Abstract
Exercise-induced arterial hypoxemia (EIAH), characterized by decline in arterial oxyhemoglobin saturation (SaO2), is a common phenomenon in endurance athletes. Acute intensive exercise is associated with the generation of reactive species that may result in redox status disturbances and oxidation of cell macromolecules. The purpose of the present study was to investigate whether EIAH augments oxidative stress as determined in blood plasma and erythrocytes in well-trained male rowers after a 2,000-m rowing ergometer race. Initially, athletes were assigned into either the normoxemic (n = 9, SaO2 >92%, \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \): 62.0 ± 1.9 ml kg−1 min−1) or hypoxemic (n = 12, SaO2 <92%, \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \): 60.5 ± 2.2 ml kg−1 min−1, mean ± SEM) group, following an incremental \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test on a wind resistance braked rowing ergometer. On a separate day the rowers performed a 2,000-m all-out effort on the same rowing ergometer. Following an overnight fast, blood samples were drawn from an antecubital vein before and immediately after the termination of the 2,000-m all-out effort and analyzed for selective oxidative stress markers. In both the normoxemic (SaO2: 94.1 ± 0.9%) and hypoxemic (SaO2: 88.6 ± 2.4%) rowers similar and significant exercise increase in serum thiobarbituric acid-reactive substances, protein carbonyls, catalase and total antioxidant capacity concentration were observed post-2,000 m all-out effort. Exercise significantly increased the oxidized glutathione concentration and decreased the ratio of reduced (GSH)-to-oxidized (GSSG) glutathione in the normoxemic group only, whereas the reduced form of glutathione remained unaffected in either groups. The increased oxidation of GSH to GSSG in erythrocytes of normoxemic individuals suggest that erythrocyte redox status may be affected by the oxygen saturation degree of hemoglobin. Our findings indicate that exercise-induced hypoxemia did not further affect the increased blood oxidative damage of lipids and proteins observed after a 2,000-m rowing ergometer race in highly-trained male rowers. The present data do not support any potential link between exercise-induced hypoxemia, oxidative stress increase and exercise performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxemia is defined as a condition characterized by low oxygen concentration in the arterial blood, whereas hypoxia is the low oxygen availability to the tissues as a result of hypoxemia. The type of hypoxia that is caused by hypoxemia is referred to as hypoxemic hypoxia. Exercise-induced arterial hypoxemia (EIAH) is characterized by decline in the partial pressure of arterial oxygen (PaO2) as well as decline in arterial oxyhemoglobin saturation (SaO2) (Dempsey et al. 2008; Dempsey and Wagner 1999). EIAH is a common phenomenon in athletes with high maximal oxygen consumption (\( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \)) during heavy (Hopkins and McKenzie 1989; Powers et al. 1988) or even submaximal exercise (Durand et al. 2000; Rice et al. 1999). Each step in the transport of O2 from air to cells is considered as a barrier for O2 transport (Nielsen 2003). Among these various steps in the transport chain, the circulation has been considered to limit \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) during whole-body exercise (Nielsen 2003). Indeed, it has been suggested that an exercise-induced reduction in SaO2 to about 92% in highly-trained men was sufficient to cause a measurable decline in \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) (Powers et al. 1989a, b). Such a limitation would be relevant especially during rowing (a whole-body exercise) where the demand for O2 transport becomes extreme (Guenette and Sheel 2007; Nielsen 2003).
Acute intensive exercise is associated with the generation of reactive oxygen and nitrogen species (collectively called reactive species), which may result in redox status disturbances and oxidation of cell macromolecules (Bloomer 2008; Bloomer et al. 2007; Fisher-Wellman and Bloomer 2009; McBride et al. 1998; Nikolaidis et al. 2008, 2011). Environmentally induced hypoxemia (i.e., by exposure to hypobaric environment) can also result in an imbalance between reactive species production and antioxidant defense mechanisms thereby leading to oxidative damage (Bailey et al. 2001; Moller et al. 2001). It has been hypothesized that this increased production of reactive species during hypoxia exposure is, at least partly, the cause of hypoxemia and the resulting decline in physical ability (Guenette and Sheel 2007; Nielsen 2003). There is significant number of studies that have investigated the effect of high altitude-induced hypoxia on oxidative stress at rest and after exercise (Joanny et al. 2001; Pialoux et al. 2009, 2010; Vasankari et al. 1997; Wang et al. 2007; Wang and Lin 2010). Nevertheless, to our knowledge, no study has tested whether EIAH, that is hypoxemia elicited by exercise itself at a sea level, is accompanied by changes in redox status.
The 2,000-m rowing ergometer maximal performance test is one of the most widely utilized protocols for evaluating physical capacity in rowing at a competitive level, because it resembles the conditions of the actual competition in terms of action, duration and intensity (Hagerman 1984; Hagerman et al. 1996). An all-out 2,000-m rowing race simulation test markedly induces blood oxidative stress in highly fit rowers (Kyparos et al. 2009; Skarpanska-Stejnborn et al. 2006; Zembron-Lacny et al. 2006). Furthermore, reductions in PaO2 and SaO2 seem to be consistent findings in response to maximal ergometer rowing (Hanel et al. 1994; Nielsen et al. 1998; Rasmussen et al. 1991). Based on the above, it appears that the 2,000-m rowing ergometer maximal performance is an ideal physiological model for studying the interplay between exercise-induced hypoxemia and exercise-induced oxidative stress.
Therefore, the purpose of the present study was to investigate whether or not EIAH, namely hypoxemia elicited during heavy exercise at a sea level, a common phenomenon in elite endurance athletes, augments oxidative stress in blood plasma and erythrocytes in well-trained male rowers following a 2,000-m rowing ergometer maximal test. The interest for examining redox status in erythrocytes under hypoxemia is the potential influence that redox status exerts on the erythrocyte’s ability to transfer oxygen to tissue.
