Abstract
Recent efforts to establish a role for plasma matrix metalloproteinase-9 (MMP-9) as a marker of exercise-induced muscle damage have been inconsistent. Methodological and experimental design issues have contributed to confusion in this area. The purpose of this study was to use a damaging eccentric arm task to evaluate the relationship between activity-induced muscle damage and plasma MMP-9 levels in humans while controlling for physical activity history and quantifying day-to-day variability of the dependent variables. Fourteen physically inactive males performed 6 sets of 10 eccentric contractions of the elbow flexors at 120% of their voluntary concentric maximum. Soreness ratings, maximum voluntary isometric strength, range of motion (ROM), limb circumference, and plasma creatine kinase (CK) and MMP-9 levels were measured at 2 time points before, immediately after, and 1, 2, 4, and 7 days post-exercise. Changes in traditional markers of muscle damage mirrored patterns previously reported in the literature, but plasma MMP-9 concentration and activity measured by ELISA and gelatin zymography were unchanged at all time points examined. Plasma levels of the MMP-9 inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), were also unchanged post-exercise. Finally, although mean MMP-9 levels were not significantly different between the two pre-exercise timepoints, the high total error of measurement and low day-to-day correlation suggest substantial within and between subject variability. Plasma MMP-9 levels are not a robust or reliable marker for eccentric exercise-induced damage of the elbow flexor musculature, though this may not preclude a role for MMPs in skeletal muscle remodeling in response to injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eccentric exercise, especially when the task is novel, produces muscle soreness and strength loss as a secondary consequence of strain-induced muscle damage (Armstrong et al. 1983; Fríden et al. 1983; Newham et al. 1983). Eccentric exercise-induced muscle damage has been associated with disruptions to the muscle fiber contractile machinery (Fríden et al. 1981, 1983; Yu et al. 2004), post-exercise infiltration of immune cells to the site of injury (Armstrong et al. 1983), and damage to, and degradation of, the muscle extracellular matrix (ECM; Brown et al. 1997; Kjaer et al. 2006; Kovanen 2002). Increased degradation of the ECM may contribute to decrements in force transmission (Gao et al. 2008) and may also be a contributing factor to the recruitment of immune cells during the immediate post-exercise inflammatory response (Adair-Kirk and Senior 2008).
Matrix metalloproteinases, or MMPs, are secreted proteinases that degrade ECM proteins and thus play a prominent role in the remodeling of the ECM during adaptive states (Sternlicht and Werb 2001). Expression and/or activity of several MMPs, and of the inducible gelatinase MMP-9 in particular, is increased in rodent models of eccentric muscle damage (Koskinen et al. 2002). Studies of systemic MMP-9 levels following eccentric exercise in humans have been more equivocal (Koskinen et al. 2001b; Mackey et al. 2004) and the relationship between muscle damage and systemic MMP-9 levels is still not clear. In human studies using lower limb eccentric activity, MMP-9 was unchanged by downhill running at room temperature (Koskinen et al. 2001b), but increased approximately equally (50%) by either downhill running in a cold room (Koskinen et al. 2001b) or by high force isokinetic eccentric contractions (Mackey et al. 2004) despite substantial differences in a different marker of muscle injury, plasma creatine kinase levels, between these two tasks (Koskinen et al. 2001b; Mackey et al. 2004). Moreover, the timing differed considerably in these studies, with MMP-9 increasing 1 day after downhill running in the cold (Koskinen et al. 2001b) and 8 days following a single bout of 100 isokinetic eccentric contractions of the knee (Mackey et al. 2004). In these studies it is unclear whether or not the protective repeated bout effect was controlled for because the subjects in the downhill running study were described as physically active, young adult males (Koskinen et al. 2001b), while the subjects in the knee extensor study were described as healthy, young adult males and females (Mackey et al. 2004), without reference to specific training history or physical activity screening criteria in either case. Finally, studies on the biological and methodological variation in systemic MMP-9 levels, particularly in a well-defined physically inactive but healthy young male population, are limited, and thus it is not clear whether MMP-9 may be a reliable index of post-exercise damage in humans. Systemic creatine kinase (CK) levels have been traditionally used as blood indicators of muscle injury following damage, but the CK response is highly variable in the general population (Clarkson and Ebbeling 1988; Hortobagyi and Denham 1989) and may not correspond to the magnitude of muscle injury that has occurred (Clarkson and Hubal 2002; Warren et al. 1999).
The purpose of this study was to determine the effects of a well-characterized, highly damaging eccentric arm task on systemic levels and activity of MMP-9 and on levels of its inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), in humans while rigorously controlling for the confounding factors such as prior activity level associated with this experimental model. We chose to utilize the eccentric arm task because of its frequent use in muscle damage research as well as for its well-documented effects producing robust changes in traditional indices of muscle damage such as maximal concentric force loss and, importantly, increased plasma creatine kinase levels. In addition, the upper extremity musculature in physically inactive individuals may be less protected from muscle damage by the repeated bout effect than the lower extremity musculature involved in locomotion. Direct comparisons between eccentric arm and lower limb tasks have amply demonstrated that arm tasks produce greater changes in force production and plasma creatine kinase (Jamurtas et al. 2005) as well as greater changes in intracellular signaling protein concentration (Thompson et al. 2003). We therefore hypothesized that MMP-9 activity would increase following a bout of upper-extremity eccentric exercise in physically inactive males.
