary tubes, which were placed in a 1 ml haemolysing solution and analysed according to the manufacturer’s instructions (EKF diagnostic, C-line system, Biosen, Germany).
Statistical analyses
Conventional statistical methods were used to obtain means, standard deviations, standard error and area under the curve. Volume load during the repetition to failure test was calculated as repetitions × load. Total work during acute loadings was calculated by repetitions × sets × average concentric force. Differences for all dependent variables were assessed by two-way and three-way ANOVA with repeated measures (training variables = group × time; loading variables = group × loading × time), using Bonferroni adjustments as post hoc tests. Relative loading-induced changes were assessed by one-way ANOVA. Growth hormone was not normally distributed and, therefore, was log transformed before applying statistical tests. Significance was defined as P ≤ 0.05.
Results
Training study: training-induced improvements
Significant main effects for time (F = 97, P < 0.001, effect size 0.77) and interaction (F = 8.2, P = 0.001, effect size 0.35) were observed in 1RM performance. Both training groups increased their 1RM performance during the first 10 weeks of training (P < 0.01, Fig. 2a) and this improvement was maintained over the second 10-week period. The magnitude of improvement was greater in both training groups than the control group (P < 0.05). 10RM performance, using the groups’ training-specific device, increased in both training groups and again most of the improvements occurred during the first 10 weeks of training (Fig. 2b).
Mean (±SD) 1RM load (a), 10RM load (b), number of repetitions using 75 %RM (c), volume load during the repetition to failure test (d), lean leg mass (e) and vastus lateralis cross-sectional area (f) before, after 10 weeks (“Mid”) and after 20 weeks of training. *P < 0.05, **P < 0.01 versus before training
A significant main effect for time (F = 10.6, P = 0.003, effect size 0.27) but not interaction (F = 2.8, P = 0.07, effect size 0.16) was observed in repetition to failure performance. Significant improvements in the repetition to failure test were observed only in the variable resistance training group as assessed either by the total number of repetitions (P < 0.05, Fig. 2c) or volume load (P < 0.05, Fig. 2d). Furthermore, the relative changes in the number of repetitions and volume load were significantly greater in the variable compared to the control group (reps 41 ± 46 vs. −4 ± 40 %, volume load 54 ± 39 vs. 1 ± 40 %, P < 0.05). No changes were observed in the constant resistance training group (Fig. 2d).
Significant main effects for time (F = 20.7, P < 0.001, effect size 0.41) and interaction (F = 6.1, P = 0.006, effect size 0.29) were observed in lean mass of the legs and VL cross-sectional area (time: F = 37.6, P < 0.001, effect size 0.56, interaction: F = 4.5, P = 0.019, effect size 0.23). Lean mass of legs (Fig. 2e) and VL cross-sectional area (Fig. 2f) increased in both training groups after training (P < 0.05–0.01), and the relative changes in lean leg mass and VL cross-sectional area in both training groups were greater than in the control groups (P < 0.05).
Acute loading study: overall loading responses before and after training
Results reported here are the combined data for subjects who trained with constant and variable resistance devices (n = 14). Large significant main effects for time (F = 48–887) in all variables, and for interaction in blood lactate (F = 3.3, P = 0.028, effect size 0.16) and in ERK 1/2 phosphorylation (F = 3.4, P = 0.025, effect size 0.18), were observed.
Total work was greater during variable versus constant resistance loading both before (73,674 ± 15,635 vs. 64,578 ± 12,564 N) and after (89,101 ± 18281 vs. 78,310 ± 13,063 N) the training intervention (P < 0.01). The initial 80 % 1RM load during variable resistance loading before training could not be lifted for ten repetitions and so the load (represented by concentric force) was reduced during subsequent sets (P < 0.05, Table 1). Reductions in vastus lateralis EMG median frequency between repetition 2 and 8 occurred in set 3 (57 ± 14 vs. 47 ± 9 Hz, P < 0.05) and set 5 (53 ± 11 vs. 47 ± 6 Hz, P < 0.05) during variable resistance loading before training only.
Large reductions in maximal bilateral isometric leg extension force were observed following all loadings before and after training (P < 0.01). However, following variable resistance loading after the training intervention, reductions were greater than the constant resistance loading after training and variable resistance loading before training (Table 1). Reduced vastus lateralis EMG median frequency occurred following variable resistance loading before and after training only (P < 0.05, Table 1), and their relative changes were significantly different compared to constant resistance loadings (P < 0.05). No changes were observed in vastus medialis or biceps femoris median frequency or EMGrms following any loading.