Materials and methods
Subjects
Twenty-one highly-trained international level male rowers volunteered to participate in this study. All athletes were members of the Greek National Rowing Team and the measurements were carried out during the pre-season preparatory training period. A written informed consent was provided by the athletes after they were fully informed of the nature, potential risks, discomforts and benefits involved in this investigation. All experimental procedures were performed in accordance with the European Union Guidelines laid down in the 1964 Declaration of Helsinki as well as the policy statement of the American College of Sports Medicine on research with human subjects as published in Medicine and Science in Sports and Exercise and were approved by the Institutional Review Board.
Preliminary measurements
Each athlete was tested in two occasions separated by 7–10 days. In the first visit anthropometric parameters were assessed and \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) on a rowing ergometer was determined in the laboratory. Body mass was measured to the nearest 0.1 kg (Beam Balance 710; Seca, Birmingham, UK) with subjects lightly dressed and barefoot. Standing height was measured to the nearest 0.5 cm (Stadiometer 208; Seca, UK). In the second visit rowers reported to the indoor training facility and performed a simulated 2,000-m rowing race on the rowing ergometer. In order to determine oxemia status, arterial oxygen saturation of all rowers was assessed non-invasively by ear oximetry at rest, during the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test on a rowing ergometer as well as during the 2,000-m rowing race. All measurements took place at the sea level in a clean, well-aired and temperature-controlled indoor training facility. Athletes were nonsmokers and advised to abstain from consuming alcohol and caffeine-containing beverages for 24 h and fast overnight before the next morning’s measurements. Rowers were also instructed to avoid taking any vitamin supplements or changing their regular diet the last week before testing. In order to control any potential effect of previous diet on the parameters measured, and ensure that participants of both groups had similar levels of macronutrient and antioxidant intake, swimmers were asked to record their diet for three consecutive days preceding the exercise protocol. Each subject had been provided with a written set of guidelines for monitoring dietary consumption and a record sheet for recording food intake. Dietary records were analyzed by the means of the nutritional analysis system Science Fit Diet 200A (Sciencefit, Athens, Greece).
Determination of maximal oxygen consumption
On the first laboratory visit, a \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test was performed on an air-resistance rowing ergometer (Concept IIc, Morrisville, USA) using the following protocol: the initial exercise load was set at 150 W and it was increased by 50 W every 2 min. Expired gases were measured and analyzed by an automated gas analysis system at 15 s intervals and the higher value recorded within the last minute was used as \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) (Oxycon Pro-Jeager, Wirzburg Germany). The pneumotach of the system was calibrated with a 2-l syringe and the gas analyzers were calibrated with calibration gases prior to the test. Heart rate was continuously monitored via electrocardiography (MedilogAR12, Digital ECG Recorder, Germany). Arterial oxygen saturation was assessed noninvasively by ear oximetry (Nanox2-Medlab, Karsruhe, Germany) at rest and during the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test at 15 s averages. Based on SaO2 measured during the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test, rowers were then categorized into either the normoxemic (SaO2 >92%) or the hypoxemic (SaO2 <92%) group (Powers et al. 1988, 1989a, b). Exercise was terminated when the subjects were no longer able to maintain the required intensity. Blood samples were obtained from fingertips 5 min following the completion of the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test and blood lactate concentration was determined using a portable lactate photometer analyzer (Accusport, Boehringer Mannheim, Germany). To ensure that \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) had been reached, it was required that each subject had met three of the following criteria: (1) a plateau in VO2 against exercise intensity; (2) a respiratory exchange ratio exceeding 1.15; (3) blood lactate concentration exceeding 8 mmol/L; (4) achievement of age-predicted maximal heart rate; and (5) a perceived exertion rate of 19 or 20 (American College of Sports Medicine 2000; Bassett and Howley 2000).
2,000 m rowing test
In the second visit rowers performed a simulated 2,000-m rowing race on the same rowing ergometer. The rowers were fully familiarized with the use of this apparatus. A standardized 5-min warm-up on the rowing ergometer, followed by a 5-min stretching exercises program was performed by the athletes before the maximal 2,000-m maximal rowing test. The subjects were asked to cover the 2,000-m distance on the rowing ergometer in the least time possible. In order to simulate race conditions and enhance motivation, two ergometers were placed side by side so that athletes performed the test in pairs. Rowers were instructed to compete with each other as if they participated in an actual race and they were verbally motivated by assistants to stroke at maximal effort throughout the race. Power and stroke frequency, total freewheel revolutions, and elapsed time were delivered continuously by the computer display of the rowing ergometer. Drag factor was set at 135.
To reconfirm the oxemia status of all rowers, arterial oxygen saturation was again assessed non-invasively by ear oximetry at rest and during the rowing test at 15 s averages as above. Heart rate was continuously monitored via electrocardiography using the same instrument as above. Heart rate before the test and at the completion of the 2,000-m rowing test for each subject was recorded. Time and power average for 2,000 m was also recorded. Blood samples were drawn from fingertip, and the concentration of blood lactate was determined in the fifth min of recovery by using the same analyzer as above. Subjects were supplemented with water (3 ml kg−1 body mass) after the warm-up, as well as after completing the rowing test. During testing the temperature and relative humidity in the indoor facility where the test was performed were kept constant at 21°C and 55%, respectively.