Methods
Subjects
All subjects gave written, informed consent to participate in the study, which was approved by the University of Colorado (CU) Institutional Review Board. Subjects were 18–30 year-old, physically inactive males (n = 14). Prior to participation in the exercise experiment, a physician at the CU-Boulder Clinical Translational Research Center (CTRC) performed a medical history and physical exam to screen subjects for the determination of health/disease status, the presence of pathological conditions that could influence systemic levels of markers of regeneration and repair (muscle and liver enzymes), and the ability to safely perform the upper-extremity exercise task. The physical exam included complete blood chemistries, and resting and exercise ECG during a maximal graded treadmill exercise test. In addition, subjects were asked about the nature, frequency, duration, and intensity of physical and occupational activities performed in the preceding 12 months through the use of an interviewer-administered Modifiable Activity Questionnaire (MAQ) (Kriska 1997). Subjects were excluded from the study if they met or exceeded the ACSM’s definition of “physically active,” participating in moderate intensity aerobic physical activity for a minimum of 30 min per day, 5 days per week or vigorous activity for 20 min per day, 3 days per week (Pollock et al. 1998). In addition, subjects were excluded if they performed any weight lifting, resistance training, or similar activities in the previous 6 months. Subjects who passed the physical activity screening criteria and were included in the study were told to avoid any exercise or physical activities, the use of ice and/or anti-inflammatory medications, therapeutic stretching of the upper-extremities, and to maintain normal dietary habits for the duration of the study.
Experimental protocol
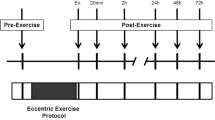
Following successful screening, subjects reported to the laboratory six times to complete the experimental procedures. These visits were scheduled at the same time of day and required subjects to adhere to the physical activity, anti-inflammatory, and dietary restrictions for the duration of the study. A summary of the study timeline is provided in Fig. 1. The first visit served to determine baseline values for the dependent variables and determine each subject’s maximal concentric strength. The second visit involved repeating baseline dependent variable measures followed by the performance of the eccentric exercise task and subsequent measurement of the dependent variables. Visits 4 to 7 involved measurement of the dependent variables. The dependent variables assessed were rating of perceived soreness, elbow flexor strength during a maximum voluntary isometric contraction of the non-dominant upper arm, range of motion (ROM), limb circumference, plasma CK activity, plasma MMP-9 activity, plasma MMP-9 concentration, and plasma TIMP-1 concentration.
Schematic outlining the study visits. The Screening Visit included the informed consent, a medical history and physical including blood chemistry screening and a graded exercise test, and a physical activity inventory. The Baseline Visit was scheduled 7–10 days after the screening visit and served to determine baseline values for the dependent variables including plasma CK, MMP-9 and TIMP-1, soreness rating, range of motion (ROM), limb circumference, maximum voluntary isometric contraction force (MVIC). In addition, maximum voluntary concentric contractions (MVCC) were performed to determine a subjects maximal concentric force production. The Exercise Visit was scheduled 1–3 days after the Baseline Visit and involved repeating baseline dependent variable measures followed by the performance of the eccentric exercise task and subsequent measurement of the dependent variables. All remaining visits (24 h post, 48 h post, 4 days post, and 7 days post) involved measurement of the dependent variables
For the baseline session, a blood sample was collected using standard phlebotomy procedures from the antecubital region of the dominant arm. A rigid wrist orthotic (Orthomerica Products, Inc., Newport Beach, CA) was then placed on the subject’s non-dominant arm for the remainder of each experimental session to prevent excessive wrist movement during the strength measurement and/or eccentric exercise injury task. Rating of perceived soreness was assessed with a computer-based Visual Analog Scale (VAS) controlled by the subject moving a 100-mm-long slider bar to indicate “least discomfort” versus “most discomfort.” This assessment was performed while the subject’s non-dominant arm was at rest and during 5-elbow flexion/extension motions. The subject was then seated on a stool at a custom-built (SuperStrut®, Thomas & Betts, Memphis, TN) strength test apparatus with the non-dominant arm supinated and supported at 90° of shoulder flexion in the sagittal plane by a padded platform. Maximal voluntary isometric strength at an angle of 90° of elbow flexion was measured using a force transducer interfaced to an A-D converter (OMEGA Engineering, INC., Stamford, CT), and recording software (LabView, National Instruments, Austin, TX). Next, subjects performed a maximal concentric elbow flexion test on the same custom-built apparatus by holding onto a rigid handle affixed to a cable and pulley system to which weight could be incrementally added until the subject could no longer complete the concentric portion only of a single arm curl in the sagittal plane. Two minutes of rest were given between each consecutive attempt to perform the concentric arm curl and the weight was lowered back to the starting position by the investigator. The weight associated with this task was deemed the 1-repetition maximum (1-RM). This 1-RM value was used in a calculation to determine the amount of weight to use in the next laboratory session for the eccentric exercise task. ROM of the elbow joint was measured by positioning the fulcrum of a manual goniometer on the lateral epicondyle of the humerus and adjusting the goniometer arms to align with the lateral midline of the humerus and the lateral midline of the radius while the subject was seated at a stool. These anatomical references were marked with permanent marker for identification at follow-up visits. Limb circumference was measured at two different marked locations (mid-belly of the biceps brachii and at the elbow joint) using a soft tape measure.
