Abstract
We examined the effect of heat acclimation (HA) on endurance capacity and blood prolactin (PRL) response to moderate intensity exercise in the heat in young male subjects (n = 21). Three exercise tests (ET) were completed on a treadmill: H1 (walk at 60% VO2peak until exhaustion at 42°C), N (walk at 22°C; duration equal to H1) and H2 (walk until exhaustion at 42°C after a 10-day HA program). Heart rate (HR), skin (T sk) and core (T c) temperatures and body heat storage (HS) were measured. Blood samples were taken immediately before, during and immediately after each ET. HA resulted in lower HR, T sk, T c and HS rate (P < 0.05) during ET, whereas endurance capacity increased from 88.6 ± 27.5 min in H1 to 162.0 ± 47.8 min in H2 (P < 0.001). Blood PRL concentration was lower (P < 0.05) during exercise in H2 compared to H1 but the peak PRL level observed at the time of exhaustion did not differ in the two trials. Blood PRL concentration at 60 min of exercise in H1 correlated with time to exhaustion in H1 (r = –0.497, P = 0.020) and H2 (r = –0.528, P = 0.014). In conclusion, HA slows down the increase in blood PRL concentration but does not reduce the peak PRL level occurring at the end of exhausting endurance exercise in the heat. Blood PRL response to exercise in the heat in non-heat-acclimated subjects is associated with their endurance capacity in the heat in a heat-acclimated state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A hot environment may induce remarkable decrements in physical performance and increase the risk of developing serious health problems during exercise (Coris et al. 2004; Nybo 2008; Wendt et al. 2007). Repeated exposure to exercise-heat stress evokes physiological adaptations that improve thermal homeostasis and working capacity, and reduce the risk of heat illness and injury during exercise in a warm environment (Wendt et al. 2007). The major physiological adaptations occurring during heat acclimatisation or acclimation (both are abbreviated as HA in the current paper) are well described (for review, see Sawka et al. 1996) and include, for example, adjustments in plasma and stroke volume, heart rate, cutaneous blood flow, sweat rate, sweat composition and urine electrolyte content.
Data on endocrine aspects of HA are comparatively scarce (Armstrong and Boulant 2006). An increased resting level of aldosterone (Marshall et al. 2007; Nielsen et al. 1993, 1997) and a slower rate of increase in blood concentrations of catecholamines, growth hormone, arginine-vasopressin, aldosterone and renin activity during exercise in the heat (Nielsen et al. 1993) have been observed in heat-acclimated compared to non-acclimated subjects. Cortisol has been used as a hormonal marker of HA (Watkins et al. 2008). However, plasma cortisol response to identical exercise in temperate and warm environments in non-acclimated subjects did not differ, whereas an increase in plasma prolactin (PRL) in the heat exceeded that observed in temperate conditions almost threefold (Bridge et al. 2003a). Therefore, considering PRL is a much more sensitive hormonal marker of acute exercise-heat stress than cortisol (Bridge et al. 2003a), it could also be expected to serve as a more reliable marker of HA status.
Acute exercise may be a potent stimulus for PRL secretion, but the extent of an increase in blood PRL concentration depends on many factors, including the intensity and duration of exercise (for review, see McMurray and Hackney 2000). Low et al. (2005) found that an identical increase in core temperature (T c) induced by cycle exercise or passive heating caused a similar rise in blood PRL concentration in young men. Others have also demonstrated that a rise in T c is an important stimulus for PRL release during exercise showing a linear (Brisson et al. 1986) or curvilinear (Bridge et al. 2003a; Strachan et al. 2004) relationship between blood PRL concentration and T c. These data suggest that an increase in T c is the key stimulus for PRL release during exercise in the heat as well as during passive heat stress.
Nevertheless, ambient temperature has a major effect on modulating blood PRL response to acute exercise (Bridge et al. 2003a). For example, cycling at equal relative intensity induced significantly higher blood PRL concentrations in young men at 35°C compared to 20°C and the between-trial difference in PRL response became evident much earlier than the between-trial difference in T c occurred (Bridge et al. 2003a). At the same time, mean skin temperature (T sk) at 35°C was higher than at 20°C by 3–4°C throughout the exercise period (Bridge et al. 2003a).
The data of Bridge et al. (2003a) suggest that mean T sk, which is influenced by ambient temperature, significantly modulates PRL release during exercise. Furthermore, Mündel et al. (2007) demonstrated that cooling of the face, which only makes up a small part (ca. 10%) of the total skin area, significantly suppresses the rise in blood PRL level during cycling exercise in the heat. Facial T sk seems to be more important in respect of PRL secretion regulation than some other skin areas, as Tyler et al. (2010) found no impact of neck cooling on blood PRL response to exercise in young men in hot conditions.
