Abstract
Our aim was to examine the effect of betaine supplementation on selected circulating hormonal measures and Akt muscle signaling proteins after an acute exercise session. Twelve trained men (age 19.7 ± 1.23 years) underwent 2 weeks of supplementation with either betaine (B) (1.25 g BID) or placebo (P). Following a 2-week washout period, subjects underwent supplementation with the other treatment (B or P). Before and after each 2-week period, subjects performed an acute exercise session (AES). Circulating GH, IGF-1, cortisol, and insulin were measured. Vastus lateralis samples were analyzed for signaling proteins (Akt, p70 S6k, AMPK). B (vs. P) supplementation approached a significant increase in GH (mean ± SD (Area under the curve, AUC), B: 40.72 ± 6.14, P: 38.28 ± 5.54, p = 0.060) and significantly increased IGF-1 (mean ± SD (AUC), B: 106.19 ± 13.45, P: 95.10 ± 14.23, p = 0.010), but significantly decreased cortisol (mean ± SD (AUC), B: 1,079.18 ± 110.02, P: 1,228.53 ± 130.32, p = 0.007). There was no difference in insulin (AUC). B increased resting Total muscle Akt (p = 0.003). B potentiated phosphorylation (relative to P) of Akt (Ser473) and p70 S6 k (Thr389) (p = 0.016 and p = 0.005, respectively). Phosphorylation of AMPK (Thr172) decreased during both treatments (both p = 0.001). Betaine (vs. placebo) supplementation enhanced both the anabolic endocrine profile and the corresponding anabolic signaling environment, suggesting increased protein synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle adaptations to exercise training are specific to the exercises performed and the motor units recruited (Kraemer and Ratamess 2005). The mechanical signal of exercise activates a specific signaling cascade that results in a transient change in gene transcription, which over frequent bouts of exercise will result in increased protein synthesis (Coffey and Hawley 2007). The two ends of the exercise spectrum, resistance training and endurance training, result in different mechanical signals, and thus different molecular activation and overall protein synthesis (Coffey et al. 2009).
Resistance exercise creates an anabolic environment by mediating both hormonal (Kraemer and Ratamess 2005) and molecular signaling cascades (Spiering et al. 2008b). An acute bout of resistance exercise increases growth hormones (GHs), testosterone, Insulin-like Growth Factor 1 (IGF-1), and insulin, where testosterone, IGF-1, and insulin are well established as anabolic hormones (Kraemer and Ratamess 2005). Interestingly, however, although GH has been traditionally accepted as providing a powerful anabolic signal, it may be considered a less assured marker of anabolic activity, as suggested by Doessing et al. (2010) and Liu et al. (2008).
Cumulative messages from GH, IGF-1 (Spiering et al. 2008a), and insulin (Coffey and Hawley 2007) coincide downstream at phosphatidylinositol 3-kinase (PI-3K). The intersection of many upstream signals, PI-3K potentiates an anabolic response through protein kinase B (Akt), which is considered a key regulator of muscle signaling and protein synthesis (Spiering et al. 2008a). Akt phosphorylates and activates the mammalian target of rapamycin (mTOR), which further signals downstream to the 70 kDa ribosomal protein S6 kinase (p70 S6k) to initiate the translation of mRNA and protein synthesis (Hawley 2009; Spiering et al. 2008a, b).
Endurance training adaptations which signal through the adenosine monophosphate-activated kinase (AMPK)/peroxisome-proliferator-activated receptor γ coactivator 1α (PGC-1α) pathway (Kimball 2006) inhibit the mTOR pathway and decrease protein synthesis (Coffey et al. 2006). Cortisol and other glucocorticoids inhibit p70 S6k phosphorylation, a downstream event in mTOR signaling (Shah et al. 2000a, b, 2002). Nutrition and supplementation that enhances anabolic signaling will optimize performance and exercise benefits.
Betaine is a neutral, zwitterionic compound (Lever and Slow 2010) and a methyl derivative of glycine (Craig 2004). Betaine is known to have two major roles in the body, as a methyl group donor and an organic osmolyte. It is found naturally in a variety of food sources including sugar beets, wheat bran, spinach, shrimp, and many others (Craig 2004). Humans also obtain betaine from dietary choline, as the liver and kidney can oxidize choline to betaine in a two-step enzyme-dependent reaction (Craig 2004; Ueland 2011). It is estimated that the average daily intake of betaine is between 100 and 300 mg (Lever and Slow 2010; Ueland 2011), but studies have shown that supplemented dietary intakes of 9–15 g per day are still safe (Craig 2004).
Betaine supplementation has been investigated as a performance aid. Betaine supplementation improves force and power production, number of repetitions (total and those performed at higher intensities), and muscular endurance in trained men (Hoffman et al. 2009; Lee et al. 2010), but mechanisms for betaine ergogenicity have not yet been defined. The hypotheses of increased phosphocreatine stores (Del Favero et al. 2011) or nitric oxide (Bloomer et al. 2011; Trepanowski et al. 2011) for ergogenicity have been investigated, but not supported in recent research.
Betaine is commonly included in animal feed, serving to increase muscle mass and/or decrease fat mass in poultry (Eklund et al. 2005; Zhan et al. 2006) and pigs (Eklund et al. 2005; Matthews et al. 2001a, b). In addition, betaine supplementation has been shown to increase serum GH, IGF-1, and insulin in pigs (QiChun et al. 2006) and serum IGF-1 and gene expression in hen liver tissue (Choe et al. 2010). Increased GH, IGF-1 and insulin are likely to stimulate the Akt/mTOR pathway to promote protein synthesis, but this has not yet been demonstrated with betaine supplementation.