Loadings caused acute increases in serum TT following variable resistance loading before and after training and constant resistance loading after training only (P < 0.05, Fig. 3a). These elevations dissipated 15 min post-loading, with the exception of variable resistance loading after training (Fig. 3a). The differences in relative changes 15 min post-loading in TT were significant between loadings after the training intervention (P < 0.05, Fig. 3d). Serum cortisol concentrations were increased throughout 30 min post-loading (Fig. 3b). However, area under the curve analysis showed that the cortisol response following variable resistance loading before training was significantly greater than constant resistance loading (P < 0.05, Fig. 3e). Serum growth hormone increased acutely and to a similar magnitude following all loadings (Fig. 3c). Loading-induced blood lactate concentrations were higher (P < 0.05) after the training intervention compared to loadings before training (P < 0.05, Fig. 3f) with no differences between variable versus constant resistance loadings.
Mean (±SD) serum total testosterone (a), cortisol (b), 22-kDa growth hormone (SE bars) (c) and blood lactate (f) concentration during loadings, with relative changes (mean ± SE) in total testosterone (d) and cortisol area under the curve responses (e) in the combined training groups (n = 14). *P < 0.05, **P < 0.01 versus pre-loading. † P < 0.05 versus the same time point before training
Post-loading phosphorylation of p70S6K, rpS6 at both Ser235/236 and at Ser240/244, MAPKAPK-2, and p38 increased following all loadings (Fig. 4). Increased phosphorylation of ERK1/2 occurred before training only, and the level of phosphorylation was greater following variable resistance loading compared to constant resistance loading (P < 0.05, Fig. 4f). The level of phosphorylation of p38 was greater following variable resistance loading before training compared to after training (Fig. 4g). The phosphorylation of Akt decreased (P <0.05–0.01) following both loadings before training and constant resistance loading after training (Fig. 4a). There were no statistically significant changes in phosphorylation of mTOR (Fig. 4b) or eEF2 (data not shown) following any loading. There were no changes in total protein or baseline phosphorylation values, apart from increased ERK 1/2 phosphorylation after the training intervention (P < 0.05, Fig. 4f), during loadings.
Mean (±SE) phosphorylation of Akt at Ser473 (a), mTOR at Ser2481 (b), p70S6K at Thr389 (c), rpS6 at Ser240 (d), rpS6 at Ser235 (e), ERK 1/2 at Thr202 (f), p38 at Thr108 (g) and MAPKAPK-2 at Thr334 (h) in the combined training groups (n = 14). *P < 0.05, **P < 0.01, ***P < 0.001 versus pre-loading. † P < 0.05 versus the same time point before training. Representative western blot images for signalling proteins, phosphorylation sites and α-actin and ponceau staining are shown following the same loading order as the bars in the figures. The two p38 bands (42 and 44 kDa) were averaged. The arrow depicts the MAPKAPK (47 kDa) band that was used for analysis
Training-specific loading responses after training
The results reported here are the data separated for the constant (n = 7) and variable (n = 7) training subjects. Lower reductions in post-loading maximal isometric force (P < 0.05, Fig. 5a) and a lower magnitude of increased TT concentration immediately post-loading (P < 0.05, Fig. 5b) and 15 min post-loading (P < 0.05, Fig. 5b) occurred during constant resistance loading in those subjects who had trained using variable resistance devices. Responses to constant and variable resistance loadings were similar in the group that trained using constant resistance (Fig. 5b).
Discussion
The present study showed greater training-induced adaptations in fatigue resistance, as determined by the repetition to failure test, in the variable resistance training group only. Similar improvements occurred in maximum strength and hypertrophy in both training groups. Indications of greater acute neuromuscular fatigue were observed following variable resistance loadings and were accompanied by greater acute responses in serum TT, cortisol and ERK 1/2 phosphorylation. These findings may be related to greater resistance and more fully activated leg extensors at extended knee angles when performing variable resistance leg press loadings.