For the eccentric exercise session, subjects returned to the lab at the same time of day and within 3 days of the Baseline session. Subjects were also reminded to adhere to the study procedures regarding exercise, diet, and anti-inflammatory medications. Prior to the exercise, all dependent parameters were re-assessed (Pre-Ex) for the determination of day-to-day (Baseline vs. Pre-Ex) biological variation. For the eccentric exercise task, subjects used their non-dominant arm to perform the eccentric portion only of an arm curl task. They did this by lowering a weight corresponding to 120% of the maximal concentric 1-RM performed at the Baseline session. The eccentric task involved 6 sets of 10 repetitions each lasting 10 s with 2 min of rest between sets. Between each repetition, the handle and cable were returned to the fully flexed elbow joint position by the investigator so that the subject was not performing the concentric portion of the task. All 60 repetitions were completed over the entire range of motion, even if a subject was unable to lower the weight at a controlled velocity. Immediately-post (Post-Ex) eccentric exercise, blood was collected again and soreness and strength were re-assessed. Subjects then returned to the lab on 4 additional occasions for follow-up visits at 1 day-, 2 days-, 4 days-, and 7 days-post eccentric exercise task. Each follow-up visit occurred at approximately the same time of day as the completion of the eccentric exercise session and included the collection of a blood sample and the assessment soreness and strength.
Blood sampling and storage
Blood samples were drawn from an antecubital vein in the dominant arm by venipuncture at each visit and collected in lithium heparin-coated vacutainer tubes (BD®, Franklin Lakes, NJ) then immediately centrifuged for 15 min at 1,500×g. Blood plasma was separated and immediately frozen and stored at −20°C in small volume aliquots until used for the assays described, while avoiding more than 3 freeze–thaw cycles for any given aliquot as recommended by a recent publication (Souza-Tarla et al. 2005). All blood samples, basal and post-exercise, were collected, handled, and stored under identical conditions.
Gelatin zymography for MMP-9 activity
Gelatin zymography was used to quantify MMP-9 activity in blood plasma as previously described (Allen et al. 2002). SDS polyacrylamide gels (10%) containing 1 mg/ml gelatin were casted and overlaid with a 4% stacking gel. Blood plasma was mixed with 1:1 volume sample buffer consisting of 50 mM Tris, pH 6.8, 2% SDS, 20% glycerol, and 0.2% bromophenol blue without reducing agent or heat. Electrophoresis was carried out at 100 volts until the dye front had reached the bottom of the gel. Gels were removed from the glass plates and washed in 2.5% Triton-X 100 three times for 15 min each time to remove SDS from the gel. The gels were then incubated at 37°C for 18 h in 50 mM Tris, pH 7.5, 10 mM CaCl2. Gels were then stained with Coomassie brilliant blue for 30 min and de-stained with 40% methanol/10% acetic acid for 1 h. Gels were imaged using a ChemiDoc-It imaging system (UVP, LLC., Upland, CA). Enzymatic activity was quantified for integrated density using ImageJ (NIH) software. Integrated density values for MMP-9 activity were normalized for each individual using his Baseline value.
Plasma MMP-9 and TIMP-1 ELISA assay
Total plasma MMP-9 and TIMP-1 were measured in duplicate by sandwich ELISA using commercially available kits (Quantikine, R&D Systems, Minneapolis, MN). Samples were prepared per the kit recommendations with a 40-fold plasma dilution for MMP-9 and a 100-fold plasma dilution for TIMP-1 into Calibrator Diluent RD5-10 and RD5P, respectively (Quantikine, R&D Systems, Minneapolis, MN), and measured using a microplate reader set to 450 nm with a correction of 540 nm.
Creatine kinase activity assay
A commercially available kit (Sigma–Aldrich, St. Louis, MO) was used for spectrophotometric analysis of creatine kinase activity with a peristaltic water pump maintaining a temperature of 37°C during analysis. Samples were prepared according to the kit and spectrophotometrically read at 340 nm for three minutes. Activity per minute was recorded and translated into U/L using a simple equation provided with the kit (Sigma–Aldrich, St. Louis, MO). Each sample was quantified in duplicate. Control samples (DC-TROL level 1 &2, Sigma–Aldrich, St. Louis, MO) were also analyzed each day creatine kinase analyses were performed.
Statistics
The stability of the dependent variables was determined using paired t tests to compare means, the calculation of total measurement error (TEM), and correlational analysis using the Pearson r value. Coefficient of variation (CV) and upper and lower 95% confidence limits (95% CI) were calculated using a statistical package that is available online (A New View of Statistics, Will G. Hopkins, 2009, <http://www.sportsci.org/resource/stats/index.html>). The PASW statistical packages version 17.0 was used for all other statistical evaluations (SPSS Inc, Chicago, IL, USA). One-way (Fixed factor = laboratory session) repeated-measures analysis of variance (1-RM ANOVA) using Greenhouse-Geiser correction with Least Significant Difference (LSD) post-hoc analysis was performed on all continuous dependent measures. Data are presented as means ± SEM unless otherwise stated.
Results
Indices of muscle damage
In total, 18 subjects were recruited for this study; however, dependent measures from all time points were assessed in just 14 of 18 subjects, and only these 14 are reported here. Two subjects did not show up for their last visit and blood samples were difficult to collect from the other 2 subjects, so measures from these subjects were excluded from the RM-ANOVAs. Individuals were 18–29 years in age (21.4 ± 3.2 years), 180.43 ± 5.7 cm in height, and 70.81 ± 10.3 kg in mass. A schematic of the study timeline and parameters measured at each visit is provided in Fig. 1.
Soreness, strength, and CK responses to the eccentric arm task are summarized in Fig. 2. There was no significant difference in soreness from Baseline (1.80 ± 3.10 mm) to Pre-Ex (4.71 ± 8.82 mm). Although the P-value was close to being significant (P = 0.092), the mean soreness values were all on the very low end of a 100-mm scale at both of the pre-exercise time points, and the correlation between the two values was very strong (r = 0.885). Soreness ratings were significantly increased from both Baseline and Pre-Ex values following the exercise task (Post-Ex, 1 day, 2 days, 4 days) and peaked at 1 day post-exercise (Fig. 2a). By 7 days post-exercise, soreness ratings were not significantly different from Baseline or Pre-Ex values.