HA reduces the rate of increase in T c during exercise in the heat (Nielsen et al. 1993). Considering the significant relationship between T c and PRL secretion in the aforementioned studies (Brisson et al. 1986; Strachan et al. 2004), a reduction in PRL response to exercise in the heat could be expected to occur in heat-acclimated compared to non-acclimated subjects. On the other hand, Pitsiladis et al. (2002) demonstrated that similar peak PRL concentrations may occur at different T c at the end of exhausting endurance exercise in the heat. Hence, although the impact of HA on T c is well documented, the effect of HA on PRL response to exercise in the heat cannot be predicted on the basis of changes in T c. According to Armstrong and Boulant (2006) and to the best of our knowledge, no study has investigated the potential effects of HA on PRL response to exercise in the heat. However, such data could contribute to a better understanding of the endocrine aspects of HA. Considering PRL secretion is inhibited by dopamine (Ben-Jonathan and Hnasko 2001) and stimulated by serotonin (Tuomisto 1985) and an increased ratio of the concentration of serotonin to dopamine has been observed in the brain at the time of exhaustion in prolonged exercise (Davis and Bailey 1997), an increase in blood PRL level may reflect changes in brain levels of these neurotransmitters. Both dopamine and serotonin are likely to be involved in the development of centrally mediated fatigue in prolonged exercise (Roelands and Meeusen 2010; Newsholm et al. 1987) and the central component of fatigue is an important factor limiting endurance capacity in the heat (Nybo 2008). Thus, changes in the rate of development of central fatigue apparently contribute to the increased endurance capacity in the heat observed following HA (Wendt et al. 2007) and these changes may be reflected in PRL response to exercise.
Therefore, the primary purpose of the present study was to examine the effect of HA on endurance capacity and blood PRL response to moderate intensity exercise in the heat in previously non-acclimated subjects. We hypothesised that HA would reduce the rate of increase in blood PRL concentration without changing the peak level achieved at the end of exhausting exercise. The secondary purpose of the study was to assess whether inter-individual differences in blood PRL response to moderate intensity exercise were associated with inter-individual variability in endurance capacity in the heat. Physical capability in a hot environment is a particular problem in some situations, such as sports competitions and military operations.
Methods
Subjects
Twenty-two young healthy male military college cadets and university students volunteered to participate in the study, the protocol of which was approved by the Research Ethics Committee of the University of Tartu. All the subjects signed an informed consent form and were examined by a physician prior to participation in any experimental procedure. All of them were non-smokers, they neither had history of heat illness nor had they travelled to a warm climate within at least 2 months before the study. One subject was excluded from the study due to very low exercise tolerance in the heat. Mean (±SD) physical characteristics of the 21 subjects who completed the study protocol were: age 24.9 ± 3.7 years, height 1.83 ± 0.06 m, body mass 80.3 ± 9.4 kg, and peak oxygen uptake (VO2peak) 53.8 ± 7.1 mL kg−1 min−1.
Experimental protocol
The study, which consisted of preliminary proceedings and experimental trials, was carried out in winter–spring period (December–March). The highest average outdoor temperature was registered in March (−1.1°C) and the lowest in January (−12.7°C).
Preliminary proceedings
During the first visit to the laboratory, the subjects were familiarised with the equipment to be affixed to their bodies as well as with all the experimental procedures except the venipuncture. They completed a 30-min treadmill walk in hot–dry environmental conditions (air temperature 42°C, relative humidity, RH, 18%). These measures were undertaken in order to minimise any potential learning and anxiety effects during the main phase of the study.
One week later, anthropometric measures of the subjects were taken and their VO2peak was determined. Body height using Martin’s metal anthropometer, and body mass using an electronic scale (CH3G-150I Combics, Sartorius AG, Goettingen, Germany) were measured to the nearest 0.001 m and 0.001 kg, respectively. VO2peak was determined in a temperate environment using an online breath-by-breath metabolic system (MasterScreen CPX, Viasys Healthcare GmbH, Hoechberg, Germany) and a motorised treadmill (Viasys/Jaeger LE300 C, Viasys Healthcare GmbH, Hoechberg, Germany). After a 10-min warm-up at 5 km h−1 on a level surface, the speed of the treadmill belt was increased to 6 km h−1 and grade to 5%. Thereafter, the speed was kept constant, but the grade was increased by 2.5% every 2 min until self-determined exhaustion. VO2peak was considered to be the mean of the three highest consecutive 15-s recordings at the end of the test. Secondary criteria for achieving VO2peak included respiratory exchange ratio (RER) >1.15 and HR >95% of the subject’s age-predicted maximum.
Experimental trials
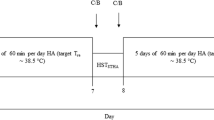
After the preliminary proceedings, the participants were subjected to a battery of physiological and performance tests three times in two environmental conditions. Firstly, a week after the preliminary proceedings, the subjects walked on a treadmill until volitional exhaustion in a hot–dry environment (42°C, RH 18%) at 60% of their predetermined VO2peak (trial H1). Secondly, a week later, they performed a walk of exactly the same duration and intensity in temperate (22°C, RH 35%) environmental conditions (trial N). Thirdly, the 2nd day after completion of a 10-day HA protocol, the subjects walked until volitional exhaustion at the same absolute exercise intensity level in a hot–dry environment (trial H2). All the trials were carried out in a climatic chamber (Design Environmental Ltd., Gwent, South Wales, UK).
