Abstract
Caffeine, an adenosine receptor antagonist, has shown to improve performance in normal ambient temperature, presumably via an effect on dopaminergic neurotransmission through the antagonism of adenosine receptors. However, there is very limited evidence from studies that administered caffeine and examined its effects on exercise in the heat. Therefore, we wanted to study the effects of caffeine on performance and thermoregulation in high ambient temperature. Eight healthy trained male cyclists completed two experimental trials (in 30°C) in a double-blind-randomized crossover design. Subjects ingested either placebo (6 mg/kg) or caffeine (6 mg/kg) 1 h prior to exercise. Subjects cycled for 60 min at 55% W max, immediately followed by a time trial to measure performance. The significance level was set at p < 0.05. Caffeine did not change performance (p = 0.462). Rectal temperature was significantly elevated after caffeine administration (p < 0.036). Caffeine significantly increased B-endorphin plasma concentrations at the end of the time trial (p = 0.032). The present study showed no ergogenic effect of caffeine when administered 1 h before exercise in 30°C. This confirms results from a previous study that examined the effects of caffeine administration on a short (15 min) time trial in 40°C. However, caffeine increased core temperature during exercise. Presumably, the rate of increase in core temperature may have counteracted the ergogenic effects of caffeine. However, other factors such as interindividual differences in response to caffeine and changes in neurotransmitter concentrations might also be responsible for the lack of performance improvement of caffeine in high ambient temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human studies using a variety of protocols have shown performance improvements after caffeine intake (Tarnapolski and Cupido 2000; Hulston and Jeukendrup 2008; Glaister et al. 2008; Hogervorst et al. 2008; Walker et al. 2008). The ergogenic effects of caffeine are mainly mediated through central blockade of the adenosine receptors, thereby counteracting the inhibitory effects of adenosine on neuroexcitability, neurotransmitter release and arousal, while there will only be a smaller effect due to the metabolic changes induced by caffeine in the periphery (Davis et al. 2003). This was shown by an animal study by Davis et al. (2003) who administered caffeine directly into the CNS of the rat and found that even a very low dose increased treadmill running performance by 60% while a peripheral dose was ineffective. Davis et al. (2003) also administered an adenosine agonist and found a reduced run time to fatigue, evidencing a strong link between adenosine and physical performance.
Caffeine is known to enhance feelings of well-being, motivation, energy, and concentration, thereby showing similar effects as amphetamines and cocaine (Griffiths and Mumford 1996). In the past caffeine has been shown to influence the catecholamines dopamine (DA; Morgan and Vestal 1989) and noradrenaline (NA; Minana and Grisolia 1986; Whitham et al. 2006). Morgan and Vestal (1989) found significantly higher DA concentrations in many regions of the brain due to caffeine administration. Since it has been shown that DA agonism or the manipulation with a DA reuptake inhibitor has a positive effect on exercise performance (Roelands et al. 2008a), the performance enhancing effect of caffeine can be explained through the dopaminergic pathway.
Considering the ergogenic effects of caffeine previously reported in normal ambient temperature, it is surprising how little research has been performed in high ambient temperatures. Subjects performed more work after administration of a caffeinated sports drink containing carbohydrates in the heat (Cureton et al. 2007), and in a recent study Del Coso et al. (2008) have found that caffeine, in combination with carbohydrates and water, is able to increase maximal leg force by increasing muscle voluntary activation. The ergogenic effect in the last mentioned studies results from the combined effects of carbohydrates and caffeine since the effect of carbohydrates or caffeine alone was less pronounced (Del Coso et al. 2008). The only laboratory based study that administered caffeine without additional carbohydrates in a time trial protocol in a warm environmental setting (40°C; Cheuvront et al. 2009), showed no difference in a 15 min TT. In another trial, quercetin (an adenosine receptor antagonist) did not induce any difference in performance (Cheuvront et al. 2009). Previously, two field studies reported no benefits of caffeine on endurance performance in hot, humid conditions (Cohen et al. 1996; Ferreira et al. 2005).