Traditional indicators of muscle damage across multiple time points following an upper extremity eccentric exercise task. a Visual analog scale soreness rating during 5 repetitive elbow flexion/extension tasks was significantly increased from both Baseline and Pre-Ex values at the Post-Ex, 24, 48, and 4 day time points. By 7 days post-exercise, soreness rating was not significantly different from Baseline or Pre-Ex values. b Elbow flexor maximal voluntary isometric force measured at an arm angle of 90° of elbow flexion was significantly decreased from both Baseline and Pre-Ex values at all time points following the eccentric exercise task, but there was a trend toward a restoration of force by 7 days post-exercise. c Plasma creatine kinase (CK) activity (u/l) was significantly increased from Baseline and Pre-Ex values at 4 days post-exercise. Bars represent means ± SEM. *Significantly different from Baseline and Pre-Ex, P < 0.05
There was no significant difference in maximum isometric strength from Baseline (126.53 ± 42.87 N) to Pre-Ex (119.70 ± 36.45 N; P = 0.110), and the values were strongly correlated (r = 0.919). The TEM for the pre-exercise values was 12.13 N (95% CI: 9.11–18.19 N), and the coefficient of variation (CV) was 10.9% (95% CI: 8.1–16.7%). Maximum isometric elbow flexion force was significantly decreased from Pre-Ex values at all time points following the exercise task, with the peak of maximal force loss of approximately 51 and 44% occurring at the Post-Ex and 1 day-post time points, respectively (Fig. 2b). By 7-days post-exercise, maximum isometric force remained significantly decreased compared to Baseline and Pre-Ex strength, although there was a trend toward a restoration to Baseline values.
There was no significant difference in CK activity from Baseline to Pre-Ex (P = 0.615), but the Baseline and Pre-Ex values were poorly correlated (r = 0.115), TEM for CK activity was 162.81 U/L (95% CI: 121.26–247.79 U/L), and the coefficient of variation (CV) was 112.3% (95% CI: 75.2–214.4%). CK activity was highly variable (Supplementary Figure 1), but significantly increased 15-fold from Baseline to 4 days post-exercise. By 7-days post-exercise, CK activity was not significantly different from Pre-Ex or Baseline levels (Fig. 2c).
ROM and limb circumference responses to the eccentric arm task are summarized in Fig. 3. There was no significant difference in ROM from Baseline to Pre-Ex (P = 0.171) and the two pre-exercise time points were strongly correlated (r = 0.777). The TEM for the pre-exercise ROM values was 3.16° (95% CI: 2.38–4.75°) and the CV was 2.6% (95% CI: 2.0–4.0%). There was a significant decrease (P < 0.05) in ROM from pre-exercise to all post-exercise time points and by 7 days post-exercise, ROM was still decreased by approximately 5° (Fig. 3a).
Changes in range of motion (ROM) and limb circumference across multiple time points following an upper extremity eccentric exercise task. a Range of motion decreased significantly at all post-exercise time points. Bars represent means ± SEM. *Significantly different from Baseline and Pre-Ex. b Limb circumference measured at the mid-belly of the biceps brachii (light bars) increased significantly from both Baseline and Pre-Ex values at 48 h and 4 days post exercise. Limb circumference measured at the elbow joint (dark bars) increased significantly from both Baseline and Pre-Ex values at all post-exercise time points with a peak increase at 7 days post exercise. Bars represent means ± SEM. *Mid-belly measurements significantly different from Baseline and Pre-Ex, P < 0.05. #Elbow joint measurements significantly different from Baseline and Pre-Ex, P < 0.05
There was no significant difference in limb circumference at either the belly of the biceps brachii (P = 0.898) or the elbow joint (P = 0.302) from Baseline to Pre-Ex and the pre-exercise time points were strongly correlated for both parameters (r = 0.990 and r = 0.988, respectively). The TEM for the biceps belly pre-exercise values was 3.29 mm (95% CI: 2.47–4.94 mm) and the CV was 1.1% (95% CI: 0.8–1.7%). The TEM for the elbow joint pre-exercise values was 2.26 mm (95% CI: 2.00–3.99 mm) and the CV was 1.1% (95% CI: 0.8–1.6%). Arm circumference at the mid-belly of the biceps brachii was significantly increased (P = 0.020) from pre-exercise values by +6.58 mm at 48 h post exercise and by +6.47 mm at 4 days post exercise. By 7 days post exercise, mid-belly arm circumference was not different from pre-exercise values. Arm circumference at the elbow joint was significantly increased (P < 0.05) at all post-exercise time points and was maximal at 7 days post exercise (+10.16 mm) (Fig. 3b).
Baseline and post-exercise MMP-9 levels
Basal plasma MMP-9 concentration ranged from 13.12 to 150.75 ng/ml with mean values of 59.10 ng/ml at Baseline and 59.27 ng/ml at Pre-Ex. A literature search for studies examining plasma MMP-9 values in control subjects from a variety of ages and ethnicities using the same kit and blood sampling methodology as the present study yielded over 25 publications with a range of MMP-9 values from 17.2 (Castellano et al. 2008) to 156.7 ng/ml (Gai et al. 2009), with most values clustered within 40–100 ng/ml. Thus the baseline values of ~60 ng/ml reported in the present study are well within both the range of values reported by the manufacturer (R & D Systems) for control populations (13–105 ng/ml) and the range of published values by other laboratories.