Both training groups showed large improvements in strength and muscle hypertrophy over the first 10-week period, which plateaued thereafter. This is typical in subjects unaccustomed to resistance training, although some improvements beyond 10 weeks may have been expected with a more progressive training programme (Häkkinen and Komi 1983). Additionally, acute signalling responses after the training period were somewhat lower compared to before training, namely in rpS6 at Ser235, p38 and ERK 1/2, independent of the type of loading. These blunted acute responses have rarely been studied in humans, but similar patterns in some proteins have been observed (e.g. Wilkinson et al. 2008). Also, it may be that the improvements within the first 10 weeks were so large that there was a need to alter the training programme, for example VL CSA increased by ~17 % which is in the upper range of previous studies (Wernbom et al. 2007). These findings may explain, in part, the observed plateau in strength and hypertrophy in the present study and suggests that additional improvements would have required a change in training programme, perhaps by increasing the training intensity and/or volume.
Regarding variable versus constant resistance training, the results of the present study are in agreement with previous studies that have observed similar increases in maximum strength (Pipes 1978; Manning et al. 1990; Cronin et al. 2003) and muscle hypertrophy (Pipes 1978). Our findings suggest that, in subjects unaccustomed to resistance training, variable resistance devices may provide no further benefit above that achieved through initiating a systematic resistance training programme. It may be that the use of variable resistance does not provide enough additional stimuli to cause a greater magnitude of improved maximum strength in previously untrained individuals. Specifically, force and muscle activation have been shown to be similar between variable versus constant resistance over 60°–120° knee angles in the leg press exercise and only differ from 120° to 180° (Walker et al. 2011a, b). This may explain why variable resistance has been shown to cause greater increases in maximum strength in well-trained athletes (Anderson et al. 2008), where further improvements are elicited through modifying/varying the training stimulus (e.g. Häkkinen and Kallinen 1994; Drinkwater et al. 2005). Alternatively, it is possible that group differences may have been attained if the training programme had varied for the second 10-week period, for example, by increasing intensity and/or volume, as a plateau in 1RM performance and hypertrophy occurred. It should be noted that hypertrophic training does not aim to increase maximum strength per se and that a lower magnitude of maximum strength improvement would be expected compared to higher intensity training programmes. Nevertheless, we observed similar increases in 10RM performance (training-specific strength) in both training groups, suggesting that improvements in strength were similar between the training groups.
A similar magnitude of phosphorylation was observed in proteins of the mTOR pathway, which may have led to similar protein synthesis translation and, ultimately, muscle growth between variable and constant resistance training. Although increased total work has been shown to increase p70S6K and rpS6 (Terzis et al. 2010; Hulmi et al. 2012), the observed responses in the phosphorylation of these and other purportedly anabolic signalling proteins did not reach the level of statistical significance between loadings in the present study. As the magnitude of acute p70S6K and growth hormone did not differ between loadings (neither before or after training) and these responses have been shown to be associated with the magnitude of muscle hypertrophy (Baar and Esser 1999, McCall et al. 1999; Terzis et al. 2008), it is not surprising that hypertrophy was equivalent between the variable and constant resistance training groups.
The novel finding of the present study is that variable resistance training of high volume, medium intensity protocols induced greater adaptations in fatigue resistance. The variable training group could perform more repetitions with 75 % 1RM, thereby increasing their volume load in the repetition to failure test after training (Fig. 2). This finding may be a consequence of greater total work performed during training, as demonstrated by the loading tests, due to greater resistance at more extended knee angles whilst performing the same number of sets and repetitions as the constant group. Hence, it is possible that by inducing greater fatigue during training sessions, the variable resistance groups were exposed to a greater stimulus to adapt fatigue resistance compared to the constant resistance training group.
Further evidence that training with variable resistance improves fatigue resistance was observed when evaluating the training-specific loading after the training period (Fig. 5). The variable resistance group, despite performing ten repetitions to failure during all loadings, demonstrated a lower magnitude of force decrease and a lower response in serum TT when this group performed constant resistance loading after training. Hypertrophic resistance training has been shown to increase glycogen, ATP, phosphocreatine and creatine concentrations (MacDougall et al. 1977), increase mitochondrial enzyme activity (Tang et al. 2006), improve buffering capacity (Tallon et al. 2005), and induce capillarization (Campos et al. 2002), which likely improves the endurance capacity and/or fatigue resistance of the muscle. However, the present study cannot confirm the exact cause of improved fatigue resistance in the variable resistance group, which could be a focus of future studies.