Blood collection and handling
Before exercise and within 5 min after the completion of exercise, a venous blood sample was drawn from the left antecubital vein of the reclining subjects using standard phlebotomy procedures. A portion of blood was collected into tubes containing dipotassium ethylenediamine tetraacetic acid (K2EDTA) as the anticoagulant and used for the determination of hematocrit (Hct) and haemoglobin concentration [Hb]. Whole-blood lysate was produced by adding 5% trichloroacetic acid to whole blood (1:1 vol/vol) collected in K2EDTA tubes, vortexed vigorously, and then centrifuged at 4000g for 10 min at 4°C. The supernatant was removed and centrifuged again at 28,600g for 5 min at 4°C. The latter step was repeated twice. The clear supernatant was then transferred to eppendorf tubes and used for reduced (GSH) and oxidized (GSSG) glutathione determination. Another portion of blood was collected and allocated to serum separation tubes, left on ice for 20 min to clot, and centrifuged at 1500g for 10 min at 4°C. The resultant serum was transferred to eppendorf tubes and used for the determination of thiobarbituric acid-reactive substances (TBARS), protein carbonyls, catalase and total antioxidant capacity (TAC).
Plasma volume
Post-exercise plasma volume changes were calculated on the basis of Hct and [Hb] using the method employed by Dill and Costill (1974). Hematocrit was measured by microcentrifugation, and hemoglobin was measured using a kit from Spinreact (Santa Coloma, Spain).
Biochemical assays
GSH and GSSG were measured according to Reddy et al. (2004) and Tietze (1969), respectively. For GSH, 20 μL of whole blood lysate treated with 5% TCA was mixed with 660 μL of 67 mM sodium–potassium phosphate (pH 8.0) and 330 μL of 1 mM 5,5′-dithiobis-2 nitrobenzoate (DTNB). The samples were incubated in the dark at room temperature for 45 min, and the absorbance was read at 412 nm. GSSG was assayed by treating 50 μL of whole blood lysate with 5% TCA and neutralized up to pH 7.0–7.5 with NaOH. One microliter of 2-vinyl pyridine was added, and the samples were incubated for 2 h at room temperature. Five microliters of whole blood lysate treated with TCA was mixed with 600 μL of 143 mM sodium phosphate (6.3 mM EDTA, pH 7.5), 100 μL of 3 mM NADPH, 100 μL of 10 mM DTNB and 194 μL of distilled water. The samples were incubated for 10 min at room temperature. After the addition of 1 μL of glutathione reductase, the change in absorbance at 412 nm was read for 1 min.
For serum TBARS, a slightly modified assay of Keles et al. (2001) was used. One hundred microliters of serum was mixed with 500 μL of 35% TCA and 500 μL of Tris–HCl (200 mM, pH 7.4) and incubated for 10 min at room temperature. One milliliter of 2 M Na2SO4 and 55 mM thiobarbituric acid solution was added, and the samples were incubated at 95°C for 45 min. The samples were cooled on ice for 5 min and were vortexed after adding 1 mL of 70% TCA. The samples were centrifuged at 15,000g for 3 min at 25°C and the absorbance of the supernatant was read at 530 nm. A baseline shift in absorbance was taken into account by running a blank along with all samples during the measurement.
Serum protein carbonyls were determined based on the method of Patsoukis et al. (2004). Fifty microliters of 20% TCA was added to 50 μL of serum, and this mixture was incubated in an ice bath for 15 min and centrifuged at 15,000g for 5 min at 4°C. The supernatant was discarded and 500 μL of 10 mM 2,4-dinitrophenylhydrazine (in 2.5 N HCl) for the sample, or 500 μL of 2.5 N HCl for the blank, was added in the pellet. The samples were incubated in the dark at room temperature for 1 h, with intermittent vortexing every 15 min, and were centrifuged at 15,000g for 5 min at 4°C. The supernatant was discarded and 1 mL of 10% TCA was added, vortexed, and centrifuged at 15,000g for 5 min at 4°C. The supernatant was discarded and 1 mL of ethanol–ethyl acetate (1:1 vol/vol) was added, vortexed, and centrifuged at 15,000g for 5 min at 4°C. This washing step was repeated twice. The supernatant was discarded and 1 mL of 5 M urea (pH 2.3) was added, vortexed, and incubated at 37°C for 15 min. The samples were centrifuged at 15,000g for 3 min at 4°C and the absorbance was read at 375 nm. Total serum protein was assayed using a Bradford reagent from Sigma-Aldrich.
Catalase activity was determined using the method of Aebi (1984). According to this protocol, 2,975 μL of 67 mM sodium–potassium phosphate (pH 7.4) were added to 20 μL of serum and the samples were incubated at 37°C for 10 min. Five microliters of 30% hydrogen peroxide were added to the samples, and the change in absorbance was immediately read at 240 nm for 2 min.
The determination of TAC was based on the method of Janaszewska and Bartosz (2002). Four hundred and eighty microliters of 10 mM sodium–potassium phosphate (pH 7.4) and 500 μL of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical were added to 20 μL of serum, and the samples were incubated in the dark for 60 min at room temperature. The samples were centrifuged for 3 min at 20,000g and the absorbance was read at 520 nm.
Each assay was performed in duplicates on the same day to minimize variation in assay conditions and within a month of the blood collection. Blood samples (serum and whole blood lysate) were stored in multiple aliquots at −80°C and thawed only once before analysis. The intra- and inter-assay coefficient of variation (CV) for each measurement were 4.3 and 4.6% for GSH, 3.0 and 4.3% for GSSG, 5.6 and 8.9% for TBARS, 2.3 and 6.8% for protein carbonyls, 4.2 and 6.8% for catalase, 2.2 and 5.3% for TAC, 2.1 and 4.1% for lactate, respectively. All reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Statistical analysis
Data were analyzed using SPSS version 14 (SPSS, Chicago, IL, USA) and presented as mean ± SEM. The distribution of all dependent variables was examined by the Shapiro–Wilk test, and was found not to differ significantly from normal values. Any potential differences in anthropometric and physiological characteristics between normoxemic and hypoxemic groups were determined using an independent Student’s t test. To evaluate any differences in the blood redox status markers within (pre- vs. post-exercise) and between (normoxemic vs. hypoxemic) groups, a 2 × 2 repeated measure on time analysis of variance (ANOVA) followed by simple main effect analysis was applied. The test–retest reliability of arterial oxygen saturation was determined by performing the intra-class reliability test. The intra-class correlation coefficient was calculated through a random-effect two-way ANOVA model from the SaO2 values obtained from five separate occasions in twelve of the participants of this study. The level of statistical significance was set at α = 0.05.