There was no significant difference in MMP-9 concentration from Baseline to Pre-Ex (P = 0.721). The TEM for the pre-exercise values was 35.45 ng/ml (95% CI: 26.40–53.95 ng/ml) and the CV was 75% (95% CI: 51.0–134.3%). The correlation between the Baseline and Pre-Ex MMP-9 levels was 0.100. Figure 4a shows the individual changes in plasma MMP-9 concentration for the subjects in this study and illustrates the wide inter-subject variation in basal MMP-9 levels from day-to-day. Since all time point samples for each subject were handled identically and analyzed on the same plate, these day-to-day differences likely reflect biological and not methodological variation. Two subjects showed increases in MMP-9 concentration between the Baseline and Pre-Ex time points, from 20–40 ng/ml to 100–140 ng/ml (Fig. 4a). Conversely 3 subjects showed decreases in plasma MMP-9 concentration from ~100–150 ng/ml to 30–80 ng/ml (Fig. 4a). The rest of the subjects showed only modest changes in plasma MMP-9 concentration from Baseline to Pre-Ex (Fig. 4a).
Variation in pre-exercise plasma MMP-9 (a) and TIMP-1 (b) levels. Lines represent the individual changes in plasma MMP-9 (a) and TIMP-1 (b) concentration for two resting time points (Baseline and Pre-Ex) and all post-exercise time points (Post-Ex, 24, 48 h, 4, and 7 days). The group mean values are represented by the shaded bars. There was no main effect for sampling time for MMP-9 concentration (P = 0.375) or for TIMP-1 concentration (P = 0.106), but this figure illustrates the wide inter-subject variation in basal MMP-9 and TIMP-1 concentration from day-to-day (Baseline vs. Pre-Ex) and how that compares with the post-exercise changes
There was no significant post-exercise change in circulating levels of MMP-9 as measured by gelatin zymography (P = 0.294) (Fig. 5a) or in either MMP-9 or TIMP-1 as measured by ELISA over the time course of the study (Fig. 5b, c). There was a trend toward a decrease in plasma TIMP-1 activity from Pre-Ex (96.62 ± 23.03 ng/ml) to Post-Ex (80.06 ± 28.10 ng/ml), but the difference was not significant (P = 0.106) and was heavily influenced by one individual (Fig. 4b).
Plasma MMP-9 concentration and activity and TIMP-1 concentration post-exercise. a Plasma MMP-9 activity (fold change relative to Baseline) quantified by gelatin zymography and b MMP-9 concentration (ng/ml) quantified by ELISA at multiple time points following eccentric exercise of the elbow flexors in humans. c TIMP-1 concentration quantified by ELISA at multiple timepoints following eccentric exercise of the elbow flexors in humans. There was no main effect for sampling time for MMP-9 activity (P = 0.294) or concentration (P = 0.375) or TIMP-1 concentration (P = 0.106). Bars represent mean ± SEM
Discussion
The results from the present study demonstrate that plasma MMP-9 is not a reliable or robust systemic marker for exercise-induced muscle damage or repair following an eccentric arm task in humans. As mentioned above, we chose this eccentric arm task in part because despite the smaller volume of muscle involved (arm flexors vs. knee extensors), this type of task has been demonstrated to produce greater changes in damage indices, most notably in another systemic marker of muscle damage, plasma creatine kinase, than lower limb tasks of the same relative intensity (Jamurtas et al. 2005; Thompson et al. 2003). If systemic MMP-9 levels are indeed a robust and reliable measure of muscle damage, then we predicted that the eccentric arm task used here would produce an equal or even greater increase in plasma MMP-9 levels than those reported previously for lower limb eccentric tasks (Koskinen et al. 2001b; Mackey et al. 2004). However, while changes in traditional markers of muscle damage (soreness rating, CK, peak isometric force) were significant and indicated that the upper-arm eccentric exercise task was indeed a damaging stimulus, neither plasma MMP-9 concentration nor activity was significantly changed at any post-exercise time point.
Moreover, while mean pre-exercise plasma MMP-9 levels were within the expected range of published values (Castellano et al. 2008; Gai et al. 2009), individual variation in pre-exercise plasma MMP-9 levels was high both across- and within-subjects (Fig. 4). Other investigators have also reported a large range of basal systemic MMP-9 values. Specifically, Tayebjee and colleagues reported that resting blood plasma MMP-9 levels to ranged from 17 to 115 ng/ml in their subjects (Tayebjee et al. 2005). Similarly, in a human study involving eccentric contractions of the knee extensors, mean pre-exercise serum MMP-9 was 112 ng/ml with a standard deviation of ±42 ng/ml (Mackey et al. 2004). A key strength of the present work is the fact that we examined two pre-exercise time points for all measures, including plasma MMP-9 levels, and thus were able to monitor day-to-day fluctuations in plasma MMP-9 levels within subjects independent of the exercise stimulus. Our results demonstrate that much of the variation in plasma MMP-9 levels appears to be due to wide day-to-day variation within subjects, with several subjects showing dramatic changes (increases or decreases) in plasma MMP-9 levels between the Baseline and Pre-Ex visits, which were always less than 3 days apart (Fig. 4a).
Part of the large variation in day-to-day MMP-9 activity observed in any study can be attributed to biological variation and part to methodological variation. As mentioned above, in the present study all samples for a given subject were processed and frozen identically and analyzed at the same time on the same ELISA plate. Thus differences in plasma MMP-9 values between the Baseline and Pre-Ex time points for a given subject were unlikely to reflect major methodological differences in sample storage or analysis. However, because each sample in the present study was assayed in duplicate, an estimate of the intra-sample methodological variability can be made. The mean CV for plasma MMP-9 activity measured between duplicates in the present study was 7.79%. This CV value represents a reasonable estimate of the methodological variation associated with the assay performed, but it is only a small contributor to the CV between the Baseline and Pre-Ex (75%), and thus much of the variability within subjects is likely due to biological variability.