Thus, from the limited amount of studies in the heat it appears that results are not at all as conclusive as results at normal ambient temperature. Since no studies thus far investigated the effects of the combination of caffeine (administered without additional carbohydrates) long duration exercise (+60 min) and heat, the purpose of the present study is to determine the effects of caffeine administration before the start of prolonged exercise on performance, thermoregulation and hormonal variables in high ambient temperature (30°C). Based on the available literature in high ambient temperature, we hypothesize that caffeine consumption (alone) will not improve performance.
Methods
Subjects
Eight healthy, trained and mild caffeine consuming males (108 ± 47 mg day−1; age 23 ± 5 years; Ht 1.83 ± 0.07 m; mass 77 ± 8 kg; W max 331 ± 20 W) participated in this investigation. All subjects were trained cyclists or triathletes, but were not accustomed to exercise in a warm environment. Prior to the start of the study, all volunteers received written information regarding the nature and purpose of the experimental protocol. Following an opportunity to ask questions, a written statement of consent was signed. The protocol employed was approved by the Research Council of the Vrije Universiteit Brussel, Belgium.
Experimental protocol
The experimental protocol applied in this study is similar to the protocol used previously (Roelands et al. 2008a, b), therefore we will address it briefly. All subjects completed a preliminary maximal exercise test, a familiarization trial and two experimental trials. The preliminary trial consisted of continuous incremental cycle exercise to volitional exhaustion and was used to determine the power output required to elicit 55 and 75% of maximal workload (W max). A familiarization trial was undertaken to ensure the subjects were accustomed to the procedures employed during the investigation and to minimize any potential learning or anxiety effects. This trial was performed in temperate environmental conditions and was identical to the experimental trials in all respects. Experimental trials were undertaken in warm (30°C) conditions, with relative humidity maintained between 50 and 60%. All subjects had to complete the experimental trials, which were separated by 7 days to minimize the development of heat acclimation and to ensure drug washout (Whitham et al. 2006; Cheuvront et al. 2009). Subjects were instructed to record dietary intake and physical activity during the 2 days before the first trial, and to replicate this in the 2 days prior to the subsequent experimental trials. Subjects were provided with a list of dietary sources of caffeine and asked to refrain from consuming these 48 h before each trial. No exercise and alcohol consumption was permitted in the 24 h before each trial.
The subjects entered the laboratory in the morning approximately 90 min after consuming a standardized breakfast (90–100 g CHO). Subjects provided a urine sample before nude post-void body mass was measured and an indwelling venous cannula was introduced into a superficial forearm vein to enable repeated blood sampling at rest and during exercise. Subjects inserted a rectal thermistor (Gram Corporation LT-8A, Japan) 10 cm beyond the anal sphincter for the measurement of core temperature. Surface skin temperature probes (Gram Corporation LT-8A, Japan) were attached to four sites (chest, upper arm, thigh and calf) to enable the determination of weighted mean skin temperature (Ramanathan 1964) and a heart rate telemetry band (Polar Accurex, Finland) was positioned. Subjects were dressed in cycling shorts, socks, and shoes for all trials.
Subjects then entered a climatic chamber maintained at the appropriate environmental conditions and rested in a seated position for 15 min. During this period, temperatures and heart rate were recorded at 5 min intervals, a resting venous blood sample was drawn, and blood pressure measured immediately before the start of exercise. The exercise protocol consisted of 60 min constant load exercise at a workload corresponding to 55% W max, followed by a TT to measure exercise performance. There was a 2–3 min delay between the end of the constant load exercise and the beginning of the TT, to program the ergometer (Lode Excalibur Sport, Holland). The TT required the subjects to complete a predetermined amount of work equal to 30 min at 75% W max as quickly as possible (Jeukendrup et al. 1996). Subjects began the TT at a workload corresponding to 75% W max, but were free to increase or decrease their power output as desired. During the TT a computer program displayed a bar indicating the percentage of total work completed to give the subject an indication of their progress. Throughout the protocol no feedback was provided regarding time elapsed, power output, pedal cadence or heart rate. During exercise subjects had ad libitum access to plain water.