The increase in anabolic hormones and growth factors associated with betaine ingestion has been recognized as the potential mechanism of favorable growth in animal studies (Choe et al. 2010; Huang et al. 2007; QiChun et al. 2006). Thus, the purpose of this study was to examine the effect of betaine supplementation on selected circulating hormonal measures and Akt muscle signaling proteins after an acute exercise session. This is the first study to explore these effects in humans. We hypothesized that betaine supplementation would increase anabolic versus catabolic endocrine signaling following acute exercise bouts performed at maximal intensity.
Methods
Experimental approach
To assess betaine supplementation effects, we performed a randomized, double-blinded crossover study. Subjects underwent 2 weeks of supplementation with either betaine or placebo (randomized order) dissolved in a sports drink (Gatorade, fruit punch flavor, Chicago, Illinois). Following a 14-day washout period, subjects crossed over to the other supplementation condition. Before and after each supplementation period, subjects completed an acute bout of exercise (Acute Exercise Session, AES) prior to collecting hormonal and signaling variables.
Subjects
Twelve recreationally trained men (age 19.7 ± 1.2 years; height 172.6 ± 5.6 cm; weight 84.3 ± 15.3 kg; lean body mass 65.2 ± 8.8 kg; fat mass 15.6 ± 8.5 kg; body fat percentage 18.7 ± 7.0 %; BMI 28.2 ± 4.0) participated in the study. Subjects had been resistance training with the inclusion of the bench press and back squat exercises at least 2–3 times per week for the preceding 3 months, and subjects averaged over 4 years of general resistance training and were familiar with the exercises of the AES. Subjects signed a University Institutional Review Board approved informed consent after receiving verbal and written explanation of the study requirements and associated risks.
Supplement
Subjects consumed either 1.25 g of betaine (DuPont Nutrition & Health, Tarrytown, NY,USA) dissolved in 300 ml of a sports drink (Gatorade, fruit punch flavor) or placebo (300 ml of a sports drink (Gatorade, fruit punch flavor) alone) twice daily for 2 weeks. Following the second AES subjects discontinued supplementation for 2 weeks as a washout period, after which, subjects crossed over to the other supplement condition (betaine or placebo) for 2 weeks. Betaine is fully soluble in our beverage solution and concentration. According to subject inquiry, betaine did not alter the taste of the sports drink, and subjects were not aware of the supplementation condition. This betaine dosing regime has been utilized in previous studies and has been shown to significantly enhance plasma betaine concentrations (Lee et al 2010), despite variable individual dietary betaine intakes. We used this dietary protocol under the assumption that our subjects likewise experienced betaine supplementation above and beyond their individual, varying dietary intakes. No supplement was ingested on the morning of the AES to prevent any confounding results due to acute pre exercise feeding.
Procedures
Experimental controls
For 3 days prior to the first AES, subjects recorded their daily food and beverage intake and replicated this diet before each additional AES. Also during the 3 days preceding an AES, subjects refrained from alcohol, caffeine, and any exhaustive exercise. Subjects maintained a physical activity log throughout the study and standardized their physical activity and exercise between both supplementation phases of the study. In addition, subjects performed a standardized whole-body resistance workout weekly during the study to ensure that their level of conditioning was maintained (Lee et al. 2010). Subjects fasted for 12 h prior to their arrival for the scheduled AES (water intake was allowed). Hydration was measured by urine refractometry (Model A300CL, Spartan, Japan) prior to each AES, and specimen collection and the exercise protocol did not begin until they were euhydrated (urine specific gravity (USG) ≤1.020) to control for any variations due to hydration status. In addition, to control for diurnal variations in hormone concentrations, subjects’ arrival times were between 6:00 and 9:00 a.m, and were consistent for each subject among the AESs.
Prior to the AESs, subjects came to the laboratory for a familiarization visit to allow them to become accustomed to the exercise protocol. At this time the subjects’ height was measured via a tape measure to the nearest 0.1 cm and total body weight was recorded to the nearest 0.1 kg on a digital scale (OHAUS Corp., Fordham Park, NJ, USA). In addition, body composition was determined via Dual Energy X-ray Absorptiometry (DEXA) (Lunar, Prodigy, GE). Following anthropometric assessments, the subjects were taught a standardized light warm-up that was performed prior to each AES. Furthermore, during this visit subjects were familiarized with the specific exercises comprising the AES.
Experimental protocol
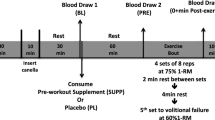
Subjects performed the standardized warm-up and then the AES, consisting of ten maximal vertical jumps without pause, a 10-s isometric squat, a 10-s isometric bench press on a Smith Machine (LifeFitness, Schiller Park, IL, USA), and finally 10 min of repeated box lifting (RBL) (Nindl et al. 1998). As such, the AES was designed to elicit a full body exercise response, requiring both anaerobic and high-intensity aerobic metabolic pathways. Subjects were given an average of 3 min rest between each exercise of the protocol. The RBL consisted of lifting an 18.14 kg metal box with handles on to a 1.32 m high platform from 0.914 m away. Two identical boxes were used and an assistant immediately returned each box to the start position using a slide system, which allowed a box to always be available for the subject to lift. During this 10-min period the subjects lifted the box to the platform as many times as possible. Subjects were instructed to perform maximally during each AES. Thus, the timed portions of the AES may have resulted in different absolute exercise volumes according to individual performance. The context of hormonal responses here is thus in response to a prescribed AES performed at each individual’s respective maximal intensity.
