Abstract
We investigated the prevalence of exercise-associated hyponatremia (EAH) in 145 male ultra-marathoners at the ‘100-km ultra-run’ in Biel, Switzerland. Changes in body mass, urinary specific gravity, haemoglobin, haematocrit, plasma [Na+], and plasma volume were determined. Seven runners (4.8%) developed asymptomatic EAH. Body mass, haematocrit and haemoglobin decreased, plasma [Na+] remained unchanged and plasma volume increased. Δ body mass correlated with both post race plasma [Na+] and Δ plasma [Na+]. Δ plasma volume was associated with post race plasma [Na+]. The athletes consumed 0.65 (0.30) L/h; fluid intake correlated significantly and negatively (r = −0.50, p < 0.0001) to race time. Fluid intake was neither associated with post race plasma [Na+] nor with Δ plasma [Na+], but was related to Δ body mass. To conclude, the prevalence of EAH was low at ~5% in these male 100 km ultra-marathoners. EAH was asymptomatic and would not have been detected without the measurement of plasma [Na+].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise-associated hyponatremia (EAH)–defined as serum [Na+] <135 mmol/L and described first in 1985 by Noakes et al. (1985) as being due to ‘water intoxication’–is a well known and well described fluid and electrolyte disorder in marathoners, where the prevalence of EAH amounts to ~22% depending upon the number of investigated athletes, their gender and fitness level (Almond et al. 2005; Chorley et al. 2007; Davis et al. 2001; Hew 2005; Hew et al. 2003; Kipps et al. 2009; Reid et al. 2004). Risk factors for EAH are weight gain, exercise duration of more than 4 h, a slow exercise pace, female gender, a low body mass, excessive drinking of more than 1.5 L/h while racing, pre-exercise hyperhydration, abundant availability of drinking fluids at the event, nonsteroidal anti-inflammatory drugs, and an extremely hot or cold environment (Hew-Butler et al. 2005; 2008a; Irving et al. 1991; Noakes et al. 2005; Rosner 2009; Rosner and Kirven 2007). In a data analysis of 2,135 athletes, weight gain as a consequence of excessive fluid consumption was the principal cause of a reduced serum [Na+] after exercise (Noakes et al. 2005). In recent years, non-osmotic secretion of arginine-vasopressin, combined with high fluid availability, plus sustained fluid intake, has been hypothesised as leading to an increase in EAH (Hew-Butler 2010; Rosner 2009). Other potential mechanisms in the pathogenesis of EAH include sweat sodium loss, inability to mobilise exchangeable sodium stores, metabolic water production, and impaired renal blood flow and glomerular filtration rate (Rosner 2009).
While there is abundant literature about the prevalence of EAH in marathoners (Almond et al. 2005; Chorley et al. 2007; Davis et al. 2001; Hew et al. 2003; Kipps et al. 2009), studies investigating EAH in ultra-marathoners are rare (Fallon et al. 1999; Glace et al. 2002; Noakes and Carter 1976; Noakes et al. 1990; Page et al. 2007; Reid and King 2007). Since ultra-marathoners run at a slow pace (Glace et al. 2002; Knechtle et al. 2009a; Knechtle et al. 2010), they may be at an especially high risk of fluid overload, and subsequently developing EAH (Hew-Butler et al. 2005, 2008a; Rosner 2009; Rosner and Kirven 2007). In a recent study of 45 male ultra-marathoners in a 161 km ultra-marathon, 51.2% of the finishers presented with EAH (Lebus et al. 2010). The authors discussed the significantly longer nature of a 161 km ultra-marathon as a main reason for the increased prevalence of EAH.
Studies investigating EAH in ultra-marathoners have been conducted in South Africa (Irving et al. 1991; Noakes et al. 1990; Noakes and Carter 1976), United States of America (Glace et al. 2002; Lebus et al. 2010; Stuempfle et al. 2003), Australia (Fallon et al. 1999; Reid and King 2007), and New Zealand (Page et al. 2007). However, no study has investigated the prevalence of EAH in a European ultra-marathon. The aim of this study was to investigate the prevalence rate of EAH in male ultra-marathoners in the ‘100-km ultra-run’ in Biel, Switzerland. This race is the most famous 100 km ultra-marathon in Europe with ~1,200 finishers each year from all over Europe.