Core and skin temperatures and heart rate were recorded at 5 min intervals during exercise. Ratings of perceived exertion (RPE; Borg 1982) and thermal stress (assessed using a 21-point scale ranging from unbearable cold to unbearable heat) were assessed every 15 min during the initial 60 min and at 10 min intervals during the TT. Venous blood samples were drawn and blood pressure measured after 60 min of constant load exercise and at the end of the TT. Following the completion of the TT, subjects returned to a seated position where recovery was monitored for 15 min. The probes and cannula were then removed and nude body mass was then re-measured to allow the estimation of body weight losses. The sweat rate (ml min−1) was calculated from the weight loss of the subject over time. Immediately afterwards subjects provided a urine sample. After that subjects completed a caffeine side-effect questionnaire (Hogervorst et al. 2008).
Drugs
Caffeine, after oral ingestion, is rapidly and almost completely (99%) absorbed from the gastrointestinal tract into the bloodstream (Arnaud 1993). Peak plasma concentrations are reached in about 30–60 min after consumption (Lorist and Tops 2003). Subjects ingested 6 mg/kg caffeine or a placebo (lactose) 1 h before the start of exercise (after the first urine sample). The treatment order was randomized and administered in a double-blind crossover manner. Caffeine and placebo capsules were prepared by a pharmacy to appear indistinguishable with regard to dimensions, weight and color.
Blood and urine collection, analysis and blood pressure
Urine samples were collected before the drug administration and after the experimental protocol. The plasma and urinary caffeine analyses were undertaken using a HPLC technique, according to the methods of Delbeke and De Backer (1996). Venous blood samples were drawn directly into pre-cooled vacutainer tubes (BD Vacutainer, UK). Ten milliliter samples were collected into a plain tubes and left to clot for 1 h at room temperature before centrifugation. The resulting serum was stored at −20°C for the determination of prolactin (PRL; Roche Diagnostics, Germany), cortisol (Diasorin, USA) and growth hormone (GH; Pharmacia and Upjohn Diagnostics, Sweden). Samples for plasma adrenocorticotropic hormone (ACTH) and beta-endorphins (B-end) were collected into 4.5 ml tubes containing K3EDTA. An additional 7.5 ml was added to lithium heparin. In order to measure the blood lactate concentration blood samples (20 μl) were drawn from an arterialized ear lobe. Lactate concentration was determined enzymatically (EKF; BIOSEN 5030, Germany). Blood pressure was measured with an automatic unit at the upper arm (Bosos, Bosch+Sohn; Germany).
Statistical analysis
Data are presented as mean ± standard deviation (SD). The One-sample-Kolmogorov–Smirnov test showed that all outcome variables had a normal distribution. To evaluate differences in TT performance and power output, a paired t test was employed. Pearson correlation analysis was used to examine the association between performance on the TT and the rise of core temperature.
Data collected over time were analyzed using two-factor (drug × time) ANOVA with repeated measures. Statistical significance was accepted at p < 0.05. Paired t tests were used to identify pairwise differences, no Bonferroni correction was applied.
Results
All eight subjects completed all experimental trials, no side effects were reported. Caffeine administration clearly increased urinary caffeine concentration (pla: 0.3 ± 0.4 μg/ml; caf: 6.0 ± 2.6 μg/ml).
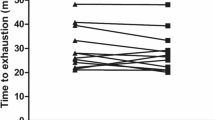
Acute caffeine administration did not significantly influence TT time (pla: 36.6 ± 3.3 min; caf: 37.7 ± 5.2 min; Fig. 1; p = 0.462). As the TT required the completion of a predetermined amount of work, the time taken to complete the protocol was directly related to the power output maintained throughout this period. There was no influence of the drug administration (p = 0.989).
Core temperature increased during exercise in all trials. The combination of caffeine and exercise in the heat significantly increased core temperature (p = 0.001; Fig. 2). Skin temperature increased during exercise, reaching a plateau after 15 min in pla and caf. No differences in weighted mean skin temperature were present between placebo and caffeine (p = 0.463; Fig. 2b). No association was found between performance on the TT and the rise of core temperature during this TT (r = −0.243; p = 0.561).