Results
Anthropometry, physiology and arterial oxygen saturation
In preliminary measurements, twelve of our subjects (6 classified as normoxemic and 6 classified as hypoxemic) were tested for arterial oxygen saturation in five separate occasions during a near-maximal and maximal rowing exercise. It was observed that all subjects in all five occasions were consistent to their either normoxemic or hypoxemic response detected during exercise. The intraclass correlation coefficient test (measured in 12 subjects on 5 separate occasions) was 0.98, denoting very high test–retest reliability of arterial oxygen saturation. This is in agreement with the statement of Powers et al. (1988) that exercise induced hypoxemia is highly reproducible among highly trained endurance athletes who show a marked decline in SaO2 during heavy exercise. The validity of a pulse oximiter similar to the one used in our study was R 2 = 0.90 with a mean error = 0.3% (compared to the reference method, i.e., cooximetry in the arterial blood) (Mollard et al. 2010; Yamaya et al. 2002).
As expected, exercise induced a certain degree of arterial hypoxemia, ranging from mild to severe in the subjects. As mentioned above, rowers were divided into either the normoxemic (SaO2 >92%) or the hypoxemic (SaO2 <92%) group (Powers et al. 1988, 1989a, b) based on SaO2 measured during the incremental \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test performed on rowing ergometer. In the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test the mean difference in SaO2 between the normoxemic (SaO2 = 94.5 ± 0.6%; range 93–95) and the hypoxemic (SaO2 = 88.4 ± 3.1%; range 83–91) athletes was significant (P < 0.001). As expected, no significant differences were found in SaO2 resting values between the two groups (normoxemic 98.5 ± 0.4% vs. hypoxemic 98.4 ± 0.5%). The difference in SaO2 values after the \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) test between the two conditions were also confirmed when the subjects performed the rowing 2,000 m test. Indeed, hypoxemic subjects had significantly lower SaO2 values at the end of the simulated 2,000-m rowing race compared with the respective values of the normoxemic subjects (88.6 ± 2.4% vs. 94.1 ± 0.9%, P < 0.001) (Fig. 1). However, again no significant differences were found between normoxemic and hypoxemic rowers in either the resting values of SaO2 (98.6 ± 0.5% vs. 98.3 ± 0.5%, respectively), or any other anthropometric and physiological variable measured (Table 1 and Table 2).
Plasma volume changes
A significant increase in Hct (41.0 ± 2.5% post-exercise vs. 40.3 ± 1.7% pre-exercise in normoxemic individuals and 41.8 ± 2.7% post-exercise vs. 40.6 ± 1.9% pre-exercise in hypoxemic individuals, P < 0.05) and [Hb] (14.5 ± 1.1 g dL−1 post-exercise vs. 14.4 ± 0.8 g dL−1 pre-exercise in normoxemic individuals and 14.4 ± 0.9 g dL−1 post-exercise vs. 14.3 ± 1.2 g dL−1 pre-exercise in hypoxemic individuals, respectively, P < 0.05) were detected. Post-exercise plasma volume relatively to pre-exercise value was 0.967 ± 0.04 in normoxemic and 0.977 ± 0.05 in hypoxemic individuals. All post-exercise values were corrected for plasma volume changes.
Blood redox status
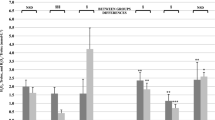
No differences in dietary macronutrient and antioxidant intake between the normoxemic and hypoxemic groups were found. Data of the redox status markers examined are presented in Figs. 2, 3, 4. No significant main effect of group (normoxemic vs. hypoxemic) in any of the examined blood oxidative stress parameters was found (P > 0.05). No significant group (normoxemic vs. hypoxemic)-by-time (pre- vs. post-exercise) interaction was found in any of the examined blood redox status parameters (P > 0.05), except for GSSG (P = 0.007) and GSH/GSSG (P = 0.047). Exercise significantly increased GSSG concentration and decreased GSH/GSSG ratio in the normoxemic group only. However, significant main effect of time was found in all blood oxidative stress parameters, but GSH (P < 0.05). No significant post-exercise change in GSH in either the normoxemic or the hypoxemic group was observed (Fig. 2). Furthermore, exercise significantly increased the concentration of TBARS and protein carbonyls (Fig. 3), as well as catalase activity and TAC (Fig. 4) in both the normoxemic and hypoxemic groups.
Exercise Performance
No statistically significant differences in performance or other physiological responses following the rowing 2,000 m test were found between the normoxemic and the hypoxemic athletes (P > 0.05) (Table 2).
Discussion
To our knowledge, this is the first study to investigate the effect of exercise-induced hypoxemia, which is hypoxemia elicited during heavy exercise at a sea level, on blood redox status. The findings of this study indicated that rowing for 2,000 m at maximum intensity modified plasma redox status in well-trained athletes in a similar manner for both hypoxemic and normoxemic individuals. On the other hand, GSH was oxidized to GSSG at a higher rate (and/or did not turn back to GSH or exported from the erythrocyte) after exercise in the normoxemic group compared to the hypoxemic group indicating that erythrocyte redox status is affected by the oxygen saturation degree of hemoglobin.