It is not clear what might have caused the high biological variability between the two pre-exercise time points. Polymorphisms exist in the MMP-9 promoter, but are not linked to plasma MMP-9 variation in healthy subjects (Demacq et al. 2008). The acute effect of diet on plasma MMP-9 levels has not been adequately explored, and thus it is not clear whether day-to-day variations in diet may have explained the differences observed in plasma MMP-9 in the present study. In addition, subjects were asked to maintain their regular diet and came in at the same approximate time for each visit, and thus it is unlikely that changes in diet or alimentation account for the majority of the variability reported here. Similarly, subjects were also screened so that they had not performed upper-extremity weight-lifting, resistance training, or similar activities in at least the previous 6 months and were told to avoid any exercise or physical activities, the use of ice and/or anti-inflammatory medications, and/or therapeutic stretching of the upper-extremities for the duration of the experiment. It therefore seems unlikely that differences in day-to-day physical activity levels accounted for an appreciable amount of the biological variance in pre-exercise plasma MMP-9 levels reported here. Thus at the present time there is not a clear explanation for the high biological variability that contributes to the remainder of the TEM for pre-exercise plasma MMP-9 levels within subjects.
Creatine kinase has often been criticized as a marker for exercise-induced muscle injury because it has been shown to be highly variable in the general population (Clarkson and Ebbeling 1988; Hortobagyi and Denham 1989) and because it may not appropriately reflect the magnitude of muscle damage that has occurred (Clarkson and Hubal 2002; Warren et al. 1999). Moreover, like the pre-exercise plasma MMP-9 levels reported here, basal blood creatine kinase levels can also vary substantially across subjects (Strømme et al. 2004). However, in the present study the experimental effect of damaging arm exercise caused a robust and significant CK response, as unlike plasma MMP-9 levels, peak plasma CK levels exceeded the methodological and biological variability of this parameter as demonstrated by the statistically significant 15-fold increase that we report here at 4 days post-exercise. Thus despite similar limitations in terms of pre- and post-exercise variance, eccentric arm exercise was sufficient to produce an increase in systemic CK but not MMP-9 levels. And while differences in assay sensitivity may contribute to some of this discrepancy, it nevertheless supports the conclusion that plasma MMP-9 levels are not a robust indicator of muscle damage. Biopsy studies and/or microdialysis may provide better insights into whether local changes in MMP-9 expression occur within the muscle following damaging exercise, but these are considerably more invasive and time consuming than blood collection.
Despite the high variability in pre-exercise systemic MMP-9 levels, the lack of a post-exercise systemic MMP-9 response in the present study was unexpected because of previous work demonstrating a significant increase in MMP-9 levels following eccentric activity of the lower limb(s) (Koskinen et al. 2001b; Mackey et al. 2004). However, the significant systemic increases in MMP-9 that have been reported by others occur at inconsistent time points, are sometimes dependent upon the room temperature where the exercise is performed (Koskinen et al. 2001b), and are within the observed range of day-to-day variability that we report here. These inconsistencies are likely one of the reasons Koskinen and colleagues concluded that serum levels of MMP-9 do not sensitively respond to exercise induced muscle damage (Koskinen et al. 2001b), which is consistent with the findings of the present study. We tried to maximize our likelihood of observing a systemic MMP-9 response by incorporating rigorous controls into our subject selection and study design. For example, we attempted to control for the well-documented “repeated bout effect” (Byrnes et al. 1985), in which prior bouts of eccentric exercise reduce the magnitude of muscle soreness and damage in subsequent bouts, by recruiting subjects who had not participated in any physical or occupational activities with their upper-extremities for a minimum of 6 months prior to the experiment, which was not explicitly described in other similar studies (Koskinen et al. 2001b; Mackey et al. 2004). In addition, we chose to produce injury in the upper- versus lower-extremity muscles because the arm muscles, particularly in the non-dominant arm, tend to get used less frequently than those of the lower limb which are used daily for locomotion, going down stairs, etc. By selecting an inactive subject population and an appropriate task, we felt that we would minimize the effects of the repeated bout effect in a way that would maximize our likelihood of observing a systemic MMP-9 response despite the high variance in basal MMP-9 levels, yet we still did not observe an increase in systemic MMP-9 following eccentric exercise.
It is not clear why eccentric arm exercise failed to produce an increase in systemic MMP-9 levels in the present study while at least one study using the lower limbs elicited increases in systemic MMP-9 levels following exercise (Mackey et al. 2004). One possibility is that the single arm eccentric task used here, while damaging, did not involve sufficient tissue volume to elicit systemic increases in MMP-9 levels. However, given that systemic CK levels are typically greater following an eccentric arm task compared to a leg task of the same relative intensity (Jamurtas et al. 2005), this seems unlikely. Another possibility is that the duration of our study was too short, since Mackey et al. reported a significant increase in MMP-9 levels at 8 days post-exercise. However, given the dramatic and significant changes occurring to the traditional markers of muscle damage (soreness, arm circumference, ROM, maximal force production, CK) within the 7 day time range used in this study, we feel that 7 days should have been more than ample time to detect a change in MMP-9. Indeed, most of these other damage markers had resolved back to baseline values by 7 days, and thus it seems unlikely that a change in MMP-9 would occur outside this time frame. In addition, we observed no trend toward an increase in MMP-9 levels at 7 days post-exercise, which might suggest that a significant change might have occurred at 8 days or some later time point. Another possibility is that changes in systemic MMP-9 levels may be greater for low-force, high repetition activities like running. Consistent with this, in a human study, marathon running produced an increase in serum MMP-9 immediately following a race (Saenz et al. 2006). This possibility might explain why MMP-9 mRNA and activity were increased in humans after a single bout of cycling exercise (Rullman et al. 2007) and a single bout of knee extensions (Rullman et al. 2009), two tasks that are typically not associated with muscle damage. Third, it is possible that differences in ECM composition and/or turnover exist between the upper and lower limb musculature, but if this is the case it has not been well documented scientifically. Fourth, differences in leakage rates into the systemic vasculature and/or local clearance of MMP-9 between the upper and lower extremities may also exist though, again, scientific confirmation of these is lacking.