To ensure standardisation of nutritional and hydration status and physical activity prior to the trials, the subjects were instructed to record their dietary intake and physical activity during the day before H1 and to replicate this in the day prior to N and H2. They were instructed to refrain from alcohol and strenuous exercise for 24 h and ingesting caffeine 12 h prior to each trial. For purposes of being adequately hydrated, the subjects were instructed to consume an additional 500 mL of water during the previous night and in the morning of each trial day.
On the day of each trial, the subjects reported to the laboratory at 8:00 am after breakfast. They voided and donated a urine sample for specific gravity (USG) measurement, which was performed by means of a digital clinical refractometer (PDX-CL, VeeGee Scientific Inc., Kirkland, WA, USA). The subjects with USG above 1.030 were deemed dehydrated and were instructed to consume an additional 250 mL of water. The subjects’ nude body mass was measured. They dressed in shorts, socks and athletic shoes and positioned a rectal probe (TX-2, Columbus Instruments, Columbus, OH, USA) 10 cm beyond the anal sphincter to enable T c measurement. Skin thermocouples (TX-4, Columbus Instruments, Columbus, OH, USA) were attached to four sites on the left-hand side of the body (chest, upper arm, thigh and calf) for calculation of weighted mean T sk according to Ramanathan (1964). The subjects were also fitted with a heart rate transmitter strap (Suunto Dual Belt, Suunto OY, Finland).
An indwelling venous cannula was inserted into an antecubital vein of right hand, fixed firmly and kept patent with 2–5 mL isotonic saline after insertion and during the test exercise.
To ensure standardisation of physiological parameters, the subjects stood still on the treadmill in the climatic chamber for 20 min prior to the start of the test exercise. The intensity of walking exercise was individually adjusted to 60% of the subject’s personal VO2peak by employing constant speed of 6 km h−1 and regulating the grade of the treadmill in the range of 7–15%. Each subject was instructed to walk until complete exhaustion and given equal verbal encouragement by the same experimenter during trials H1 and H2. The test exercise was terminated earlier if one of the following conditions was observed: (1) a rise of T c to 40°C for 5 min, (2) a rise of HR to 95% of maximal HR for 5 min, (3) occurrence of symptoms of exertional heat illness, such as nausea, dizziness or headache. The subjects were encouraged to drink water ad libitum during the test exercise.
HA protocol
Following one rest day after N, the subjects completed a 10-day HA protocol. Nineteen subjects completed all the sessions within 10 consecutive days. On two occasions, the HA protocol was accomplished within 12 days. Each session comprised walking on a treadmill for two 50-min periods separated by 10 min of rest. Exercise intensity was adjusted individually to 55% of VO2peak during the first 5 days and raised to 60% of VO2peak for the last 5 days of the HA protocol. The intensity of exercise was controlled by changing the grade of the treadmill in the range of 5–15%, whereas speed was kept constant at 6 km h−1. All HA sessions were carried out in the climatic chamber, where the temperature and RH were maintained at 42°C and 18%, respectively. The subjects were allowed to drink water ad libitum, and their HR and T c were monitored throughout each session. The termination criteria were the following: (1) completion of the protocol, (2) a rise of T c to 39.5°C for 5 min, (3) a rise of HR to 95% of maximal HR for 5 min, (4) symptoms of exertional heat illness and (5) a subject’s request to stop.
Measurements
During each experimental trial, HR, T c and T sk were monitored continuously and sampled every 5 min. T c and T sk were recorded using an electronic data logger (Iso-Thermex 256, Columbus Instruments, Columbus, OH, USA) and HR was registered using a telemetry system (Suunto PC POD, Suunto OY, Finland).
Temperature data were used for the calculation of mean body temperature (T b) according to Colin et al. (1971): T b = 0.2T sk + 0.8T re.
Body heat storage (HS) was calculated according to the modified version of the equation of Adams et al. (1992): HS (W m−2) = 0.965 BW ∆T b A −1D , where 0.965 W °C−1 kg−1 is the specific heat constant of body tissues, BW is the subject’s body weight in kg, ∆T b is the change in mean body temperature according to Celsius scale, and A D is the subject’s body surface area in m2 (DuBois and DuBois 1916). HS rate (W m−2 min−1) was calculated by dividing HS by trial duration in minutes.
Ratings of overall perception of exertion (RPE; Borg 1982) and thermal sensation (TS; BSI 2001) were recorded at 15 min intervals throughout each test exercise.
The physiological strain index (PSI) was calculated according to Moran et al. (1998) to provide an objective and comparable indication of heat strain between the trials.
Blood sampling
Blood samples were taken immediately before (Pre), after 30 (Ex30) and 60 (Ex60) min of exercise and at the time of exhaustion in H1 (ExhH1) in all three experimental trials. An additional blood sample (ExhH2) was taken in H2 at the time of exhaustion. Venous blood was collected into sterile Vacutainer tubes (Becton Dickenson & Co., Plymouth, UK) as well as into Vacutainer tubes containing EDTA. The blood from the EDTA tube was used for the measurement of haemoglobin concentration (Dr. Lange cuvette test LKM 143; miniphotometer LP 20 Plus, Dr. Lange, Düsseldorf, Germany) and packed cell volume (by spun haematocrit). The values obtained were used for calculating relative changes in plasma volume (Dill and Costill 1974).