This study was the first to investigate MAPKAPK-2 phosphorylation, a target of p38 (Ronkina et al. 2007), before and after a prolonged resistance training period. The greater phosphorylation of MAPK proteins following variable resistance loading before training, especially in ERK 1/2 and to a certain extent p38 (~100 % greater response n.s.) and MAPKAPK-2 (~30 % greater response n.s.), may indicate physiological mechanisms for greater endurance capacity of the muscle (Nadar and Esser 2001; Gavin et al. 2007; Zhou et al. 2007). This pattern of greater MAPK signalling response may have been due to the greater resistance at more extended knee angles (Wretman et al. 2001; Frey et al. 2009) and concentric muscle activation (shown in Walker et al. 2011a, b), and also related to the greater increases in serum hormone concentrations (e.g. cortisol and TT). Greater resistance at more extended knee angles may also have led to greater muscle damage, which may be reflected in the greater decreases in force and reduced EMG median frequency. After the training period, the pattern of larger MAPK response was not observed and in fact reversed with regard to p38 phosphorylation, perhaps suggesting that the advantage of using variable resistance to develop fatigue resistance properties is realized early in the training period.
Greater increases in serum TT and cortisol were observed following variable resistance loading before the training period (Fig. 3). The present observations support previous findings (Kraemer et al. 1990; Gotshalk et al. 1997) that greater total work during loadings causes a higher response in serum hormones. As cortisol response is thought to reflect a psycho-physiological stress, as well as metabolic demand, it is no surprise that variable resistance induced a greater response compared to constant resistance before training, which accompanied greater acute neuromuscular fatigue as reported by Walker et al. (2011b). A similar acute hormone response following both loadings after the training period may suggest that, although variable resistance induces greater neuromuscular fatigue, trained subjects have an increased tolerance to loading-induced stress that may represent an overall training-induced adaptation.
Conclusions
Maximum strength and lower limb muscle mass increased similarly due to variable and constant resistance hypertrophic training in previously untrained subjects. However, acute variable resistance loadings led to greater neuromuscular fatigue in the leg extensors and greater responses in serum hormone concentrations and MAPK intramuscular signalling. These acute responses may have contributed to the greater fatigue resistance properties observed during the repetition to failure test due to variable resistance training. Therefore, it could be suggested that variable resistance training is an advantageous training strategy if the goal is to improve fatigue resistance and work capacity.
References
Ahtiainen JP, Hoffren M, Hulmi JJ, Pietikäinen M, Mero AA, Avela J, Häkkinen K (2010) Panoramic ultrasonography is a valid method to measure changes in skeletal muscle cross-sectional area. Eur J Appl Physiol 108(2):273–279
Anderson CF, Sforzo GA, Sigg JA (2008) The effects of combining elastic and free weight resistance on strength and power in athletes. J Strength Cond Res 22(2):567–574
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276(1):C120–C127
Campos GER, Luecke TJ, Wendeln HK et al (2002) Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88(1–2):50–60
Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA (2006) Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB 20(1):190–192
Cronin J, McNair PJ, Marshall RN (2003) The effects of bungy weight training on muscle function and functional performance. J Sports Sci 21(1):59–71
Drinkwater EJ, Lawton TW, Lindsell RP, Pyne DB, Hunt PH, McKenna MJ (2005) Training leading to repetition failure enhances bench press strength gains in elite junior athletes. J Strength Cond Res 19(2):382–388
Dudley GA (1988) Metabolic consequences of resistive-type exercise. Med Sci Sports Exerc 20(5 suppl):S158–S161
Eloranta V, Komi PV (1980) Function of the quadriceps femoris muscle under maximal concentric and eccentric contractions. Electromyogr Clin Neurophysiol 20(2):159–174
Frey JW, Farley EE, O’Neil TK, Burkholder TJ, Hornberger TA (2009) Evidence that mechanosensors with distinct biomechanical properties allow for specificity in mechanotransduction. Biophys J 97(1):347–356
Frost DM, Cronin J, Newton RU (2010) A biomechanical evaluation of resistance: fundamental concepts for training and sports performance. Sports Med 40(4):303–326
Gavin TP, Drew JL, Kubic CJ, Pofahl WE, Hickner RC (2007) Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol 191(2):139–146
Gotshalk LA, Loebel CC, Nindl BC, Putukian M, Sebastianelli WJ, Newton RU, Häkkinen K, Kraemer WJ (1997) Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol 22(3):244–255