Another possibility is that the eccentric arm exercise task, while damaging, may not be sufficient to cause alterations to the muscle ECM. In a recent review by Butterfield, the distinction between exercise-induced muscle damage and severe strain-induced muscle injury was emphasized, and the author pointed out that in vivo exercise protocols often involve less mechanical strain to muscle fibers when compared with in vitro or in situ muscle damaging protocols typically done in rodents (Butterfield 2010). It is thus possible that MMP activity is increased and detectable in the systemic circulation only following a protocol that more closely resembles traumatic muscle injury, rather than exercise-induced injury. In studies using severely damaging interventions such as cardiotoxin injection (Kherif et al. 1999), ischemia-reperfusion (Roach et al. 2002), crush (Zimowska et al. 2008), or denervation (Chattopadhyay et al. 2007), an increase in muscle MMP-9 activity is almost always reported. Similarly, pathological muscle diseases resulting in a chronic state of muscle damage and inflammation are also associated with increased basal levels of MMP-9, both locally (Kieseier et al. 2001; Schoser et al. 2002) and systemically (Koskinen et al. 2001b). Marathon running is associated with increases in systemic MMP-9 levels (Saenz et al. 2006), but in this case the ground reaction force produced by the feet over an extended period of time may induce additional traumatic injury to muscles or other limb structures (bones, tendons, ligaments) that result in greater connective tissue damage. In the present study, the fact that the eccentric arm exercise task did not result in a detectable systemic MMP-9 response may indicate that in vivo eccentric arm exercise is not, or is only minimally disruptive to, the muscle ECM. While the results from the present study suggest that systemic MMP-9 was not changed after an upper-extremity eccentric task, there may still be a role for MMP-9 in muscle damage and repair. As mentioned above, levels of MMP-9 activity may have been increased locally within the tissue and been involved in immune cell infiltration, connective tissue repair/remodeling, or both. Secondly, other MMPs, and in particular MMP-2, have also been implicated in the remodeling of the ECM proteins surrounding skeletal muscle (Kherif et al. 1999; Koskinen et al. 2001b; Sternlicht and Werb 2001). Though MMP-2 is constitutively expressed (Sternlicht and Werb 2001), some investigators have reported changes in local (Koskinen et al. 2001a) and systemic MMP-2 following eccentric exercise (Koskinen et al. 2002). By visual inspection of the gelatin zymograms from our eccentric arm exercise experiment, there was no indication that MMP-2 activity was changed at any specific time point throughout the experiment reported here (data not shown).
This study was designed to evaluate systemic MMP-9 levels as an index of muscle damage following eccentric contractions. Through a series of experiments, we have concluded that plasma MMP-9 is not a robust systemic index for eccentric arm exercise-induced injury in humans. Whether or not MMP-9 or other MMPs might be involved in the cellular adaptations that occur at the level of the tissue in response to eccentric exercise is not clear at this time. In future studies, establishing a relationship between the magnitude of disruption to the muscle ECM and the tissue MMP-9 response may provide more support for the role of MMPs in skeletal muscle injury and repair.