The remaining blood was allowed to clot for 45 min at room temperature and centrifuged for 10 min at 3,000 rpm at 4°C. The separated serum was stored at −25°C until PRL concentration was measured by chemiluminescence immunoassay on IMMULITE 2000 System Analyser (Siemens Medical Solution Diagnostics, Los-Angeles, CA, USA). The intra- and inter-assay coefficients of variance were 3.1 and 4.8%, respectively, with analytical sensitivity of 10.6 mU L−1.
Statistical analysis
The SPSS version 18.0 for Windows software was used for performing statistical analysis. All data were checked for normal distribution using the Kolmogorov–Smirnov test. Two-factor (time × trial) repeated measures ANOVA was used to evaluate the differences within and between the trials. Where a significant main effect was observed, Bonferroni post hoc analysis was used to locate differences between the means. The mean values of different parameters registered at the time of exhaustion in H1 and H2 were compared using Student’s paired t test. A Pearson and Spearman product moment coefficient of correlation (r and ρ, respectively) with alpha level set at 0.05 was applied to determine the relationship between variables. Significance was set at P < 0.05 level. Data are presented as mean ± SD.
Results
Pre-exercise hydration status and endurance capacity
Urine specific gravity (1.0178 ± 0.0080, 1.0208 ± 0.0058 and 1.0171 ± 0.0083 in H1, H2 and N, respectively; P > 0.05) and body mass of the subjects (80.36 ± 9.27 kg in H1, 80.69 ± 9.23 kg in H2 and 80.45 ± 9.39 kg in N; P > 0.05) measured before the exercise test did not differ in the three experimental trials. As a result of the HA programme, time to exhaustion increased from 88.6 ± 27.5 min in H1 to 162.0 ± 47.8 min in H2 (P < 0.001) revealing an improvement in endurance capacity by 85.9 ± 35.2%.
Prolactin response to exercise
Significant main effects of time (F = 54.32, P < 0.001), trial (F = 62.20, P < 0.001) and time by trial interaction (F = 46.59, P < 0.001) were evident for changes in serum PRL concentration (Table 1). Pre-exercise serum PRL concentration did not differ in the three trials. During the exercise test in H1 and H2, a significant increase in serum PRL concentration above the pre-exercise level was found after 60 min of exercise. PRL concentration remained significantly lower in H2 compared to H1 after 60 min of exercise, but reached similar values in H1 and H2 at the time of exhaustion. In N, serum PRL concentration did not change during exercise.
Thermoregulatory and cardiovascular responses
Significant main effects for time (F = 366.92, P < 0.001), trial (F = 38.65, P < 0.001) and time by trial interaction (F = 55.82, P < 0.001) were evident for changes in T c (Fig. 1). Pre-exercise T c was lower in H2 (37.0 ± 0.2°C) compared to H1 (37.2 ± 0.2°C, P = 0.001). T c increased with duration of exercise in both H1 and H2, but remained significantly lower in H2 compared to H1 at all time points after 25 min, except at the time of exhaustion when T c reached the same value (39.7 ± 0.4°C) in both trials carried out in the heat. In N, T c increased slowly from the pre-exercise value of 37.1 ± 0.3 to 38.3 ± 0.5°C (P < 0.001) at the end of the exercise test.
Core temperature during test exercise. Data are presented as mean ± SD. Empty arrow and filled arrow indicate time to exhaustion in H1 and H2, respectively. At the time point of occurrence of exhaustion in H1, core temperature was also recorded in N and H2. Difference between all three trials, *P < 0.05; difference between H1 and N, # P < 0.05; difference between H1 and H2, $ P < 0.05
Pre-exercise mean T sk was similar in H1 and H2 and significantly higher in comparison with N (Table 2). There were significant main effects for time (F = 170.52, P < 0.001), trial (F = 880.97, P < 0.001) and time by trial interaction (F = 9.73, P < 0.001). Mean T sk increased with duration of the exercise both in H1 and H2, but remained significantly lower in H2 compared to H1 after 60 min of exercise. At the time of exhaustion, mean T sk reached similar values in both trials carried out in the heat. In N, mean T sk increased significantly during the initial 30 min of exercise and then remained stable until the end of the test.
Significant main effects for time (F = 137.18, P < 0.001), trial (F = 24.00, P < 0.001) and time by trial interaction (F = 23.59, P < 0.001) were evident for HS rate. The HS rate was similar in the three trials over the initial 30 min of the exercise test (Table 2). In the later stages of exercise, HS rate was significantly lower in N compared to both H1 and H2, and in H2 compared to H1.
Pre-exercise HR in H1 (80.4 ± 6.1 beats min−1) was significantly higher compared to both H2 (75.3 ± 7.4 beats min−1) and N (72.8 ± 7.4 beats min−1) (Fig. 2). Significant main effects for time (F = 823.66, P < 0.001), trial (F = 71.86, P < 0.001) and time by trial interaction (F = 33.07, P < 0.001) were evident for HR. HR increased during the test exercise in all three trials, but remained steadily lower in H2 compared to H1 after the initial 10 min of exercise. A significant difference in HR was also observed at the time of exhaustion (183.0 ± 6.9 beats min−1 in H1 and 176.1 ± 7.3 beats min−1 in H2; P < 0.001).