Comparison of blood redox status between the normoxemic and hypoxemic rowers at rest
There is no doubt that erythrocytes of the hypoxemic rowers have been chronically exposed to decreased levels of oxygen tension. However, no differences were found with regard to baseline redox status between the two groups of trained individuals, indicating that repeated episodes of hypoxemia does not affect resting redox status in blood. A previous study in male subjects reports that acute hypoxemia produced by inhalation of a hypoxic gas mixture does not modify the production of reactive species and oxidative stress either at rest or after static handgrip at 60% of maximal voluntary contraction until exhaustion (Dousset et al. 2002). However, other investigations showing almost unanimously that environmentally induced hypoxemia (i.e, due to reduced partial pressure of oxygen in the air inspired) induced oxidative stress both at rest and after exercise (Joanny et al. 2001; Pialoux et al. 2009, 2010; Vasankari et al. 1997; Wang et al. 2007; Wang and Lin 2010).
It has been recently demonstrated that elite athletes who lived in hypoxemic conditions and were aerobically trained for 18 days under normoxemic conditions (“live high-train low” method), magnified the exercise-induced decrease in antioxidant status that normally observed when athletes live and train in normoxemic conditions (“live low-train low” method) (Pialoux et al. 2009, 2010). More interestingly, the decreased antioxidant status in blood of the athletes who lived under hypoxemic conditions was not fully restored after 14 days of recovery (Pialoux et al. 2010). This difference indicates that it is important to distinguish between environmentally- and exercise-induced hypoxemia with regard to their effects on blood redox status. Although in both conditions, the final outcome is the reduced oxygen saturation in arterial blood, thus less oxygen is available to be delivered to the tissues leading to hypoxia, yet the mechanism of action is different. In environmental hypoxemia, the oxygen unavailability is due to a reduction in partial pressure of oxygen in the air inspired, whereas in exercise-induced hypoxemia this is due to either diffusion limitations and/or ventilation-perfusion inequality (otherwise term as ventilation perfusion mismatch) (Dempsey et al. 1984; Torre-Bueno et al. 1985).
Comparison of blood redox status between the normoxemic and hypoxemic rowers post-exercise
Our data demonstrate that maximal 2,000-m rowing ergometer performance increases oxidative stress in well-trained rowers, as evidenced by the significant increase in serum TBARS, protein carbonyls, catalase activity and TAC in both the normoxemic and hypoxemic groups. These findings are in agreement with the results of relevant studies (Kyparos et al. 2009; Skarpanska-Stejnborn et al. 2006; Zembron-Lacny et al. 2006). More specifically, Skarpanska-Stejnborn et al. (2006) found a 7% increase in plasma TAC and a 94% increase in erythrocyte TBARS immediately after a rowing test compared with a 9% increase in plasma TAC and 45% increase in plasma TBARS observed in our study. Accordingly, Zembron-Lacny et al. (2006) showed a 76% increase in erythrocyte TBARS and a 12% increase in catalase activity compared with the more than onefold increase in serum catalase activity detected in our study. Therefore, based on the findings reported here and in the relevant studies, it is evident that high intensity rowing exercise increases oxidative stress in well-trained rowers (Kyparos et al. 2009; Skarpanska-Stejnborn et al. 2006; Zembron-Lacny et al. 2006). However, it was not clear as yet whether a decrease in arterial oxyhemoglobin saturation—normally induced by maximal exercise—may further affect redox status and oxidative stress in elite athletes.
It appears that the degree of exercise-induced decrease in arterial oxygen saturation produced differential effects in erythrocytes, but not in plasma. Both hypoxemic and normoxemic athletes increased plasma oxidative stress markers in a similar fashion immediately after exercise. In addition, normoxemic individuals also showed increased oxidation of GSH to GSSG in erythrocytes indicating that exercise was capable of inducing oxidative stress isolated in erythrocytes. This was somehow expected because the relative amount of oxygen—a potential source of oxidative stress—that is transported with erythrocytes and utilized during exercise was greater in normoxemic compared to hypoxemic individuals. Acute exercise has been repeatedly shown to decrease the level of GSH and increase the level of GSSG, and thereby decrease the glutathione redox ratio (GSH/GSSG) (Nikolaidis et al. 2008; Nikolaidis and Jamurtas 2009). Glutathione is largely known to minimize the lipid peroxidation of cellular membranes and other such targets, thus alleviating oxidative stress (Kerksick and Willoughby 2005). Thus, the increased oxidation of GSH implies induction of oxidative stress. GSH acts both as the cofactor for glutathione peroxidase, thus reducing hydrogen peroxide to water, and also as a direct radical-scavenging antioxidant. The calculated proportion of superoxide reacting with GSH has been estimated to be about 22% (Jones et al. 2003). This can reduce the power of glutathione to convert GSSG to GSH, thus indirectly leading to increased GSSG concentration.
The effect of exercise-induced hypoxemia on rowing performance
In athletes performing maximal exercise, the capacity for breathing and lactate metabolism determines the extent to which hypoxemia limits VO2 and exercise capacity (Nielsen 2003). Hypoxemia exacerbates peripheral fatigue of limb locomotor muscles and this effect may contribute, in part, to the early termination of exercise. Also, endurance capacity decreases in severe hypoxia are due, in part, to fatigue-induced changes within the working muscles (Romer and Dempsey 2006). Finally the normal exercise-induced O2 desaturation during heavy-intensity endurance exercise contributes significantly to exercise performance limitation in part because of its effect on locomotor muscle fatigue (Romer and Dempsey 2006).