Blood draws
A trained phlebotomist inserted a 21-gauge Teflon cannula into the antecubital vein. The cannula was kept patent with a 10 % heparin/saline solution lock. Prior to each blood draw, 3 ml of blood was extracted and discarded to avoid inadvertent saline dilution of the blood. During each AES, blood was drawn prior to any exercise (Pre), immediately following the isometric back squat (Mid), immediately after completion of the RBL (Post), 5 min (+5) and 15 min (+15) post completion. During each blood draw, 15 ml of blood was collected into appropriate tubes for obtaining plasma and serum. Blood tubes were centrifuged at 3,000 rpm for 15 min at 4 °C and resultant serum and plasma were aliquoted and stored at −80 °C for later analyses.
Muscle biopsies
Following the Pre blood sample, but prior to exercise (Resting) and at 10-min post-exercise (+10 Post Exercise), a physician performed the percutaneous needle biopsy procedure on the subjects’ vastus lateralis muscle using the technique described by Bergstrom (1962) modified with suction to obtain a small (~100 g) muscle sample. Briefly, the skin was cleaned and disinfected using betadine and a local anesthetic (8–10 cc of 1 % Lidocaine) was injected with proper technique to minimize damage to extracted muscle tissue. A small incision (~1 cm) was made (#11 surgical scalpel) and a sterile biopsy needle acquired the biopsy sample via suction. The incision was treated and sutured. The muscle sample obtained was cleared of excess blood and connective tissue, flash frozen in liquid nitrogen, and stored at −80 °C for later analyses.
During each AES, both the Resting and +10 Post Exercise muscle biopsies were obtained from the same leg, approximately 3 cm apart. These separate sites were used to avoid local immune/inflammatory responses that might confound the muscle signaling proteins of interest. For the subsequent AES, the opposite leg was used to allow the maximum recovery time.
Circulating hormone concentrations
Biochemical analyses for circulating GH, IGF-1, cortisol (ALPCO Diagnostics, Salem, NH, USA), and insulin (Calbiotech, Spring Valley, CA,USA) were performed in duplicate via enzyme-linked immunosorbent assays (ELISA). Coefficients of variation for the assays were 7.3, 1.5, 2.8, and 5.5 %, respectively. Serum and plasma samples were thawed only once before analysis. In addition, whole blood plasma samples were analyzed for glucose and lactate concentration [glucose and lactate analyzer (2300 YSI Stat Plus, Yellow Springs, OH, USA)].
Western blot protocol
Muscle samples were homogenized in lysis buffer (Tissue Protein Extraction Reagent (TPER), 10 μl/ml Protease and Phosphatase Inhibitor and 10 μl/ml EDTA Solution (Cat # 78510 and #78444, Thermo Scientific, Rockford, IL, USA)) over liquid nitrogen and centrifuged at 10,000×g (10 min, 4 °C). Supernatants were extracted and a small portion was used to determine total protein concentration via the Bradford assay method (Cat #23236, Thermo Scientific).
Samples (standardized for a total protein concentration of 3 mg/ml in lysis and Laemmli buffer; 30 μg per well) were loaded in duplicate on a 4–15 % Tris–HCl gradient gel (Cat #345-0029, Bio-Rad Laboratories, Hercules, CA, USA) with Precision Plus Kaleidoscope Protein Standards (Cat #161-0375, Bio-Rad Laboratories). Following SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), protein was transferred to PVDF membrane (Cat #162-0177, Bio-Rad Laboratories). Although membranes were not stripped and re-probed for control antibodies, due to concerns that our stripping protocols can result in uneven membrane stripping, non-specific bands in each membrane (data not shown) indicated to us that equal volumes were loaded successfully.
Membranes were incubated with blocking solution for 1 h at room temperature. For measurement of Total Akt, phospho-Akt (Ser473), or Total p70 S6K, the blocking solution was Tris Buffered Saline (TBS) with 0.5 % Tween-20 (Cat # P7949, Sigma-Aldrich, St. Louis, MO, USA) (TBS-T) and 5 % non-fat dry milk (Cat #170-6404, Bio-Rad Laboratories). For phospho-p70 S6K (Thr389) or phospho-AMPK (Thr172), the blocking solution was TBS-T and 5 % bovine serum albumin (BSA) (Cat # A3059, Sigma-Aldrich).
Following blocking, the membrane was rinsed with TBS-T and then incubated overnight at 4 °C with agitation in primary antibodies to Akt Total, phospho-Akt (Ser473), p70 S6k Total, phospho-p70 S6k (Thr389), or phospho-AMPK (Thr172) (Cat #9272, 9271, 9202, 9205, and 2531, respectively, Cell Signaling Technology, Inc., Danvers, MA, USA). All the primary antibodies were rabbit anti-mouse, polyclonal, and were diluted 1:1,000 in 5 % BSA, TBS-T solution. Following the overnight incubation, the membrane was rinsed in TBS-T and incubated for 1 h at room temperature in the secondary antibody (Cat #7074, Goat anti-rabbit IgG HRP linked antibody, Bio-Rad Laboratories) diluted 1:5,000 in a 5 % BSA TBS-T solution. The membrane was rinsed and detection was via West Pico Chemiluminescent Kit (Cat #34080, Thermo Scientific). The visualized bands (Fig. 1) were captured and quantified for densitometric values using a Kodak Imager (Image Station 4000MM Pro, Kodak).