Heart rate during test exercise. Data are presented as mean ± SD. Empty arrow and filled arrow indicate time to exhaustion in H1 and H2, respectively. At the time point of occurrence of exhaustion in H1, heart rate was also recorded in N and H2. Difference between all three trials, *P < 0.05; difference between H1 and N, # P < 0.05; difference between H1 and H2, $ P < 0.05; difference between H1 and H2 at the time of occurrence of exhaustion, † P < 0.05
An increase in resting plasma volume by 11.1 ± 8.8% was observed as a result of the HA programme.
PSI increased during the exercise test in all three trials (Fig. 3). There were significant main effects for time (F = 501.18, P < 0.001), trial (F = 81.56, P < 0.001) and time by trial interaction (F = 76.11, P < 0.001). After the initial 25 min of exercise, PSI in H1 significantly exceeded that observed in both H2 and N. However, at the time of exhaustion PSI reached similar “very high” values in both trials carried out in the heat (10.6 ± 1.1 in H1 and 10.3 ± 1.0 in H2; P > 0.05).
Physiological strain index during test exercise. Data are presented as mean ± SD. Empty arrow and filled arrow indicate time to exhaustion in H1 and H2, respectively. At the time point of occurrence of exhaustion in H1, PSI was also recorded in N and H2. Difference between all three trials, *P < 0.05; difference between H1 and N, # P < 0.05
Rating of perceived exertion and thermal sensation
There were significant main effects for time (F = 113.38, P < 0.001), trial (F = 52.28, P < 0.001) and time by trial interaction (F = 22.11, P < 0.001) for the changes in RPE. The pre-exercise RPE did not differ between the three trials but the rise in RPE during the exercise test was more rapid in H1 compared to both H2 and N (Fig. 4). However, at the time of exhaustion, a similar level of RPE was reached in H1 (18.5 ± 1.4) and H2 (18.7 ± 1.3, P > 0.05). During the first 45 min of exercise, RPE did not differ between N and H2.
Rating of perceived exertion during test exercise. Data are presented as mean ± SD. Empty arrow and filled arrow indicate time to exhaustion in H1 and H2, respectively. At the time point of occurrence of exhaustion in H1, RPE score was also recorded in N and H2. Difference between all three trials, *P < 0.05; difference between H1 and N, # P < 0.05; difference between H1 and H2, $ P < 0.05
There were significant main effects for time (F = 30.56, P < 0.001), trial (F = 47.50, P < 0.001) and time by trial interaction (F = 4.14, P < 0.001) for the changes in TS. TS remained significantly lower in N compared to H1 and H2 but did not differ between H1 and H2 (Fig. 5).
Perceived thermal sensation during test exercise. Data are presented as mean ± SD (n = 15). Empty arrow and filled arrow indicate time to exhaustion in H1 and H2, respectively. At the time point of occurrence of exhaustion in H1, TS score was also recorded in N and H2. Different from N in both H1 and H2, # P < 0.05
Correlation analysis
A highly significant curvilinear relationship was found between T c and serum PRL concentration in the trials carried out in the heat (Spearman’s rank correlation coefficient ρ = 0.784, P < 0.001) (Fig. 6). A significant negative relationship was observed between serum PRL concentration at 60 min of exercise and the time to exhaustion in H1 (Pearson’s correlation coefficient r = −0.497, P = 0.020) and H2 (r = −0.481, P = 0.027) in our subjects. Moreover, there was a significant negative relationship between serum PRL concentration at 60 min of exercise in H1 and time to exhaustion in H2 (r = −0.528, P = 0.014).
Discussion
Effect of HA on PRL response to moderate intensity exercise in the heat
The main novel finding of this study was that HA slowed down the rate of increase in serum PRL concentration during moderate intensity endurance exercise in the heat, but did not reveal any impact on peak PRL level occurring at the time of exhaustion. Similar observations were reported by Pitsiladis et al. (2002), who found that a high carbohydrate diet significantly improved cycle exercise capacity at 70% of VO2max without any effect on the serum peak PRL concentrations at the time of exhaustion.
T c is known to influence PRL response to exercise and a linear (Brisson et al. 1986) or curvilinear (Bridge et al. 2003a; Strachan et al. 2004) relationship between circulating PRL concentration and T c during low to moderate intensity physical activities in the heat has been reported. In our subjects, HA slowed down the rate of increase of T c during exercise in hot conditions, but did not change the curvilinear pattern of the relationship between T c and blood PRL concentration observed before HA. Mean T sk may significantly modulate PRL release during exercise (Bridge et al. 2003a), but in our subjects, mean T sk changed similar to T c. Thus, changes in the patterns of an increase in both T c and mean T sk may explain the slower rate of increase of PRL during moderate intensity exercise in the heat after HA compared to before HA.