Methods
Subjects
Data were collected over four consecutive years in a 100 km ultra-run; the ‘100-km run’ in Biel, Switzerland, in order to increase the sample size. The Race Director contacted all the participants, in the years 2007–2010, via a separate newsletter at the time of inscription, in which they were asked to participate in the study. In 2007, 1,118 male runners finished, in 2008 1,993, in 2009 1,090 and in 2010 1,059, respectively. A total of 157 male ultra-runners volunteered to participate in our investigation over this 4 year period. The athletes were informed of the procedures and gave their informed written consent. The study was approved by the Institutional Review Board for use of Human Subjects of St. Gallen, Switzerland. Age, anthropometry, training and pre race experience of the subjects is represented in Table 1.
Race
The ‘100-km run’ in Biel, Berne, Switzerland, generally takes place during the night of the first weekend in June. The athletes start the 100 km run on Friday at 10:00 p.m. They have to climb a total altitude of 645 metres. During these 100 km, the organiser provides a total of 17 aid stations offering an abundant variety of food and beverages (see Table 2). The athletes are allowed to be supported by a cyclist in order to have additional food and clothing, if necessary. In all 4 years, the general weather conditions were comparable, with the temperature at the start being 15–18°C, night lows of 8–10°C, and daily highs of 25–28°C the following day. There was no rain or wind.
Measurements and calculations
Before the start of the race, and after arrival at the finish line, every subject underwent determination of body mass, and the collection of capillary blood and urinary samples. Body mass was measured using a commercial scale (Beurer BF 15, Beurer GmbH, Ulm, Germany) to the nearest 0.1 kg. Body height was determined using a stadiometer to the nearest 0.01 m. Capillary blood samples were taken from the fingertip; plasma [Na+], haemoglobin and haematocrit were analysed using the i-STAT® 1 System (Abbott Laboratories, Abbott Park, IL, USA). Standardisation of posture prior to blood collection was respected since postural changes can influence blood volume and therefore haemoglobin concentration and haematocrit (Strauss et al. 1951). The percentage change in plasma volume (%ΔPV) was calculated from pre- and post-race levels of haematocrit (Hct) following the equation of van Beaumont (1972). Urinary specific gravity was analysed using Clinitek Atlas® Automated Urine Chemistry Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). During the race, the athletes recorded their fluid intake on a sheet of paper which they carried with them during the run. At each aid station, they marked the number of consumed cups. In addition, all supplemental fluid intake provided by the support crew was recorded. Fluid intake was estimated according to the reports of the athletes. Upon inscription to the investigation the participants were instructed to keep a comprehensive training diary until the start of the race. All training units in running were recorded, showing distance in kilometres and duration. In addition, every athlete indicated his number of finished marathons on a flat course and his personal best marathon time. Furthermore, the number of finished 100 km runs and the personal best time in a 100 km was also recorded. Pre race, no information about fluid intake or the risk of EAH was provided, either by the race organiser or the investigator. On arrival for the post race measurements, the athletes were asked for symptoms of EAH (Hew-Butler et al. 2005, 2008a; Rosner 2009; Rosner and Kirven 2007).
Statistical analysis
Results are presented as mean (SD). The one sample Wilcoxon signed rank test was used to check for significant changes (∆) in the parameters for all finishers before and after the race. The results, between hyponatremic and non-hyponatremic finishers, were compared using the Mann–Whitney U test. Spearman correlation analysis was performed to assess the univariate association between ∆ in the anthropometric variables and ∆ in the laboratory data. For all statistical tests significance was set at a level of 0.05.
Results
Among these 157 ultra-runners, 145 athletes finished the 100 km within 706 (119) min, competing at an average running speed of 8.7 (1.4) km/h. Twelve participants did not finish within the time limit of 21 h. One athlete dropped out due to low back pain, four non-finishers suffered overuse injuries of the lower limbs, and seven runners complained about exhaustion. Based on the serum sodium classification by Noakes et al. (2005), two finishers (1.4%) were classified as hypernatremic, 136 athletes (93.8%) were normonatremic and seven ultra-runners (4.8%) were hyponatremic. Hyponatremic finishers were asymptomatic.