It has been reported that an approximate reduction of 4% from the % SaO2 baseline value has resulted in a significant decline in \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) in habitually endurance-trained men (Squires and Buskirk 1982) and women (Harms et al. 2000) performed treadmill-graded exercise test to exhaustion in a hypobaric chamber. Powers et al. (1989a, b) have also suggested that an exercise-induced reduction in SaO2 to 92–93% is sufficient to cause a detectable decline in \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \). More recently, Harms et al. (2000) has demonstrated that \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) was significantly improved in recreational endurance-trained women when arterial O2 desaturation normally induced during incremental test to exhaustion was prevented via a mild hyperoxic inhalation.
Nevertheless, in the present study, there was no difference in rowing performance between the hypoxemic and normoxemic groups. Our finding on exercise performance was not in agreement with previous reports that arterial hypoxemia induced by an artificial reduction in the inspired O2 fraction during maximal cycle test to exhaustion impaired the total work output in well-trained male cyclists (Koskolou and McKenzie 1994). These authors concluded that maximal performance was impaired at a % SaO2 level of 87%, but not under a milder desaturation level of 90%. In our study the mean SaO2 of the hypoxemic rowers was a little higher (88.6%) than the lower level (87%) set by Koskolou and McKenzie (1994) in which decreases in performance was found, a fact that may in part explain this difference in performance between the two studies. Perhaps, the potential adverse effects of hypoxemia on exercise performance would be measurable in conditions of more severe O2 arterial desaturation.
Therefore, in terms of exercise performance in the model used in the present study hypoxemia does not appear to have a physiological significance. It has been previously demonstrated in endurance athletes that vastus lateralis muscle deoxygenation was greater in those athletes who exhibited hypoxemia during an incremental test to exhaustion on a cycle ergometer (Legrand et al. 2005). \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) and maximal power output were not different between normoxemic and hypoxemic subjects, suggesting that muscle adaptation may compensate for the reduced O2 delivery to the muscle (Legrand et al. 2005). These authors suggested that muscle deoxygenation was probably due to the fact that muscle reached its maximal O2 extraction and/or that O2 delivery to the muscle cannot be further increased.
The findings of the present study should be viewed within the context that our subjects were highly trained athletes who performed an activity they were quite accustomed to. An additional point to be taken into account when evaluating the results is that the activity performed was largely comprised of concentric muscle contractions, thus minimizing the potential generation of free radicals from muscle injury induced by eccentric contractions.
This study was also delimited by the fact that redox status and oxidative stress markers assessed only immediately after the exercise. In a previous study on yet untrained male subjects, we have demonstrated that there is no “ideal” blood sampling time point than can apply to and capture simultaneously the peak values of all biomarkers after exhaustive aerobic exercise of moderate duration (Michailidis et al. 2007). Depending on the biomarker assessed in the blood, it may take up to a couple of hours after the cessation of exercise for peak oxidative stress activity to be manifested. In the light of these findings, the present work may be viewed as an oxidative stress response study pertaining well-trained athletes. In any case, if only one post-exercise sampling time point is to be chosen for measuring oxidative stress markers, then sampling immediately after the cessation of exercise may be the best compromise and the most “meaningful” sampling collection time point. In this occasion, the generalization of the findings acquired from a single sample immediately post-exercise to what may occur several hours of post-exercise recovery should be cautiously made, because we were not able to follow any potential late increase or other alteration in reactive species, neither the progression of oxidative damage throughout the recovery period.
However, experiments in isolated perfused rat and rabbit hearts (Baker et al. 1988; Zweier et al. 1987, 1989) have directly demonstrated using electron paramagnetic resonance spectroscopy that reactive oxygen-centered free radicals are generated in myocardium during ischemia and that a burst of oxygen radical generation occurs within moments of reperfusion, in fact after only 10–15 s of reflow following ischemia. More recently, oxidative stress and redox status biomarkers were measured in response to reactive hyperemia that induced by ischemia–reperfusion in exercise-trained men (Bloomer et al. 2010). Peak values of malondialdehyde concentration, total glutathione and oxidized glutathione were observed immediately post the ischemia–reperfusion protocol, hydrogen peroxide and xanthine oxidase activity at 3 min post, whereas F2-isoprostanes and total antioxidant capacity remained unaffected by treatment or protocol (Bloomer et al. 2010).
Some reservations exist with respect to the validity of the TBARS assay in detecting lipid peroxidation, as this measure is criticized for the lack of specificity (Halliwell and Gutteridge 2007). Although this may be considered as a limitation of the present work, yet many studies from our and other research groups (Fisher et al. 2011; Nikolaidis et al. 2007, 2008) have repeatedly shown that TBARS concentrations have been consistently increasing after exercise. In addition, it has been found that TBARS concentrations followed similar changes to F2-isoprostane concentrations (currently considered the reference method) after exercise (Margonis et al. 2007).
Although ischemia–reperfusion and exercise-induced hypoxemia do not share the same etiology, a common outcome is the decreased oxygen availability followed by an oxygen burst. In this regard, it would have also been interesting to look into the potential “re-oxygenation”-induced oxidative damage throughout the time course of post-exercise recovery. It might be that after the cessation of exercise and once normal arterial oxyhemoglobin saturation was again achieved in hypoxemic athletes, the generation of reactive species and subsequent oxidative damage would further increase in these subjects. Furthermore, it has been recently demonstrated that although the magnitude of changes in selective oxidative stress markers observed immediately post-strenuous exercise are large enough and they can be reliably detected biochemically, however, all markers do not follow the same time course post-exercise and their peak values do not necessarily coincide at the same time point (Michailidis et al. 2007). Therefore, to address the hypothesis of potential “re-oxygenation”-induced oxidative damage following exercise in the hypoxemic athletes, a proper experimental design should incorporate the assessment of redox status and oxidative damage markers at several time points during the post-exercise recovery period.