There was no rise in the blood PRL level in our subjects in a normal environment (22°C) during exercise throughout the observation period (88.6 ± 27.5 min). This finding differs from that reported by Bridge et al. (2003a), who found a significant increase in blood PRL concentration in their subjects after 40 min of exercise at an ambient temperature of 20°C. Differences in exercise mode (walking vs. cycling) and intensity (60 vs. 73% VO2max) could have caused the discrepancy in the data in these two studies. Work intensity above the anaerobic threshold may be necessary to elicit substantial increases in blood PRL concentration in a temperate environment (McMurray and Hackney 2000), but the anaerobic threshold was obviously not achieved in our subjects. Furthermore, the secretion of PRL during exercise is stimulated by an increase in T c only above 38.0–38.5°C (Bridge et al. 2003a; Strachan et al. 2004). Our findings support this observation as in N trial T c reached only 38.3°C at the end of exercise and this modest increase in T c was likely too small to stimulate PRL secretion.
Relationship between endurance capacity and PRL response to moderate intensity exercise in the heat
In our subjects, both peak blood PRL concentration and T c occurring at the time of exhaustion before and after HA were similar despite significantly longer exercise time to exhaustion after HA. In the subjects studied by Pitsiladis et al. (2002), the longer time to exhaustion was associated with significantly higher T c at the end of exercise but there was no difference in peak blood PRL level observed at the same time. Although the interventions in these two studies differed (HA vs. dietary manipulation), the comparison of the data suggests that blood PRL response may be even more consistently related to endurance capacity in the heat than the changes in T c. Indeed, blood PRL concentration at 60 min of exercise in H1 was moderately strongly, but persistently (i.e. in both H1 and H2) and inversely related to the time to exhaustion. In general, the subjects who exhibited a comparatively modest blood PRL response to exercise in the heat in a non-heat-acclimated state were able to exercise for the longest duration after HA. Such a relationship was not found between T c and time to exhaustion. Therefore, it seems that blood PRL response to exercise in the heat in non-heat-acclimated subjects may predict their endurance capacity in the heat in a heat-acclimated state in the range of ca. 28%.
Fatigue occurring during exercise and limiting physical performance is a complex phenomenon and involves several factors of both central (i.e. central nervous system related) and peripheral origin. The contribution of central factors appears to become more relevant during prolonged exercise in the heat (Nybo 2008). The findings that HA (current study) and dietary manipulation (Pitsiladis et al. 2002) significantly improved endurance capacity in the heat, whereas exhaustion coincided with similar peak serum PRL concentrations before and after interventions may refer to a link between blood PRL response to exercise and the central component of fatigue. According to the central fatigue hypothesis originally proposed by Newsholm et al. (1987), an increase in serotonin level in the brain contributes to fatigue. Serotonin is known to stimulate PRL secretion (Tuomisto 1985) and therefore, changes in blood PRL may provide a useful marker for changes in central serotonergic activity and central fatigue development during prolonged exercise (Strüder and Weicker 2001). However, provided an increase in central serotonergic activity stimulates PRL secretion during physical activity, administration of selective serotonin reuptake inhibitors should increase blood PRL response to exercise. The results of relevant studies do not support this assumption, because a suppressing impact (Meeusen et al. 2001) or no effect (Roelands et al. 2009; Strachan et al. 2004) of different selective serotonin reuptake inhibitors on PRL secretion during exercise has been reported. On the other hand, Bridge et al. (2003b) demonstrated that besides the serotonergic mechanism, non-serotonergic (most likely dopaminergic) activity plays an important role in regulating plasma PRL concentration. Presently, it is considered likely that central fatigue is determined by the collaboration of different neurotransmitter systems, with dopamine and noradrenaline possibly playing the most important role (Roelands and Meeusen 2010). Thus, the peripheral PRL data reported in the current study and by Pitsiladis et al. (2002) are compatible with the potential role of both serotonin and dopamine in central fatigue. The consistent relationship between blood PRL response to exercise and endurance capacity in the heat is obviously not causal, but the changes in blood PRL level may reflect the changes in the rate of development of central fatigue under the experimental conditions of the current study.
Effect of HA on endurance capacity in the heat
Similar pre-exercise USG and body mass values reveal that our subjects were in a similar hydration status at the beginning of the test exercise in the three trials. Likewise, similar values of PSI and RPE were registered at the time of exhaustion in H1 and H2. These data suggest that the time to exhaustion was measured correctly and the results were not influenced by any between-trial differences in hydration status or the level of motivation of the subjects.
The significant improvement in endurance capacity (ca. 86%) over a rather short period of HA is consistent with the findings of previous studies (Nielsen et al. 1993, 1997; Yamada et al. 2007). Considering the possibility of a small (ca. 5%) increase in VO2max that may occur over 10-day HA (Lorenzo et al. 2010), the improved endurance capacity in the heat observed in our subjects may be based on both the physiological effects of HA and on the training effects of the physical load concomitant with the HA program employed.
HS rate and reaching a critically high T c have been demonstrated to be important factors limiting endurance capacity in the heat (Gonzalez-Alonso et al. 1999; Nielsen et al. 1993). It is currently recognised that during fixed-intensity exercise, thermoregulation mediates voluntary exercise exhaustion upon the attainment of a T c of ca. 40°C, although the absolute temperature at which exhaustion occurs may vary inter-individually in a considerable range (Schlader et al. 2011). Therefore, the significantly lower pre-exercise T c and a reduced HS rate during exercise in H2 compared to H1 can be considered important physiological adaptations induced by the HA program and associated with a remarkable improvement in endurance capacity in the heat in our subjects.