Hyponatremic finishers were no slower compared to non-hyponatremic finishers (see Table 3). There was also no difference between them regarding training and pre race experience. The number of completed marathons (r = 0.23, p = 0.0077), the personal best marathon time (r = 0.71, p < 0.0001) and the personal best 100 km time (r = 0.77, p < 0.0001) were related to race time for all finishers.
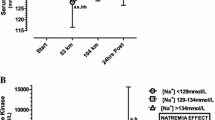
While running, the athletes consumed a total of 7.3 (3.0) L over the 100 km, equal to 0.65 (0.30) L/h. Fluid intake varied between 0.27–1.62 L/h and correlated negatively and significantly to race time (see Fig. 1). Hyponatremic finishers were drinking no more compared with non-hyponatremic ones (see Table 3). For all 145 finishers, body mass decreased, plasma [Na+] remained stable, haematocrit and haemoglobin decreased and urinary specific gravity increased (see Table 4). In both hyponatremic and non-hyponatremic finishers, haematocrit and haemoglobin decreased, and urinary specific gravity increased (see Table 5). Plasma [Na+], however, decreased in the hyponatremic finishers, but not in the non-hyponatremic finishers. Plasma volume increased by 6.1 (15.6) % for all finishers. In the hyponatremic finishers, plasma volume increased significantly higher by 15.2 (9.7) %; in the non-hyponatremic finishers, by 5.5 (12.6) % (p < 0.05).
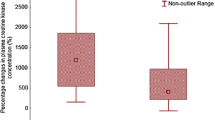
Δ body mass correlated to both post race plasma [Na+] (see Fig. 2) and Δ plasma [Na+] (see Fig. 3) but not to finishing time (see Fig. 4). Post race plasma [Na+] correlated positively and significantly with Δ plasma [Na+] (r = 0.73, p < 0.0001). Fluid intake was neither associated with post race plasma [Na+] nor with Δ plasma [Na+]. Fluid intake was related to Δ body mass (see Fig. 5), but not to post race body mass. Fluid intake showed no correlation to post race urinary specific gravity, Δ urinary specific gravity or Δ plasma volume. Δ plasma volume was associated with post race plasma [Na+] (r = −0.29, p = 0.0375), but not with Δ [Na+], post race body mass, or Δ body mass. Race time showed no association with post race body mass, Δ body mass, post race plasma [Na+], Δ plasma [Na+], post race urinary specific gravity or Δ urinary specific gravity.
Discussion
The aim of this study was to investigate the prevalence of EAH in male ultra-marathoners in a European ultra-marathon. Seven runners (4.8%) developed asymptomatic EAH. Regarding the existing literature on EAH in ultra-marathoners, the prevalence of EAH seems generally to be lower in ultra-marathoners compared to marathoners, although Stuempfle et al. (2002) described a prevalence of 44% of EAH in ultra-marathoners and Lebus et al. (2010) of 51.2%. Noakes et al. (1990) found symptomatic EAH in 0.3% of ultra-marathoners finishing a 90 km ultra-marathon with a total of 18,031 finishers. In a 161 km race, however, no case of EAH occurred, although decreased plasma [Na+] after the race was due to fluid overload caused by excessive fluid consumption (Stuempfle et al. 2003). Also Reid and King (2007) and Glace et al. (2002) found no case of EAH in ultra-marathoners.
In a very recent study of 161 km ultra-marathoners, however, the prevalence of EAH amounted to ~50% (Lebus et al. 2010). The 45 athletes in that study competed for ~26 h, considerably longer compared to our ultra-runners who completed the 100 km within ~12 h. Lebus et al. (2010) discussed the significantly longer nature of a 161 km ultra-marathon, compared to a marathon, as a main risk factor for the high prevalence of EAH. The considerably larger difference of ~5% EAH in our 100 km ultra-marathoners compared to ~50% EAH in the 161 km ultra-marathoners of Lebus et al. (2010) is, however, striking. A reason for the notably higher prevalence of EAH in Lebus et al. (2010) could be the higher ambient temperature at the ‘Rio Del Lago 100-Mile Endurance Run’ of 12.2–37.6°C, compared with the lower temperatures at Biel, Switzerland. An extremely hot or cold environment is considered as a risk factor for EAH (Hew-Butler et al. 2005, 2008a; Rosner 2009; Rosner and Kirven 2007).