Representative western blot of Total Akt protein in vastus lateralis muscle. The visualized bands from membrane probed for each analyte were captured and quantified for densitometric values using a Kodak Imager. Molecular weight markers were loaded on both sides. The image shows Total Akt protein in the Post Betaine trial at resting (a) and +10 Post Exercise (b), and in the Post Placebo trial at resting (c) and +10 Post Exercise (d)
Statistical analysis
Results were analyzed using a condition × time repeated measures ANOVA. Hormonal results were computed for area under the curve (AUC) using standard trapezoidal methods from each time point during the AES. Muscle signaling results were first log-transformed. LSD Fisher post hoc comparisons were used to determine specific pairwise differences when necessary. Significance was set at p ≤ 0.05 and statistical trends are reported if p ≤ 0.10. All data are presented as mean ± SD, and units of measurement are indicated.
Results
Circulating hormone concentrations
Growth Hormone (GH) approached a significant increase (Fig. 2a) in the Post Betaine trial compared to the Pre Betaine trial and Post Placebo trial (p = 0.089 and p = 0.067, respectively; (μmol L−1AUC), Pre Betaine 38.36 ± 5.59, Post Betaine 40.72 ± 6.14, Pre Placebo 38.88 ± 7.00, Post Placebo 38.28 ± 5.54). IGF-1 significantly increased (Fig. 2b) in the Post Betaine trial compared to the Pre Betaine trial and Post Placebo trial (p = 0.021 and p = 0.010, respectively; (nmol L−1AUC), Pre Betaine 98.89 ± 11.22, Post Betaine 106.19 ± 13.45, Pre Placebo 100.01 ± 7.46, Post Placebo 95.10 ± 14.23). Cortisol significantly decreased (Fig. 2c) in the Post Betaine trial compared to the Pre Betaine trial and Post Placebo trial (p = 0.050 and p = 0.007, respectively; (μmol L−1AUC), Pre Betaine 1,149.64 ± 80.83, Post Betaine 1,079.18 ± 110.02, Pre Placebo 1,200.77 ± 121.49, Post Placebo 1,228.54 ± 130.32). A significant time effect for insulin was seen (Peaked at +15 with a mean concentration of 6.24 ± 3.99 pmol L−1), but there was no difference in insulin between supplementation conditions.
Betaine supplementation promotes an anabolic endocrine milieu. a Betaine supplementation increased circulating Growth Hormone (GH) concentrations at post supplementation, while placebo treatment resulted in no change. b Betaine supplementation significantly increased circulating Insulin-like Growth Factor-1 (IGF-1) concentrations at post supplementation, while placebo treatment resulted in no change. c Betaine supplementation significantly decreased circulating cortisol concentrations at post supplementation, while placebo treatment resulted in no cortisol changes. Bars represent area under the curve (AUC) for hormone concentrations. Asterisk indicates significant differences between pre and post supplementation at p ≤ 0.05. Tristar symbol indicates significant differences between pre and post supplementation at p ≤ 0.10. Cross symbol indicates significant differences between Post Betaine and Post Placebo supplementation at p ≤ 0.05. Filled diamond indicates significant differences between Post Betaine and Post Placebo supplementation at p ≤ 0.10
tained during betaine supplementation
Though there was a significant time effect for lactate and glucose due to exercise (lactate peaked at Post with a mean concentration of 5.82 ± 2.2 mmol L−1 and glucose peaked at +5 with a mean concentration of 5.96 ± 1.08 mmol L−1), there were no differences between supplementation conditions, indicating that both supplementation treatment groups experienced comparable exercise intensities.
Muscle signaling results
There were no significant differences for any of the muscle signaling proteins between Resting and +10 Post Exercise per AES. Resting Akt Total was significantly increased in the Post Betaine trial compared to the Pre Betaine trial and the Post Placebo trial (p = 0.003 and p = 0.040, respectively; Pre Betaine 7.754 ± 0.065, Post Betaine 7.825 ± 0.028, Pre Placebo 7.742 ± 0.065, Post Placebo 7.777 ± 0.049). Akt Total at +10 Post Exercise was significantly increased following both betaine and placebo supplementation (p = 0.017 and p = 0.029, respectively; Pre Betaine 7.765 ± 0.071, Post Betaine 7.821 ± 0.025, Pre Placebo 7.766 ± 0.059, Post Placebo 7.817 ± 0.068).
Resting phosphorylation of Akt (Ser473) was not significantly different (Fig. 3a) among AESs (Pre Betaine 6.812 ± 0.314, Post Betaine 6.738 ± 0.195, Pre Placebo 6.945 ± 0.442, Post Placebo 6.682 ± 0.281). Phosphorylation of Akt (Ser473) at +10 Post Exercise was significantly reduced (Fig. 3b) following placebo supplementation, while Akt phosphorylation was maintained during betaine supplementation (p = 0.016; Pre Betaine 6.919 ± 0.394, Post Betaine 6.756 ± 0.397, Pre Placebo 6.946 ± 0.268, Post Placebo 6.620 ± 0.214).