Heart rate before, during and after exercise was not altered by caffeine administration (p > 0.05; Fig. 3).
RPE were similar between placebo and caffeine treatment (at the end of exercise: pla: 18.3 ± 1.3; caf: 18.3 ± 1.3; p = 0.313). The subjects’ ratings of thermal stress were not influenced by the drug treatment (at the end of exercise: pla: 7.6 ± 2.3; caf: 8.5 ± 1.6; p = 0.450). No differences in blood lactate concentrations were found (p = 0.744; Table 1). There was no drug effect on the loss of body mass, corrected for fluid intake and blood that was drawn, nor sweat rate (pla: 32 ± 9 ml min−1; caf: 32 ± 10 ml min−1) after exercise.
Caffeine supplementation did not induce any significant change in glucose concentration in the blood (p = 0.116; Table 1). Circulating concentrations of pituitary and adrenal hormones are presented in Table 1. In caf30 cortisol concentrations were not different from placebo (p = 0.429; Table 1). Plasma ACTH concentrations increased over time (p < 0.001) but no drug effect was observed (p = 0.534; Table 1). B-end concentrations were significantly higher in the caffeine trial (Table 1; p = 0.032). PRL and GH concentrations increased over time (p < 0.01), but no effect of drug administration was detected (Table 1).
Discussion
We examined the effect of the acute administration of an adenosine receptor antagonist, caffeine, 1 h before the start of exercise, on performance during prolonged exercise in high (30°C) ambient temperature. Previous research—using a similar protocol—with caffeine has shown ergogenic (Hulston and Jeukendrup 2008; Walker et al. 2008) effects on endurance performance undertaken in normal ambient temperature, while one laboratory based study applying high ambient temperature (40°C) was not capable of detecting performance differences in a short TT (Cheuvront et al. 2009). The results of the present study confirm those of the Cheuvront et al. (2009) study, showing that indeed in high ambient temperature, caffeine did not induce any significant change in performance during prolonged exercise, but caffeine significantly increased core temperature compared to the placebo trial.
The mechanism of action of caffeine is still elusive, even in normal ambient temperature. In the past it has been attributed to an increased availability of free fatty acids (Spriet et al. 1992), resulting in a glycogen sparing effect. However, this finding is far from conclusive and there is now evidence that the mechanism of action of caffeine is not due to muscle glycogen sparing (Graham et al. 2000). Caffeine may also reduce perception of effort and pain during exercise, thereby allowing subjects to perform at higher workloads for a longer period of time (Motl et al. 2003). This statement has not been comprehensively tested, and the current study results disagree with this finding as there were no differences in RPE and thermal sensation between caffeine and placebo. Current research supports a central nervous system effect mediated by antagonism of adenosine receptors as most likely cause (Glaister et al. 2008; Davis et al. 2003). Caffeine is known to antagonize adenosine receptors in the brain, while adenosine inhibits the release of DA. Logically, caffeine will induce higher DA concentrations in the brain (Davis et al. 2003).
Recently, we showed that the inhibition of the reuptake of DA significantly improved performance on a 30 min TT and increased core temperatures (Roelands et al. 2008a; Watson et al. 2005). In the present study there was a significant increase in core temperature after caffeine administration. Literature displays differing effects on core temperature, some studies (Stebbins et al. 2001; Roti et al. 2006) did not detect any effect on core temperature, while the finding in the present study is in line with other studies performed in the same environmental conditions (Del Coso et al. 2008; Cheuvront et al. 2009; Millard-Stafford et al. 2007). The lack of change in the study by Stebbins et al. (2001) might be explained by the experimental protocol, which consisted of a 35 min submaximal exercise, while the lack of increase in core temperature in the study by Roti et al. (2006) can be explained by the fact that there was an acute administration of caffeine in chronically consuming subjects. The observed effect on core temperature in the present study might reflect an increased DA concentration in the brain. We have recently shown in rodents that the preoptic and anterior hypothalamus, the thermoregulatory center of the brain, is influenced by catecholaminergic reuptake inhibition resulting in increased core temperatures (Hasegawa et al. 2005, 2008).