Considering the aid stations and the nutrition provided, the two races were comparable. Lebus et al. (2010), however, acknowledged that their investigation was limited since fluid and nutrition intake was not recorded. In the ‘Rio Del Lago 100-Mile Endurance Run’, 24 aid stations were offered at ~7 km; in the ‘100-km run’ in Biel, the athletes could feed at 17 aid stations at ~6 km. In both races salt was offered. In the ‘Rio Del Lago 100-Mile Endurance Run’ salty snacks were provided; in the ‘100-km run’ in Biel the athletes could drink soup. In contrast to Lebus et al. (2010), we were able to determine fluid intake, however, the sodium intake would also be interesting. It has been shown that the risk of EAH can be minimised by the use of replacement fluids of high sodium concentrations while running for 4 h (Twerenbold et al. 2003). However, studies at ultra-endurance races such as an Ironman triathlon showed that oral sodium supplementation was not necessary to prevent hyponatremia (Hew-Butler et al. 2006; Speedy et al. 2002). Also sodium ingestion may not be of much advantage when athletes replace only ~50% of their fluid loss during performances at 32°C (Sanders et al. 1999). Sodium supplementation might, however, be of importance in a hot environment. When fluid intake matches sweat loss, sodium intake during prolonged exercise in the heat, at 30°C, plays a significant role in preventing sodium losses that may lead to hyponatremia (Anastasiou et al. 2009). At 30°C, saline intake, however, was not associated with significantly higher plasma sodium during exercise compared to water intake (Barr et al. 1991). Sweat loss might be of importance at high ambient temperatures regarding the risk of EAH. At 34°C, decreased plasma sodium concentration can result from replacing sweat loss with plain water, when sweat losses are large, and can precipitate the development of hyponatremia (Vrijens and Rehrer 1999).
A further reason for the different findings in plasma [Na+] in Lebus et al. (2010) compared to our results might be the device used in the determination of plasma [Na+]. In both investigations, however, the i-STAT® 1 System (Abbott Laboratories, Abbott Park, IL, USA) was used to determine plasma [Na+]. Lebus et al. (2010) reported malfunctions in the pre race determination of plasma [Na+], whereas we had no malfunctions. The reason for the malfunctions might have been the higher ambient temperature at the ‘Rio Del Lago 100-Mile Endurance Run’ compared to our race.
Fluid overload due to overdrinking is the main risk factor for EAH (Hew-Butler et al. 2005, 2008a; Irving et al. 1991; Noakes et al. 2005; Rosner 2009; Rosner and Kirven 2007). Weight gain, as a consequence of excessive fluid consumption, was the principal cause of a reduced serum [Na+] after exercise when 2,135 athletes were analysed (Noakes et al. 2005). The way to minimise the risk of EAH is not to overdrink during exercise (Beltrami et al. 2008). This is clearly shown by all the studies in which athletes are advised not to overdrink during exercise. In those races, the risk of EAH is negligible. Examples are the 90 km Comrades Marathon in South Africa (Noakes and Carter 1976; Noakes et al. 1990), the Ironman South Africa (Sharwood et al. 2002; Sharwood et al. 2004) and the Ironman New Zealand (Speedy et al. 2000) where the advice to reduce fluid intake reduced the incidence of EAH dramatically.