References
Adair-Kirk TL, Senior RM (2008) Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 40:1101–1110
Allen DL, Teitelbaum DH, Kurachi K (2002) Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro. Am J Physiol Cell Physiol 284(4):C805–C815
Armstrong RB, Ogilvie RW, Schwane JA (1983) Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54:80–93
Brown SB, Child RB, Day SH, Donnelly AE (1997) Indices of skeletal muscle damage and connective tissue breakdown following eccentric muscle contractions. Eur J Appl Physiol Occ Physiol 75(4):369–374
Butterfield TA (2010) Eccentric exercise in vivo: strain-induced muscle damage and adaptation in a stable system. Exerc Sport Sci Rev 38(2):51–60
Byrnes WC, Clarkson PM, White JS, Hsieh SS, Frykman PN, Maughan RJ (1985) Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol 59(3):710–715
Castellano G, Malaponte G, Mazzarino MC, Figini M, Marchese F, Gangemi P, Travali S, Stivala F, Canevari S, Libra M (2008) Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clin Cancer Res 14:7470–7480
Chattopadhyay S, Myers RR, Janes J, Shubayev V (2007) Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain Behav Immun 21(5):561–568
Clarkson PM, Ebbeling C (1988) Investigation of serum creatine kinase variability after muscle-damaging exercise. Clin Sci 75:257–261
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81(11):S52–S69
Demacq C, Vasconcellos VB, Marcaccini AM, Gerlach RF, Silva WA Jr, Tanus-Santos JE (2008) Functional polymorphisms in the promoter of the matrix metalloproteinase-9 (MMP-9) gene are not linked with significant plasma MMP-9 variation in healthy subjects. Clin Chem Lab Med 46(1):57–63
Fríden J, Sjostrom M, Ekblom B (1981) A morphological study of delayed muscle soreness. Experientia 37:506–507
Fríden J, Sjostrom M, Ekblom B (1983) Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 4:170–176
Gai X, Lan X, Luo Z, Wang F, Liang Y, Zhang H, Zhang W, Hou J, Huang M (2009) Association of MMP-9 gene polymorphisms with atrial fibrillation in hypertensive heart disease patients. Clin Chim Acta 408:105–109
Gao Y, Wineman AS, Waas AM (2008) Mechanics of muscle injury induced by lengthening contraction. Ann Biomed Eng 36(10):1615–1623
Hortobagyi T, Denham T (1989) Variability in creatine kinase methodological, exercise, and clinically-related factors. Int J Sports Med 10:69–80
Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, Paschalis V, Koutedakis Y, Nosaka K (2005) Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol 95:179–185
Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fourneir JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS (1999) Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205:158–170
Kieseier BC, Schneider C, Clements JM, Gearing AJ, Gold R, Tyoka KV, Hartung HP (2001) Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain 124(Pt 2):341–351
Kjaer M, Magnusson P, Krogsgaard M, Moller JB, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H (2006) Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208:445–450
Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han KY, Komulainen J, Kovanen J, Takala TE (2001a) Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol 280:R1292–R1300
Koskinen SO, Hoyhtya H, Turpeeniemi-Hujanen T, Martikkala V, Makinen TT, Oksa J, Rintamaki H, Loftberg M, Somer H, Takala TE (2001b) Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports 11:9–15
Koskinen SO, Ahtikoski AM, Komulainen J, Dost MR, Takala TE (2002) Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch 444:59–72
Kovanen V (2002) Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev 30(1):20–25
Kriska AM (1997) Modifiable activity questionnaire. In: MA Pereira, SJ FitzGerald, EW Gregg, et al (eds) A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 29(Suppl 6): S73–S78
Mackey AL, Donnelly AE, Turpeenniemi-Hujanen T, Roper HP (2004) Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol 97:197–203
Newham DJ, McPhail G, Mills KR, Edwards RH (1983) Ultrastructural changes after concentric and eccentric contractions in human muscle. J Neurol Sci 61:109–122
Pollock ML, Gaesser GA, Butcher JD, Despres J-P, Dishman RK, Franklin BA, Garber CE (1998) The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30(6):975–991
Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA (2002) Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor doxycycline. Eur J Vasc Endovasc Surg 23(3):260–269
Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T (2007) A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol 102:2346–2351
Rullman E, Norrbom J, Stromberg A, Wagsater D, Rundqvist H, Haas T, Gustafsson T (2009) Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol 106:804–812
Saenz AJ, Lee-Lewandrowski E, Wood MJ, Neilan TG, Siegel AJ, Januzzi JL, Lewandrowski KB (2006) Measurement of a plasma stroke biomarker panel and cardiac troponin T in marathon runners before and after the 2005 Boston marathon. Am J Clin Pathol 126(2):185–189
Schoser BGH, Blottner D, Stuerenburg H-J (2002) Matrix metalloproteinases in inflammatory myopathies: enhanced immunoreactivity near atrophic myofibers. Acta Neurol Scand 105(4):309–313
Souza-Tarla CD, Uzuelli JA, Machado AA, Gerlach RF, Tanus-Santos JE (2005) Methodological issues affecting the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Clin Biochem 38:410–414
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Strømme JH, Rustad P, Steensland H, Theodorsen L, Urdal P (2004) Reference intervals for eight enzymes in blood of adult females and males measured in accordance with the International federation of clinical chemistry reference system at 37 degrees C: part of the Nordic reference interval project. Scand J Clin Lab Invest 64:371–384
Tayebjee MH, Lip GYH, Blann AD, MacFadyen RJ (2005) Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res 115:205–210
Thompson HS, Maynard EB, Morales ER, Scordilis SP (2003) Exercise-induced HSP27, HSP70 and MAPK responses in human skeletal muscle. Acta Physiol Scand 178:61–72
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27:43–59
Yu JG, Carlsson L, Thornell LE (2004) Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: an ultrastructural and immuno-electron microscope study. Histochem Cell Biol 121:219–227
Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczweski J (2008) Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 52(2–3):307–314
Acknowledgments
We would like to thank Zachary T. Leonard and Nicole T. Bannister for their contributions to this study. We would also like to thank the staff at the CU-Boulder Clinical Translational Research Center for their technical assistance with this study. This research was supported by the National Institutes of Health Grant K01 AR050505-01, in addition to the Clinical Translational Research Center Grant (CTRC NCRR/NIH 1UL1RR025780), the Undergraduate Research Opportunity Program and the Bioscience Undergraduate Research Skills and Training Program at CU-Boulder, and the Howard Hughes Medical Institute.
Conflict of interest
None of the authors have conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
421_2010_1806_MOESM1_ESM.tif
Supplementary Figure 1. Variation in post-exercise plasma Creatine Kinase (CK) levels. Lines represent the individual changes in plasma CK activity. The group mean values are represented by the shaded bars. CK activity was highly variable, but significantly increased 15-fold from Baseline to 4 days post-exercise. (TIFF 19772 kb)
Rights and permissions
About this article
Cite this article
Madden, M.C., Byrnes, W.C., Lebin, J.A. et al. Plasma matrix metalloproteinase-9 response to eccentric exercise of the elbow flexors. Eur J Appl Physiol 111, 1795–1805 (2011). https://doi.org/10.1007/s00421-010-1806-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1806-y