Betaine supplementation potentiates Akt phosphorylation. a There were no differences in phosphorylation of Akt(Ser473) with betaine or placebo treatments pre exercise. b Betaine supplementation maintained muscular concentrations of phosphorylated Akt(Ser473) while placebo treatment resulted in decreases in phosphorylated Akt(Ser473), at +10 Post Exercise. Bars represent log-transformed (log10) intensity values of densitometry measurements of western blots. Asterisk indicates significant differences between pre and post supplementation at p ≤ 0.05
There were no significant differences in Total p70 S6k Resting (Pre Betaine 7.374 ± 0.278, Post Betaine 7.418 ± 0.141, Pre Placebo 7.421 ± 0.119, Post Placebo 7.428 ± 0.126) or +10 Post Exercise (Pre Betaine 7.418 ± 0.204, Post Betaine 7.481 ± 0.111, Pre Placebo 7.507 ± 0.086, Post Placebo 7.472 ± 0.155). Resting phosphorylation of p70 S6 k (Thr389) was maintained with betaine (Fig. 4a), but decreased during placebo supplementation (p = 0.004; Pre Betaine 6.566 ± 0.260, Post Betaine 6.405 ± 0.168, Pre Placebo 6.675 ± 0.357, Post Placebo 6.360 ± 0.318). Similarly, phosphorylation of p70 S6k (Thr389) at +10 Post Exercise was maintained with betaine (Fig. 4b), but decreased during placebo supplementation (p = 0.005; Pre Betaine 6.614 ± 0.224, Post Betaine 6.538 ± 0.142, Pre Placebo 6.714 ± 0.259, Post Placebo 6.406 ± 0.291).
Betaine supplementation potentiates downstream a signaling target of Akt signaling, p70 S6k. a Betaine supplementation maintained muscular concentrations of phosphorylated downstream mTOR kinase target, p70 S6k(Thr389) whereas placebo treatment resulted in decreases in phosphorylated Akt(Ser473) while resting. b Betaine supplementation maintained muscular concentrations of phosphorylated p70 S6k(Thr389), while placebo treatment resulted in decreases in phosphorylated p70 S6k(Thr389), at +10 Post Exercise. Bars represent log-transformed (log10) intensity values of densitometry measurements of western blots. Asterisk indicates significant differences between pre and post supplementation at p ≤ 0.05
Resting phosphorylation of AMPK (Thr172) decreased following both betaine and placebo supplementation (Fig. 5a) (p = 0.001 and p = 0.031, respectively; Pre Betaine 6.669 ± 0.124, Post Betaine 6.423 ± 0.212, Pre Placebo 6.634 ± 0.205, Post Placebo 6.477 ± 0.174). Phosphorylation of AMPK (Thr172) at +10 Post Exercise decreased following both betaine and placebo supplementation (Fig. 5b) (both p = 0.001; Pre Betaine 6.684 ± 0.168, Post Betaine 6.446 ± 0.142, Pre Placebo 6.729 ± 0.141, Post Placebo 6.482 ± 0.213).
Betaine and placebo treatment with exercise training decrease an inhibitory regulator of Akt signaling, AMPK. a Betaine supplementation and placebo treatment both decreased resting phosphorylated AMPK(Thr172), an inhibitory signal to mTOR. b Betaine supplementation and placebo treatment both decreased phosphorylated AMPK(Thr172), an inhibitory signal to mTOR, at +10 Post Exercise. Bars represent log-transformed (log10) intensity values of densitometry measurements of western blots. Asterisk indicates significant differences between pre and post supplementation at p ≤ 0.05
Discussion
Our aim was to examine the effect of betaine supplementation on selected circulating hormonal measures and Akt muscle signaling proteins after an acute exercise session performed at each subject’s respective maximal intensity. We tested this aim by measuring circulating hormones and phosphorylation of key signaling components in response to an acute bout of exercise with and without betaine supplementation. Based on animal studies (Choe et al. 2010; Eklund et al. 2005; Huang et al. 2007; Matthews et al. 2001a, b; QiChun et al. 2006; Warren et al. 1999) showing enhanced carcass characteristics and anabolic endocrine profiles, we specifically examined signaling pathways by which endocrine controls regulate protein synthesis. Our novel finding is that betaine supplementation supports an anabolic endocrine profile and preservation of this milieu perhaps through phosphorylation of the Akt signaling pathway following acute exercise sessions.
Animal studies (Choe et al. 2010; Huang et al. 2007; QiChun et al. 2006) support that betaine supplementation can increase circulating GH, IGF-1, and insulin. Our findings suggest that betaine increases IGF-1, perhaps GH, but not insulin. IGF-1, a well-characterized anabolic hormone (Adams. 2002), was increased (Fig. 2b) with betaine supplementation. We did not observe increased circulating insulin, but we concede that fasting protocols prior to our AES may have affected insulin values. It is known that fasting decreases circulating insulin concentrations (Boden et al. 1996; Guezennec et al. 1984; Marliss and Vranic 2002), and it is likely that our fasting protocol may have masked any presumed betaine-induced increases. To determine whether this is true, future protocols should include groups that address fasting and diet-related insulin effects in betaine experimental designs.
We observe that GH increases with betaine supplementation compared to placebo approached significance (Fig. 2a). GH is a hormone traditionally touted as crucial to increasing anabolic metabolism (Kraemer et al. 2010), but recent evidence suggests that GH may not be essential to increasing anabolic growth (Doessing et al. 2010; Liu et al. 2008). If betaine is acting to increase an anabolic endocrine milieu, then GH might not be as critical to that response. Also possible is that if GH is as critical to the anabolic response to exercise as previously shown in some studies (Widdowson et al. 2009), betaine supplementation may not have as widespread an anabolic endocrine effect as we presume. Further study is required to investigate this particular question, but in light of the other results, we conclude that in the context of our study design, betaine supplementation did have an overall effect on multiple anabolic signaling components, particularly IGF-1 and downstream elements.