The lack of performance effects of caffeine administration without co-ingestion carbohydrates might be explained by different factors:
A recent study by Cheuvront et al. (2009) stated that “environmental conditions may negate the efficacy of otherwise well established nutritional ergogenic aids”, thereby explaining the lack of positive effect on exercise performance after administration of two different adenosine receptor antagonists (caffeine and quercetin).
Indeed, during prolonged exercise in the heat, elevated internal body temperature and increased heat storage have been considered to be limiting factors (Gonzalez-Alonso et al. 1999) that reduce CNS drive for exercise performance and precipitate feelings of fatigue (Nielsen et al. 1990). Rather than a proposed “critical” core temperature, it appears that the rate of increase in core temperature after caffeine administration could cause a progressive inhibition of different brain regions responsible for motor activation (Belza et al. 2009), consequently leading to a disappearance of the ergogenic effect of caffeine (Nybo 2010). The present study results however did not indicate a significant correlation between the rise in core temperature during the time trials and the performance on these time trials. There are interindividual variations in the changes in exercise capacity in response to caffeine ingestion, which can be explained by the fact that there are responders and non-responders (Graham and Spriet 1995; Burke 2008). Furthermore, individual differences in the time-to-peak plasma caffeine concentrations occured with subjects reaching peak values after 60–180 min (Desbrow et al. 2009). Catecholamine concentrations might influence the effects exerted by caffeine. DA concentrations might not have increased to the level to inhibit the descending signals arising from the central nervous system to reduce resistance, or NA concentrations may have increased after caffeine administration (Minana and Grisolia 1986; Whitham et al. 2006). Piacentini et al. (2002) found a trend towards a decrease in performance after administration of a NA reuptake inhibitor (Reboxetine), and in a previous study we showed that administration of the same NA reuptake inhibitor significantly decreased TT performance in both 18 and 30°C (Roelands et al. 2008b).
The authors acknowledge that it is a limitation to the present study that the concentration of the catecholamines was not measured. Peripheral catecholamine concentrations could have provided interesting data on lipolysis and glycogenolysis and thus energy status of the miocyte. The time-to-peak plasma caffeine concentration was not measured as it has already been well described in literature. The authors have analyzed B-endorphins as they are known to be influenced by caffeine and have the ability to promote the feeling of well-being. The precursor of B-endorphin is the same as for ACTH and thus cortisol. PRL was measured because previous research (Ben-Jonathan 1985; Slattum et al. 1996) has shown that DA is known to be the most important PRL inhibiting factor. An increase in PRL concentration would suggest a decrease in DA, as was also shown in rats by Piacentini et al. (2003).
Caffeine intake has shown to result in the release of B-end by the anterior pituitary (Arnold et al. 1982). Furthermore, research performed by Laurent et al. (2000) suggests that caffeine lowers the threshold for B-end release. The same finding is found in other studies (Lane et al. 1990; Lovallo et al. 1996). The present study further confirms this result, as a significant increase in B-end concentration was found. The mechanisms responsible for the increase in B-end are not yet understood, a suggested mechanism is that caffeine ingestion promotes the release of corticotropin releasing factor from the hypothalamus, which, in turn, increases proopiomelanocortin release, resulting in increased B-end release (Laurent et al. 2000). In this scenario an increase in ACTH and cortisol concentration might occur, an effect that is also visible in the present study (although significance was not reached due to high standard deviations for both ACTH and cortisol).
In conclusion, caffeine did not induce any change in performance in high ambient temperature, but increased core temperature during exercise. Presumably, this temperature effect is mediated by the effect of caffeine raising dopaminergic receptor signaling. The reason for the lack of performance improvement in high ambient temperature is thus far not clear and might be explained by interindividual differences in response to caffeine administration or changes in brain neurotransmitter concentrations. Caffeine might have induced an increase in noradrenaline, resulting in a loss of ergogenic effects.