The mean 11% resting plasma volume expansion induced by HA in our subjects was similar to that observed by others (Nielsen et al. 1993; Yamada et al. 2007), but exceeded the 6.5% expansion reported by Lorenzo et al. (2010). Hopper et al. (1988) have demonstrated that plasma volume expansion is associated with increased stroke volume and decreased heart rate during submaximal exercise. Moreover, acute moderate expansion of plasma volume has been shown to slow down the rise in T c and to increase endurance capacity in the heat (Sims et al. 2007). Thus, expansion in plasma volume could be considered a factor that modulated heart rate and T c, and contributed to the improvement in endurance capacity in our subjects.
RPE generally parallels patterns of responses in T c during prolonged exercise (Bridge et al. 2003b; Nybo and Nielsen 2001; Nybo et al. 2003) and HA decreases RPE at fixed intensity of exercise (Weller et al. 2007). The similarity between the patterns of changes in RPE and T c observed in all three trials and the reduction in RPE in H2 compared to H1 in the current study are in accordance with these data. However, RPE in H2 was similar to that observed in N during the first 45 min of exercise, whereas T c remained at the level similar to N only for 30 min revealing that the impact of HA was stronger on RPE in comparison with T c in our subjects.
Both T c and T sk contribute to thermal comfort (Frank et al. 1999). However, in a hot environment, T sk changes more rapidly and to a greater extent. Therefore, warm sensation depends initially on T sk, then on deep body temperature (Parsons 2003). In the present study, both the mean T sk (from 60 min of exercise) and the T c (from 25 min of exercise) were consistently lower in H2 in comparison with H1. Nevertheless, the TS scores revealed that the subjects perceived themselves to feel almost equally warm throughout these two trials. The reason for such a discord between our body temperature and TS data remains obscure, but it seems that any change in TS did not contribute to the increase in endurance capacity observed in our subjects following HA.
Our study was carried out during cold winter–spring time in subjects who have lived in comparatively cool climatic conditions all their life. Therefore, our results show that significant physiological adaptations and improvement in endurance capacity in the heat could successfully be achieved within a short period of time even in subjects who can be considered naturally cold-acclimated persons.
In conclusion, HA slows down the increase in blood PRL concentration but does not reduce the peak PRL level occurring at the end of moderate intensity exhausting endurance exercise in the heat. Blood PRL response to 60 min of moderate intensity exercise in the heat is inversely associated with endurance capacity in the heat in young men in both non-heat-acclimated and acclimated state. This relationship is persistent as it also occurs between PRL response to exercise measured in a non-heat-acclimated state and endurance capacity registered in the same subjects in an acclimated state. Blood PRL can be used as a complementary marker for predicting endurance capacity in the heat together with other physiological characteristics considering that PRL alone explains about 28% of the inter-individual variance in endurance capacity in heat-acclimated subjects in a hot environment.
References
Adams WC, Mack GW, Langhans GW, Nadel ER (1992) Effects of varied air velocity on sweating and evaporative rates during exercise. J Appl Physiol 73:2668–2674
Armstrong LE, Boulant JA (2006) Neuroendocrine influences on temperature regulation in hot environments. In: Kraemer WJ, Rogol AD (eds) The endocrine system in sports and exercise. Blackwell, Malden, pp 466–486
Ben-Jonathan N, Hnasko R (2001) Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763
Borg GAV (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bridge MW, Weller AS, Rayson M, Jones DA (2003a) Ambient temperature and the pituitary hormone response to exercise in humans. Exp Physiol 88:627–635
Bridge MW, Weller AS, Rayson M, Jones DA (2003b) Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol 89:451–459
Brisson GR, Audet A, Ledoux M, Matton P, Pellerin-Massicotte J, Peronnet F (1986) Exercise-induced blood prolactin variations in trained adult males: a thermic stress more than an osmotic stress. Horm Res 23:200–206
BSI (2001) BS EN ISO 10551. Ergonomics of the thermal environment–assessment of the influence of the thermal environment using subjective judgement scales. British Standards Institution. London
Colin J, Timbal J, Houdas Y, Boutelier C, Guieu JD (1971) Computation of mean body temperature from rectal and skin temperatures. J Appl Physiol 31:484–489
Coris EE, Ramirez AM, Van Durme DJ (2004) Heat illness in athletes. The dangerous combination of heat, humidity and exercise. Sports Med 34:9–16
Davis JM, Bailey SP (1997) Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 29:45–57
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
DuBois D, DuBois EF (1916) The measurement of surface area of man. Arch Intern Med 15:868–881
Frank SM, Raja SN, Bulcao CF, Goldstein DS (1999) Relative contribution of core and cutaneous temperatures on thermal comfort and autonomic responses in humans. J Appl Physiol 86:1588–1593
Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B (1999) Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86:1032–1039
Hopper MK, Coggan AR, Coyle EF (1988) Exercise stroke volume relative to plasma-volume expansion. J Appl Physiol 64:404–408
Lorenzo S, Halliwill JR, Sawka MN, Minson CT (2010) Heat acclimation improves exercise performance. J Appl Physiol 109:1140–1147
Low D, Purvis A, Reilly T, Cable NT (2005) The prolactin responses to active and passive heating in man. Exp Physiol 90:909–917
Marshall HC, Campbell SA, Roberts CW, Nimmo MA (2007) Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol 32:341–348
McMurray RG, Hackney AC (2000) Endocrine responses to exercise and training. In: Garrett WE, Kirkendall DT (eds) Exercise and sport science. Lippincott Williams & Wilkins, Philadelphia, pp 135–161
Meeusen R, Piacentini MF, Van Den Eynde S, Magnus L, De Meirleir K (2001) Exercise performance is not influenced by a 5-HT reuptake inhibitor. Int J Sports Med 22:329–336
Moran DS, Shitzer A, Pandolf KB (1998) A physiological strain index to evaluate heat stress. Am J Physiol 275:R129–R134
Mündel T, Bunn SJ, Hooper PL, Jones DA (2007) The effects of face cooling during hyperthermic exercise in man: evidence for an integrated thermal, neuroendocrine and behavioural response. Exp Physiol 92:187–195
Newsholm EA, Acworth IN, Blomstrand E (1987) Amino acids, brain neurotransmitters and a functional link between muscle and brain that is important in sustained exercise. In: Benzi G (ed) Advances in myochemistry. John Libbey, London, pp 127–133
Nielsen B, Hales JRS, Strange S, Christensen NJ, Warberg J, Saltin B (1993) Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460:467–485
Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B (1997) Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflügers Arch Eur J Physiol 434:49–56
Nybo L (2008) Hyperthermia and fatigue. J Appl Physiol 104:871–878
Nybo L, Nielsen B (2001) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 91:2017–2023
Nybo L, Nielsen B, Blomstrand E, Moller K, Secher N (2003) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 95:1125–1131
Parsons K (2003) Human thermal environments, 2nd edn. Taylor & Francis, London
Pitsiladis YP, Strachan AT, Davidson I, Maughan RJ (2002) Hyperprolactinaemia during exercise in the heat: evidence for a centrally mediated component of fatigue in trained cyclists. Exp Physiol 87:215–226
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Roelands B, Meeusen R (2010) Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med 40:229–246
Roelands B, Goekint M, Buyse L, Pauwels F, De Schutter G, Piacentini F, Hasegawa H, Watson P, Meeusen R (2009) Time trial performance in normal and high ambient temperature: is there a role for 5-HT? Eur J Appl Physiol 107:119–126
Sawka MN, Wenger CB, Pandolf KB (1996) Thermoregulatory responses to acute exercise—heat stress and heat acclimation. In: Handbook of physiology environmental physiology. American Physiological Society, Bethesda, pp 157–186
Schlader ZJ, Stannard SR, Mündel T (2011) Exercise and heat stress: performance, fatigue and exhaustion—a hot topic. Br J Sports Med 45:3–5
Sims ST, van Vilet L, Cotter JD, Rehrer NJ (2007) Sodium loading aids fluid balance and reduces physiological strain of trained men exercising in the heat. Med Sci Sports Exerc 39:123–130
Strachan AT, Leiper JB, Maughan RJ (2004) Paroxetine administration to influence human exercise capacity, perceived effort or hormone responses during prolonged exercise in a warm environment. Exp Physiol 89:657–664
Strüder HK, Weicker H (2001) Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part I. Int J Sports Med 22:467–481
Tuomisto J (1985) Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev 37:249–332
Tyler CJT, Wild P, Sunderland C (2010) Practical neck cooling and time-trial running performance in a hot environment. Eur J Appl Physiol 110:1063–1074
Watkins AM, Cheek DJ, Harvey AE, Blair KE, Mitchell JB (2008) Heat acclimation and HSP-72 expression in exercising humans. Int J Sports Med 29:269–276
Weller AS, Linnane DM, Jonkman AG, Daanen HAM (2007) Quantification of the decay and re-induction of heat acclimation in dry-heat following 12 and 26 days without exposure to heat stress. Eur J Appl Physiol 102:57–66
Wendt D, van Loon LJC, van Marken Lichtenbelt WD (2007) Thermoregulation during exercise in the heat. Strategies for maintaining health and performance. Sports Med 37:669–682
Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider S (2007) Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol 103:1196–1204
Acknowledgments
The research project has been supported by the Estonian Ministry of Defence, research Grant No. 456/0709, by the Estonian Ministry of Education and Research, the target-financed theme No. 1787 and by the Estonian Science Foundation, research Grant No. 7395. The authors wish to thank the subjects who participated in this study, and also Merle Havik, Ainika Jakobson, Kalle Karelson, Dr Ahti Varblane and Helen Kaptein for their excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Burk, A., Timpmann, S., Kreegipuu, K. et al. Effects of heat acclimation on endurance capacity and prolactin response to exercise in the heat. Eur J Appl Physiol 112, 4091–4101 (2012). https://doi.org/10.1007/s00421-012-2371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2371-3