To support our hypothesis that betaine exerts effects through increased anabolism, we observed a significant decrease in a catabolic hormone, cortisol, following 14 days of betaine supplementation (Fig. 2c). The exercise-induced increase in circulating cortisol was attenuated following betaine supplementation. Resting cortisol between treatments did not decrease; however, suggesting that betaine reduces the production and/or release of cortisol during stressful exercise. Further study is required to explain this putative betaine-associated inhibition of cortisol. Evaluating enzymes involved in corticosterone and cortisol conversion in future investigations could clarify the exact mechanism by which betaine may exert effects on cortisol.
Considering only the hormone data, the mechanisms by which betaine may have affected the hormones measured in this study are still unclear and require further research. If betaine supplementation is indeed increasing anabolism and simultaneously inhibiting catabolism of muscle, decreased cortisol during betaine supplementation might permissively increase GH via suppression of GH inhibitory pathways. Cortisol has been shown to blunt GH release in response to growth hormone releasing hormone (GHRH) (Dinan et al. 1994; Sartin et al. 1994; Thompson et al. 1995). Furthermore, corticotropin-releasing hormone (CRH) has also been shown to inhibit GH release stimulated by GHRH, and cortisol may increase somatostatin as another point of inhibition to GH (Barbarino et al. 1990). To our knowledge, no other study has reported both cortisol and GH responses to betaine supplementation. The intricacies of specific mechanisms behind the hormone results we observed need to be further examined. Betaine supplementation effects on hormones could also be evaluated in response to different types of acute exercise bouts, including those at constant volume rather than maximal intensity, as was prescribed in our study, and perhaps with either resistance or aerobic components alone. Our results provide novel human exercise data that support future research into betaine effects on anabolic–catabolic metabolic balance in muscle tissue.
Complementing our data on upstream hormones is our observation that although there were no significant differences in Akt signaling proteins within each AES, there were significant differences following supplementation. The significantly greater Resting Total Akt following betaine supplementation indicates more potential for translation initiation. The significant increase in +10 Post Exercise Total Akt in both conditions may partially be explained by the placebo creating an overall positive energy balance due to increased caloric intake over the supplementation period. More notably, Akt (Ser473) phosphorylation at +10 Post Exercise was maintained only with betaine supplementation (Fig. 3). Studies have shown that resistance training itself decreases Akt (Ser473) phosphorylation (Hulmi et al. 2009), so we surmise that betaine supplementation may negate this typical decrease. Because there are multiple isoforms and phosphorylation sites of Akt, further study would investigate our hypothesis that betaine promotes anabolism through phosphorylation of key Akt signaling molecules, providing a greater stimulus to phosphorylate and consequently activate mTOR.
Our findings with other components of anabolic signaling pathways also support our hypothesis that betaine is affecting anabolic versus catabolic signaling. Maintenance of phosphorylation of p70 S6k (Thr389) at both Resting and +10 Post Exercise with betaine supplementation (Fig. 4) indicates greater translation initiation and protein synthesis. p70 S6k (Thr389) levels vary greatly due to training volume (Terzis et al. 2010), fed state (Wilkinson et al. 2008), type of muscle action (Eliasson et al. 2006), and cortisol inhibition (Shah et al. 2000a). The decreased cortisol levels during the post betaine supplementation trial may have decreased inhibition to allow greater p70 S6k (Thr389) phosphorylation and subsequently translation initiation. During the other trials, the higher levels of cortisol likely inhibited the phosphorylation of p70 S6k (Thr389) to a greater extent.
The RBL provided a partially oxidative component to the AES, so an AMPK response would be expected. Since AMPK is a systemic energy sensor (Kahn et al. 2005), it is regulated by cellular energy state, as well as overall energy balance. Although the placebo (Gatorade) did not contain a large amount of calories, it could have created an overall positive energy balance and decreased AMPK activation in the post supplementation AESs. In addition, a reduction in the subjects’ training volume due to the standardized weekly maintenance workout during the supplementation weeks may have contributed to an overall positive energy balance and decreased AMPK activation in the post supplementation AESs. Studies have shown that decreases in muscle protein synthesis are associated with increases in AMPK activity (Dreyer et al. 2006), so one could infer that the decrease in AMPK (Thr172) during the post supplementation trials would contribute to enhanced protein synthesis during these trials.
An enhancement of protein synthesis could be expected as betaine is an organic osmolyte (a “compensatory” solute) that stabilizes proteins by countering the denaturing effect of perturbing solutes (Craig 2004; Lever and Slow 2010; Ueland 2011). Betaine is highly effective at redistributing cellular water to maintain protein hydration and folding, and increase cytoplasmic osmolarity (Courtenay et al. 2000; Somero and Yancey 2010; Yancey et al. 1982). In particular, betaine improves muscle cell survival against hypertonic stress (Alfieri et al. 2006), prevents ammonia toxicity of neurons (Minana et al. 1996), protects against urea-induced inactivation of muscle myosin ATPases (Ortiz-Costa et al. 2002), and prevents structural changes in myosin due to urea accumulation (Ortiz-Costa et al. 2002) that may lead to inhibition of protein synthesis. Furthermore, an in vitro study showed that the addition of betaine actually increased protein translation initiation and elongation in rabbit reticulocyte lysates under stress (Brigotti et al. 2003). So, it could further be speculated that betaine supplementation could have aided protein synthesis by protecting it from the stress exercise imposes on the muscle cells.