References
Arnaud MJ (1993) Metabolism of caffeine and other components of coffee. In: Garattini S (ed) Caffeine, coffee and health. Raven Press, New York, pp 43–95
Arnold MA, Carr DB, Togasaki DM et al (1982) Caffeine stimulates beta-endorphin release in blood but not in cerebrospinal fluid. Life Sci 32:1017–1024
Belza A, Toubro S, Astrup A (2009) The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur J Clin Nutr 63:57–64
Ben-Jonathan N (1985) Dopamine: a prolactin-inhibiting hormone. Endocrine Rev 6:564–589
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Burke LM (2008) Caffeine and sports performance. Appl Physiol Nutr Metab 33:1319–1334
Cheuvront SN, Ely BR, Kenefick RW et al (2009) No effect of nutritional adenosine receptor agonists on exercise performance in the heat. Am J Physiol Regul Integr Comp Physiol 296(2):R394–R401
Cohen BS, Nelson AG, Prevost MC et al (1996) Effects of caffeine ingestion on endurance racing in heat and humidity. Eur J Appl Physiol 73:358–363
Cureton KJ, Warren GL, Millard-Stafford ML et al (2007) Caffeinated sports drink: ergogenic effects and possible mechanisms. Int J Sports Nutr Exerc Metab 17(1):35–55
Davis JM, Zhao Z, Stock HS et al (2003) Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Regul Integr Comp Physiol 284:R399–R404
Del Coso J, Estevez E, Mora-Rodriguez R (2008) Caffeine effects on short-term performance during prolonged exercise in the heat. Med Sci Sports Exerc 40(4):744–751
Delbeke FT, De Backer P (1996) Threshold level for theophylline in doping analysis. J Chromatogr B Biomed Appl 687(1):247–252
Desbrow B, Barrett CM, Minahan CL et al (2009) Caffeine, cycling performance, and exogenous CHO oxidation: a dose response study. Med Sci Sports Exerc 41(9):1744–1751
Ferreira GM, Guerra GC, Guerra RO (2005) Effect of caffeine in the performance of cyclists under high thermal risk. Acta Cir Brasil 20(S1):196–203
Glaister M, Howatson G, Abraham CS et al (2008) Caffeine supplementation and multiple sprint running performance. Med Sci Sports Exerc 40(10):1835–1840
Gonzalez-Alonso J, Teller C, Andersen S et al (1999) Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86:1032–1039
Graham TE, Spriet LL (1995) Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J Appl Physiol 78:867–874
Graham TE, Helge JW, MacLean DA et al (2000) Caffeine ingestion does not alter carbohydrate or fat metabolism in human muscle during exercise. J Physiol 529:837–847
Griffiths RR, Mumford GK (1996) Caffeine reinforcement, discrimination, tolerance and physical dependence in laboratory animals and humans. In: Schuster CR, Kuhar MJ (eds) Handbook of experimental pharmacology. Springer, Heidelberg, pp 315–341
Hasegawa H, Meeusen R, Sarre S et al (2005) Acute dopamine/noradrenaline reuptake inhibition increases brain and core temperature in rats. J Appl Physiol 99(4):1397–1401
Hasegawa H, Piacentini MF, Sarre S et al (2008) Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol 586(1):141–149
Hogervorst E, Bandelow S, Schmitt J et al (2008) Caffeine improves physical and cognitive performance during exhaustive exercise. Med Sci Sports Exerc 40(10):1841–1851
Hulston CJ, Jeukendrup AE (2008) Substrate metabolism and exercise performance with caffeine and carbohydrate intake. Med Sci Sports Exerc 40(12):2096–2104
Jeukendrup A, Saris WH, Brouns F et al (1996) A new validated endurance performance test. Med Sci Sports Exerc 28(2):266–270
Lane JD, Adcock RA, Williams RB et al (1990) Caffeine effects on cardiovascular and neuroendocrine responses to acute physiological stress and their relationship to level of habitual caffeine consumption. Psychosom Med 52:320–336
Laurent D, Schneider KE, Prusaczyk WK et al (2000) Effects of caffeine on muscle glycogen utilization and the neuroendocrine axis during exercise. J Clin Endocrinol Metab 85:2170–2175
Lorist MM, Tops M (2003) Caffeine, fatigue, and cognition. Brain Cogn 53:82–94