Häkkinen K, Kallinen M (1994) Distribution of strength training volume into one or two daily sessions and neuromuscular adaptations in female athletes. Electromyogr Clin Neurophysiol 34(2):117–124
Häkkinen K, Komi PV (1983) Electromyographic changes during strength training and detraining. Med Sci Sports Exerc 15(6):455–460
Häkkinen K, Pakarinen A (1993) Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol 74(2):882–887
Häkkinen K, Kauhanen H, Komi PV (1988) Effects of fatiguing loading with a variable resistance equipment on neural activation and force production of the knee extensor muscles. Electromyogr Clin Neurophysiol 28(2–3):79–87
Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA (2004) Mechanical stimuli regulate rapamycin-sensitive signaling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 380(3):795–804
Hulmi JJ, Volek JS, Selänne H, Mero AA (2005) Protein ingestion prior to strength exercise affects blood hormones and metabolism. Med Sci Sports Exerc 37(11):1990–1997
Hulmi JJ, Tannerstedt J, Selänne H, Kainulainen H, Kovanen V, Mero AA (2009) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 106(5):1720–1729
Hulmi JJ, Walker S, Ahtiainen JP, Nyman K, Kraemer WJ, Häkkinen K (2012) Molecular signaling in muscle is affected by the specificity of resistance exercise protocol. Scand J Med Sci Sports 22(2):240–248
Judelson DA, Maresh CM, Farrell MJ et al (2007) Effect of hydration state on strength, power, and resistance exercise performance. Med Sci Sports Exerc 39(10):1817–1824
Judelson DA, Maresh CM, Yamamoto LM et al (2008) Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol 105(3):816–824
Kraemer WJ, Marchitelli L, Gordon SE et al (1990) Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol 69(4):1442–1450
MacDougall JD, Ward GR, Sale DG, Sutton JR (1977) Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol 43(3):700–703
Manning RJ, Graves JE, Carpenter DM, Legget SH, Pollock ML (1990) Constant vs variable resistance knee extension training. Med Sci Sports Exerc 22(3):397–401
McCall GE, Byrnes WC, Fleck SJ, Dickinson A, Kraemer WJ (1999) Acute and chronic hormonal responses to resistance training designed to promote muscle hypertrophy. Can J Appl Physiol 24(1):96–107
Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J (2007) Effect if two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol 292(4):R1594–R1602
Nadar GA, Esser KA (2001) Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90(5):1936–1942
Pipes TV (1978) Variable resistance versus constant resistance strength training in adult males. Eur J Appl Physiol Occup Physiol 39(1):27–35
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3(11):1009–1013
Ronkina N, Kotlyyarov A, Dittrich-Breiholz O et al (2007) The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol Cell Biol 27(1):170–181
Tallon MJ, Harris RC, Boobis LH, Fallowfield JL, Wise JA (2005) The carnosine content of vastus lateralis is elevated in resistance-trained bodybuilders. J Strength Cond Res 19(4):725–729
Tang JE, Hartman JW, Phillips SM (2006) Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab 31(5):495–501
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavoura S, Manta P, Mascher H, Blomstrand E (2008) Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102(2):145–152
Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E (2010) The degree of p70 S6K and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol 110(4):835–843
Walker S, Peltonen H, Avela J, Häkkinen K (2011a) Kinetic and electromyographic analysis of single repetition constant and variable resistance leg press actions. J Electromyogr Kinesiol 21(2):262–269
Walker S, Taipale RS, Nyman K, Kraemer W, Häkkinen K (2011b) Neuromuscular and hormonal responses to constant and variable resistance loadings. Med Sci Sports Exerc 43(1):26–33
Wernbom M, Augustsson J, Thomee R (2007) The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 37(3):225–264
Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586(15):3701–3717
Wretman C, Lionikas A, Widegren U, Lännergren J, Westerblad H, Henriksson J (2001) Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J Physiol 535(1):155–164
Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, Patterson C, Moser M (2007) ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res 76(3):390–399
Acknowledgments
The authors would like to thank Mr. Risto Puurtinen for his assistance during data collection and Miss. Opri Jokelainen for her assistance during western blot analyses. This work was funded in part by the Department of Biology of Physical Activity, University of Jyväskylä and by a personal grant to Simon Walker from the National Doctoral Program of Musculoskeletal Disorders and Biomaterials, Finland. All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Walker, S., Hulmi, J.J., Wernbom, M. et al. Variable resistance training promotes greater fatigue resistance but not hypertrophy versus constant resistance training. Eur J Appl Physiol 113, 2233–2244 (2013). https://doi.org/10.1007/s00421-013-2653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2653-4