We conclude that betaine (vs. placebo) supplementation enhanced the anabolic endocrine milieu via increased GH and IGF-1 and decreased cortisol following acute exercise. This hormonal environment corresponded with an increased anabolic signaling environment, specifically increased Resting Total Akt, maintenance of Akt (Ser473) and p70 S6k (Thr389) phosphorylation, and decreased inhibition from AMPK (Thr172) phosphorylation, which suggests increased protein synthesis. Our findings suggest that betaine supplementation improves endocrine control of anabolic versus catabolic pathways to enhance anabolic signaling and protein synthesis, in the context of response to an acute bout of resistance and aerobic exercise performed at maximal intensity. The effect of the magnitude of quantitative fold changes we observed should be further examined in studies also assessing functional and/or clinical outcomes. Future studies will interrogate whether betaine supplementation effects on anabolic and catabolic signaling components are consistent in a variety of exercise prescriptions and among more subject sample populations, and what the mechanisms of betaine action might be in providing an ergogenic benefit.
References
Adams GR (2002) Invited review: autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol 93:1159–1167. doi:10.1152/japplphysiol.01264.2001
Alfieri RR, Bonelli MA, Cavazzoni A et al (2006) Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol 576:391–401. doi:10.1113/jphysiol.2006.115006
Barbarino A, Corsello SM, Della Casa S et al (1990) Corticotropin-releasing hormone inhibition of growth hormone-releasing hormone-induced growth hormone release in man. J Clin Endocrinol Metab 71:1368–1374
Bergstrom J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest 68:110–167
Bloomer RJ, Farney TM, Trepanowski JF, McCarthy CG, Canale RE (2011) Effect of betaine supplementation on plasma nitrate/nitrite in exercise-trained men. J Int Soc Sports Nutr 8:5. doi:10.1186/1550-2783-8-5
Boden G, Chen X, Mozzoli M, Ryan I (1996) Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81:3419–3423
Brigotti M, Petronini PG, Carnicelli D et al (2003) Effects of osmolarity, ions and compatible osmolytes on cell-free protein synthesis. Biochem J 369:369–374. doi:10.1042/BJ20021056
Choe HS, Li HL, Park JH, Kang CW, Ryu KS (2010) Effects of dietary betaine on the secretion of insulin-like growth factor-I and insulin-like growth factor binding protein-1 and -3 in laying hens. Asian Australas J Anim Sci 23:379–384
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37:737–763
Coffey VG, Zhong Z, Shield A et al (2006) Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20:190–192. doi:10.1096/fj.05-4809fje
Coffey VG, Pilegaard H, Garnham AP, O’Brien BJ, Hawley JA (2009) Consecutive bouts of diverse contractile activity alter acute responses in human skeletal muscle. J Appl Physiol 106:1187–1197. doi:10.1152/japplphysiol.91221.2008
Courtenay ES, Capp MW, Anderson CF, Record MT Jr (2000) Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 39:4455–4471
Craig SA (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549
Del Favero S, Roschel H, Artioli G et al (2011) Creatine but not betaine supplementation increases muscle phosphorylcreatine content and strength performance. Amino Acids. doi:10.1007/s00726-011-0972-5
Dinan TG, Thakore J, O’Keane V (1994) Lowering cortisol enhances growth hormone response to growth hormone releasing hormone in healthy subjects. Acta Physiol Scand 151:413–416
Doessing S, Heinemeier KM, Holm L et al (2010) Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol 588:341–351. doi:10.1113/jphysiol.2009.179325
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB (2006) Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576:613–624. doi:10.1113/jphysiol.2006.113175
Eklund M, Bauer E, Wamatu J, Mosenthin R (2005) Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev 18:31–48. doi:10.1079/NRR200493
Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E (2006) Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab 291:E1197–E1205. doi:10.1152/ajpendo.00141.2006
Guezennec CY, Serrurier B, Aymonod M, Merino D, Pesquies PC (1984) Metabolic and hormonal response to short term fasting after endurance training in the rat. Horm Metab Res 16:572–575. doi:10.1055/s-2007-1014854
Hawley JA (2009) Molecular responses to strength and endurance training: are they incompatible? Appl Physiol Nutr Metab 34:355–361. doi:10.1139/h09-023
Hoffman JR, Ratamess NA, Kang J, Rashti SL, Faigenbaum AD (2009) Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr 6:7. doi:10.1186/1550-2783-6-7
Huang QC, Xu ZR, Han XY, Li WF (2007) Effect of betaine on growth hormone pulsatile secretion and serum metabolites in finishing pigs. J Anim Physiol Anim Nutr (Berl) 91:85–90. doi:10.1111/j.1439-0396.2006.00644.x
Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA (2009) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 106:1720–1729. doi:10.1152/japplphysiol.00087.2009
Kahn BB, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25. doi:10.1016/j.cmet.2004.12.003
Kimball SR (2006) Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 38:1958–1964. doi:10.1249/01.mss.0000233796.16411.13