Conclusions
Our findings indicate that exercise-induced hypoxemia did not further affect the increased blood oxidative damage of lipids and proteins observed after a 2,000-m rowing ergometer race in highly trained male rowers. In both the normoxemic and hypoxemic rowers similar and significant post-exercise increase in serum TBARS, protein carbonyls, catalase activity and TAC concentration were observed. The present data do not support any potential link between exercise-induced hypoxemia, oxidative stress increase and exercise performance. On the other hand, the present data indicate that erythrocytes of the normoxemic individuals produced more GSSG post-exercise; suggesting that erythrocyte redox status is affected by the oxygen saturation degree of hemoglobin and those erythrocytes produced more reactive species during exercise. The compartmentalized effect of exercise on redox status also denotes that the 2,000-m rowing race is a good model to induce physiologically oxidative stress and to delineate subtle differences in redox status due to oxygen saturation at least in the erythrocytes.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
American College of Sports Medicine (2000) ACSM’s Guidelines for Exercise Testing and Prescription, 6th edn. Lippincott Williams & Wilkins, Philadelphia 117
Bailey DM, Davies B, Young IS (2001) Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin Sci 101:465–475
Baker JE, Felix CC, Olinger GN, Kalyanaraman B (1988) Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci USA 85:2786–2789
Bassett DR Jr, Howley ET (2000) Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32:70–84
Bloomer RJ (2008) Effect of exercise on oxidative stress biomarkers. Adv Clin Chem 46:1–50
Bloomer RJ, Fry AC, Falvo MJ, Moore CA (2007) Protein carbonyls are acutely elevated following single set anaerobic exercise in resistance trained men. J Sci Med Sport 10:411–417
Bloomer RJ, Smith WA, Fisher-Wellman KH (2010) Oxidative stress in response to forearm ischemia-reperfusion with and without carnitine administration. Int J Vitam Nutr Res 80:12–23
Dempsey JA, Wagner PD (1999) Exercise-induced arterial hypoxemia. J Appl Physiol 87:1997–2006
Dempsey J, Hanson P, Henderson K (1984) Exercise-induced arterial hypoxemia in healthy persons at sea level. J Physiol 355:161–175
Dempsey JA, McKenzie DC, Haverkamp HC, Eldridge MW (2008) Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest 134:613–622
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma and red cells in dehydration. J Appl Physiol 37:247–248
Dousset E, Steinberg JG, Faucher M, Jammes Y (2002) Acute hypoxemia does not increase the oxidative stress in resting and contracting muscle in humans. Free Radic Res 36:701–704
Durand F, Mucci P, Prefaut C (2000) Evidence for an inadequate hyperventilation inducing arterial hypoxemia at submaximal exercise in all highly trained endurance athletes. Med Sci Sports Exerc 32:926–932
Fisher G, Schwartz DD, Quindry J, Barberio MD, Foster EB, Jones KW, Pascoe DD (2011) Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J Appl Physiol 110:730–737
Fisher-Wellman K, Bloomer RJ (2009) Acute exercise, oxidative stress: a 30 year history. Dyn Med 8:1–25
Guenette JA, Sheel AW (2007) Exercise-induced arterial hypoxaemia in active young women. Appl Physiol Nutr Metab 32:1263–1273
Hagerman FC (1984) Applied physiology of rowing. Sports Med 1:303–326
Hagerman FC, Fielding RA, Fiatarone MA, Gault JA, Kirkendall DT, Ragg KE, Evans WJ (1996) A 20-yr longitudinal study of Olympic oarsmen. Med Sci Sports Exerc 28:1150–1156
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York, NY
Hanel B, Clifford PS, Secher NH (1994) Restricted postexercise pulmonary diffusion capacity does not impair maximal transport for O2. J Appl Physiol 77:2408–2412
Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA (2000) Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med Sci Sports Exerc 32:1101–1108
Hopkins SR, McKenzie DC (1989) Hypoxic ventilatory response and arterial desaturation during heavy work. J Appl Physiol 67:1119–1124
Janaszewska A, Bartosz G (2002) Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest 62:231–236
Joanny P, Steinberg J, Robach P, Richalet JP, Gortan C, Gardette B, Jammes Y (2001) Operation Everest III (Comex’97): the effect of simulated sever hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation 49:307–314
Jones CM, Lawrence A, Wardman P, Burkitt MJ (2003) Kinetics of superoxide scavenging by glutathione: an evaluation of its role in the removal of mitochondrial superoxide. Biochem Soc Trans 31:1337–1339
Keles MS, Taysi S, Sen N, Aksoy H, Akcay F (2001) Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can J Neurol Sci 28:141–143
Kerksick C, Willoughby D (2005) The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr 2:38–44
Koskolou MD, McKenzie DC (1994) Arterial hypoxemia and performance during intense exercise. Eur J Appl Physiol Occup Physiol 68:80–86
Kyparos A, Vrabas IS, Nikolaidis MG, Riganas CS, Kouretas D (2009) Increased oxidative stress blood markers in well-trained rowers following two thousand-meter rowing ergometer race. J Strength Cond Res 23:1418–1426
Legrand R, Ahmaidi S, Moalla W, Chocquet D, Marles A, Prieur F, Mucci P (2005) O2 arterial desaturation in endurance athletes increases muscle deoxygenation. Med Sci Sports Exerc 37:782–788
Margonis K, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Douroudos I, Chatzinikolaou A, Mitrakou A, Mastorakos G, Papassotiriou I, Taxildaris K, Kouretas D (2007) Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radic Biol Med 43:901–910