Lovallo WR, Al’Absi M, Blick K et al (1996) Stress-like adrenocorticotropin responses to caffeine in young healthy man. Pharmacol Biochem Behav 55(3):365–369
Millard-Stafford ML, Cureton KJ, Wingo JE et al (2007) Hydration during exercise in warm, humid conditions: effect of a caffeinated sports drink. Int J Sport Nutr Exerc Metab 17(2):163–177
Minana MD, Grisolia S (1986) Caffeine ingestion by rats increases noradrenaline turnover and results in self-biting. J Neurochem 47(3):728–732
Morgan ME, Vestal RE (1989) Methylxanthineeffects on caudate dopamine release as measured by in vivo electrochemistry. Life Sci 45(21):2025–2039
Motl RW, O’Connor PJ, Dishman RK (2003) Effect of caffeine on perceptions of leg muscle pain during moderate intensity cycling exercise. J Pain 4(6):316–321
Nielsen B, Savard G, Richter EA et al (1990) Muscle blood flow and metabolism during exercise and heat stress. J Appl Physiol 69:1040–1046
Nybo L (2010) CNS fatigue provoked by prolonged exercise in the heat. Front Biosci (Elite Ed) 2:779–792
Piacentini MF, Meeusen R, Buyse L et al (2002) No effect of a noradrenergic reuptake inhibitor on performance in trained cyclists. Med Sci Sports Exerc 34(7):1189–1193
Piacentini MF, Clinckers R, Meeusen R et al (2003) Effect of burpropion on hippocampal neurotransmitters and on peripheral hormonal concentrations in the rat. J Appl Physiol 95:652–656
Ramanathan LM (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–532
Roelands B, Hasegawa H, Watson P et al (2008a) Acute DA reuptake inhibition enhances performance in warm but not temperate conditions. Med Sci Sports Exerc 40(5):879–885
Roelands B, Goekint M, Heyman E et al (2008b) Acute norepinephrine reuptake inhibition decreases in normal and high ambient temperature. J Appl Physiol 105(1):206–212
Roti MW, Casa DJ, Pumerantz AC et al (2006) Thermoregulatory responses to exercise in the heat: chronic caffeine intake has no effect. Aviat Space Environ Med 77:124–129
Slattum P, Venitz J, Barr W (1996) Comparison of methods for the assessment of central nervous system stimulant response after dextroamphetamine administration to healthy male volunteers. J Clin Pharmacol 36:1039–1050
Spriet LL, MacLean DA, Dyck DJ et al (1992) Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am J Physiol 262(6Pt1):E891–E898
Stebbins CL, Daniels JW, Lewis W (2001) Effects of caffeine and high ambient temperature on haemodynamic and body temperature responses to dynamic exercise. Clin Physiol 21(5):528–533
Tarnapolski M, Cupido C (2000) Caffeine potentiates low frequency skeletal muscle force in habitual and non-habitual caffeine consumers. J Appl Physiol 89(5):1719–1724
Walker GJ, Dziubak A, Houghton L et al (2008) The effect of caffeine ingestion on human neutrophil oxidative burst responses following time-trial cycling. J Sports Sci 26(6):611–619
Watson P, Hasegawa H, Roelands B et al (2005) Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 565(Pt 3):873–883
Whitham M, Walker GJ, Bishop NC (2006) Effect of caffeine supplementation on the extracellular heat shock protein 72 response to exercise. J Appl Physiol 101(4):1222–1227
Acknowledgments
We want to acknowledge the hard work of Giullia De Ioannon, Vinciane Fontenelle, Michael Marien, Marco Iorio, Gerd Vande Velde and Eric Muyldermans during the experiments. Bart Roelands is a postdoctoral fellow of the Fund for Scientific Research Flanders (FWO). This study was supported by research funding from the Vrije Universiteit Brussel (OZR 990 and 1235).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jacques R. Poortmans.
Rights and permissions
About this article
Cite this article
Roelands, B., Buyse, L., Pauwels, F. et al. No effect of caffeine on exercise performance in high ambient temperature. Eur J Appl Physiol 111, 3089–3095 (2011). https://doi.org/10.1007/s00421-011-1945-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-1945-9