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Kraemer WJ, Dunn-Lewis C, Comstock BA, Thomas GA, Clark JE, Nindl BC (2010) Growth hormone, exercise, and athletic performance: a continued evolution of complexity. Curr Sports Med Rep 9:242–252. doi:10.1249/JSR.0b013e3181e976df
Lee EC, Maresh CM, Kraemer WJ et al (2010) Ergogenic effects of betaine supplementation on strength and power performance. J Int Soc Sports Nutr 7:27. doi:10.1186/1550-2783-7-27
Lever M, Slow S (2010) The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem 43:732–744. doi:10.1016/j.clinbiochem.2010.03.009
Liu H, Bravata DM, Olkin I et al (2008) Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med 148:747–758
Marliss EB, Vranic M (2002) Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes 51(Suppl 1):S271–S283
Matthews JO, Southern LL, Bidner TD, Persica MA (2001a) Effects of betaine, pen space, and slaughter handling method on growth performance, carcass traits, and pork quality of finishing barrows. J Anim Sci 79:967–974
Matthews JO, Southern LL, Higbie AD, Persica MA, Bidner TD (2001b) Effects of betaine on growth, carcass characteristics, pork quality, and plasma metabolites of finishing pigs. J Anim Sci 79:722–728
Minana MD, Hermenegildo C, Llsansola M, Montoliu C, Grisolia S, Felipo V (1996) Carnitine and choline derivatives containing a trimethylamine group prevent ammonia toxicity in mice and glutamate toxicity in primary cultures of neurons. J Pharmacol Exp Ther 279:194–199
Nindl BC, Sharp MA, Mello RP, Rice VJ, Murphy MM, Patton JF (1998) Gender comparison of peak oxygen uptake: repetitive box lifting versus treadmill running. Eur J Appl Physiol Occup Physiol 77:112–117
Ortiz-Costa S, Sorenson MM, Sola-Penna M (2002) Counteracting effects of urea and methylamines in function and structure of skeletal muscle myosin. Arch Biochem Biophys 408:272–278
QiChun H, ZiRong X, XinYan H, WeiFen L (2006) Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livest Sci 105:78–85. doi:10.1016/j.livsci.2006.04.031
Sartin JL, Kemppainen RJ, Coleman ES, Steele B, Williams JC (1994) Cortisol inhibition of growth hormone-releasing hormone-stimulated growth hormone release from cultured sheep pituitary cells. J Endocrinol 141:517–525
Shah OJ, Kimball SR, Jefferson LS (2000a) Among translational effectors, p70S6k is uniquely sensitive to inhibition by glucocorticoids. Biochem J 347:389–397
Shah OJ, Kimball SR, Jefferson LS (2000b) Acute attenuation of translation initiation and protein synthesis by glucocorticoids in skeletal muscle. Am J Physiol Endocrinol Metab 278:E76–E82
Shah OJ, Iniguez-Lluhi JA, Romanelli A, Kimball SR, Jefferson LS (2002) The activated glucocorticoid receptor modulates presumptive autoregulation of ribosomal protein S6 protein kinase, p70 S6K. J Biol Chem 277:2525–2533. doi:10.1074/jbc.M105935200
Somero GN, Yancey PH (2010) Osmolytes and cell-volume regulation: physiological and evolutionary principles. In: Anonymous comprehensive physiology. John Wiley & Sons, Inc, New York
Spiering BA, Kraemer WJ, Anderson JM et al (2008a) Effects of elevated circulating hormones on resistance exercise-induced Akt signaling. Med Sci Sports Exerc 40:1039–1048. doi:10.1249/MSS.0b013e31816722bd
Spiering BA, Kraemer WJ, Anderson JM et al (2008b) Resistance exercise biology: manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med 38:527–540
Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E (2010) The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol 110:835–843. doi:10.1007/s00421-010-1527-2
Thompson K, Coleman ES, Hudmon A, Kemppainen RJ, Soyoola EO, Sartin JL (1995) Effects of short-term cortisol infusion on growth hormone-releasing hormone stimulation of growth hormone release in sheep. Am J Vet Res 56:1228–1231
Trepanowski JF, Farney TM, McCarthy CG, Schilling BK, Craig SA, Bloomer RJ (2011) The effects of chronic betaine supplementation on exercise performance. skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J Strength Cond Res. doi:10.1519/JSC.0b013e318217d48d
Ueland PM (2011) Choline and betaine in health and disease. J Inherit Metab Dis 34:3–15. doi:10.1007/s10545-010-9088-4
Warren LK, Lawrence LM, Thompson KN (1999) The influence of betaine on untrained and trained horses exercising to fatigue. J Anim Sci 77:677–684
Widdowson WM, Healy ML, Sonksen PH, Gibney J (2009) The physiology of growth hormone and sport. Growth Horm IGF Res 19:308–319. doi:10.1016/j.ghir.2009.04.023
Wilkinson SB, Phillips SM, Atherton PJ et al (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586:3701–3717. doi:10.1113/jphysiol.2008.153916
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Zhan XA, Li JX, Xu ZR, Zhao RQ (2006) Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br Poult Sci 47:576–580. doi:10.1080/00071660600963438
Acknowledgments
The authors would like to thank the DuPont Nutrition & Health for funding this study. Also, we wish to thank Kathleen N. Beasley, Brittanie M. Volk, Glenn Solomon-Hill, and Dr. Beth Joseph, M.D. for their assistance in data collection and the subjects for their participation.
Conflict of interest
This study was partially funded by DuPont Nutrition & Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Apicella, J.M., Lee, E.C., Bailey, B.L. et al. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur J Appl Physiol 113, 793–802 (2013). https://doi.org/10.1007/s00421-012-2492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2492-8