McBride JM, Kraemer WJ, Triplett-McBride T, Sebastianelli W (1998) Effect of resistance exercise on free radical production. Med Sci Sports Exerc 30:67–72
Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D (2007) Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med Sci Sports Exerc 39:1107–1113
Mollard P, Bourdillon N, Letournel M, Herman H, Gibert S, Pichon A, Woorons X, Richalet JP (2010) Validity of arterialized earlobe blood gases at rest and exercise in normoxia and hypoxia. Respir Physiol Neurobiol 172:179–183
Moller P, Loft S, Lundby C, Olsen NV (2001) Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J 15:1181–1186
Nielsen HB (2003) Arterial desaturation during exercise in man: implication for O2 uptake and work capacity. Scand J Med Sci Sports 13:339–358
Nielsen HB, Madsen P, Svendsen LB, Roach RC, Secher NH (1998) The influence of PaO2, pH and SaO2 on maximal oxygen uptake. Acta Physiol Scand 164:87–89
Nikolaidis MG, Jamurtas AZ (2009) Blood as a reactive species generator and redox status regulator during exercise. Arch Biochem Biophys 490:77–84
Nikolaidis MG, Paschalis V, Giakas G, Fatouros IG, Koutedakis Y, Kouretas D, Jamurtas AZ (2007) Decreased blood oxidative stress after repeated muscle-damaging exercise. Med Sci Sports Exerc 39:1080–1089
Nikolaidis MG, Jamurtas AZ, Paschalis V, Fatouros IG, Koutedakis Y, Kouretas D (2008) The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med 38:579–606
Nikolaidis MG, Kyparos A, Vrabas IS (2011) F(2)-isoprostane formation, measurement and interpretation: The role of exercise. Prog Lipid Res 50:89–103
Patsoukis N, Zervoudakis G, Panagopoulos NT, Georgiou CD, Angelatou F, Matsokis NA (2004) Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci Lett 357:83–86
Pialoux V, Mounier R, Rock E, Mazur A, Schmitt L, Richalet JP, Robach P, Brugniaux J, Coudert J, Fellmann N (2009) Effects of the ‘live high-train low’ method on prooxidant/antioxidant balance on elite athletes. Eur J Clin Nutr 63:756–762
Pialoux V, Brugniaux JV, Rock E, Mazur A, Schmitt L, Richalet JP, Robach P, Clottes E, Coudert J, Fellmann N, Mounier R (2010) Antioxidant status of elite athletes remains impaired 2 weeks after a simulated altitude training camp. Eur J Nutr 49:285–292
Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, McKnight T, Grinton S (1988) Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol 58:298–302
Powers SK, Dodd S, Freeman JG, Ayers D, Samson H, McKnight T (1989a) Accuracy of pulse oximetry to estimate HbO2 fraction of total Hb during exercise. J Appl Physiol 67:300–304
Powers SK, Lawler J, Dempsey JA, Dodd S, Landry G (1989b) Effects of incomplete pulmonary gas exchange on VO2max. J Appl Physiol 66:2491–2495
Rasmussen J, Hanel B, Diamant B, Secher NH (1991) Muscle mass effect on arterial desaturation after maximal exercise. Med Sci Sports Exerc 23:1349–1352
Reddy YN, Murthy SV, Krishna DR, Prabhakar MC (2004) Role of free radicals and antioxidants in tuberculosis patients. Indian J Tuberc 51:213–218
Rice AJ, Scroop GC, Gore CJ, Thornton AT, Chapman MA, Greville HW, Holmes MD, Scicchitano R (1999) Exercise-induced hypoxaemia in highly trained cyclists at 40% peak oxygen uptake. Eur J Appl Physiol Occup Physiol 79:353–359
Romer LM, Dempsey JA (2006) Effects of exercise-induced arterial hypoxemia on limb muscle fatigue and performance. Clin Exp Pharmacol Physiol 33:391–394
Skarpanska-Stejnborn A, Basta P, Pilaczynska-Szczesniak L (2006) The influence of supplementation with black currant (Ribes nigrum) extract on selected prooxidative antioxidative balance in rowers. Stud Phys Cult Tour 13:51–58
Squires RW, Buskirk ER (1982) Aerobic capacity during acute exposure to simulated altitude, 914 to 2,286 m. Med Sci Sports Exerc 14:36–40
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Torre-Bueno J, Wagner P, Saltzman H, Gale G, Moon R (1985) Diffusion limitations in normal humans during exercise at sea level and simulated altitude. J Appl Physiol 58:899–905
Vasankari TJ, Kujala UM, Rusko H, Sarna S, Ahotupa M (1997) The effect of endurance exercise at moderate altitude on serum lipid peroxidation and antioxidative functions in humans. Eur J Appl Physiol Occup Physiol 75:396–399
Wang JS, Lin CT (2010) Systemic hypoxia promotes lymphocyte apoptosis induced by oxidative stress during moderate exercise. Eur J Appl Physiol 108:371–382
Wang JS, Chen LY, Fu LL, Chen ML, Wong MK (2007) Effects of moderate and severe intermittent hypoxia on vascular endothelial function and haemodynamic control in sedentary men. Eur J Appl Physiol 100:127–135
Yamaya Y, Bogaard HJ, Wagner PD, Niizeki K, Hopkins SR (2002) Validity of pulse oximetry during maximal exercise in normoxia, hypoxia, and hyperoxia. J Appl Physiol 92:162–168
Zembron-Lacny A, Szyszka K, Sobanska B, Pakula R (2006) Prooxidant-antioxidant equilibrium in rowers: effect of a single dose of vitamin E. J Sports Med Phys Fitness 46:257–264
Zweier JL, Flaherty JT, Weisfeldt ML (1987) Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84:1404–1407
Zweier JL, Kuppusamy P, Williams R, Rayburn BK, Smith D, Weisfeldt ML, Flaherty JT (1989) Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem 264:18890–18895
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Kyparos, A., Riganas, C., Nikolaidis, M.G. et al. The effect of exercise-induced hypoxemia on blood redox status in well-trained rowers. Eur J Appl Physiol 112, 2073–2083 (2012). https://doi.org/10.1007/s00421-011-2175-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2175-x