In this race, the athletes were offered 17 refreshment points over the 100 km, on average every 5.9 km. Our ultra-marathoners consumed––despite the increased availability of aid stations––on average 0.65 (0.30) L/h. In the ‘Position Statement’ of IMMDA, marathoners should drink no more than 400–800 mL/h ad libitum, according to their thirst (Noakes 2003). These ultra-marathoners met––with a mean fluid intake rate of 0.65 (0.30) L/h––the recommendations of IMMDA exactly. An interesting finding was the fact that fluid intake was significantly and negatively related to race time where faster runners were consuming more fluids than slower ones. In marathoners, EAH was associated with increased fluid consumption during the race because slower athletes showed a higher drinking frequency compared with faster ones (Almond et al. 2005; Kipps et al. 2009; Mettler et al. 2008). Although our faster athletes consumed significantly more fluids, race time was neither associated with post race plasma [Na+] nor with Δ plasma [Na+]. This is in contrast to the opinion that high fluid consumption leads to dilutional hyponatremia (Verbalis 2007). The athletes in this race, compared to a marathon race, had the opportunity of being followed by a support crew member, mainly a cyclist. This cyclist can carry food and drinks as well as clothes. We assume that the faster runners were followed by a support crew providing fluids between the aid stations so the athlete did not have to stop at aid stations to get a drink. Presumably, a runner finishing in the top 10 will not stop at the refreshment points, but rather consume fluids from the support crew while running. For example, one athlete finishing within 504 min (8 h 24 min) drank 9.8 L of fluids, equal to 1.34 L/h. Experienced ultra-runners with a fast race time were obviously able to consume rather large amounts of fluids so that neither dehydration nor fluid overload occurred. Alternatively, the faster runners had greater sweat rates, due to the higher speed, than the slower runners and, therefore, consumed more fluids.
In case of fluid overload, we expected an increase in body mass and a decrease in plasma [Na+] (Hew-Butler et al. 2005, 2008a; Rosner 2009; Rosner and Kirven 2007). Fluid intake was related to Δ body mass in these ultra-marathoners, but not to post race body mass. Furthermore, fluid intake was neither related to post race plasma [Na+] nor to Δ plasma [Na+]. Glace et al. (2002) described a significant and negative relationship between fluid intake and post race plasma [Na+] in their ultra-marathoners; high fluid intakes were correlated with lower post race plasma [Na+]. Their ultra-runners consumed 19.4 (5.6) L during the 26.2 (0.4) h, on average 0.74 L/h. This was slightly more compared with our runners consumption of 0.65 (0.30) L/h and might be the reason for the lower post race plasma [Na+] concentration. Weight gain during endurance exercise is associated with EAH (Hew-Butler et al. 2005, 2008a; Noakes et al. 2005; Rosner 2009; Rosner and Kirven 2007). The determination of Δ body mass is a useful measure of both fluid intake (Almond et al. 2005) and fluid retention (Siegel et al. 2007). These ultra-marathoners, however, lost ~1.8 kg body mass. A decrease in body mass is––apart from other parameters––a marker of dehydration (Shirreffs 2003; Kavouras 2002) and we assume that these ultra-marathoners were rather dehydrated than overhydrated. A loss of 2 kg body mass could also be purely due to energy loss and fluid associated with glycogen. Those athletes who lost 2 kg body mass would probably not have shown any change in their total body water. Nolte et al. (2010) showed recently that a reduction in body mass of 1.4% was not associated with a reduction in total body water in 15 soldiers performing a 16.4 km route march. Body mass change, however, is not always a reliable measure of changes in hydration status, and substantial loss of mass may occur without an effective net negative fluid balance (Maughan et al. 2007). Following the ‘Statement of the Consensus Conference’ and the large data analysis of 2,135 athletes, a loss of ~2% body mass seems to be preventative against EAH (Hew-Butler et al. 2008a; Noakes et al. 2005). Body mass changes correlated with both post race plasma [Na+] and Δ plasma [Na+]. This is in line with the recent findings of the marathon runners of Mettler et al. (2008), where Δ body mass correlated with both post race plasma [Na+] and Δ plasma [Na+]. Post race plasma [Na+] correlated to Δ plasma [Na+] in their marathoners as has been found in our ultra-marathoners. Mettler et al. (2008) demonstrated a significant association between both post race plasma [Na+] and post race plasma osmolality, and they speculated that the increased plasma osmolality might be due to an increased activity of vasopressin.
Haematocrit decreased during this ultra-marathon and plasma volume increased by 6.0 (12.6) %. During a marathon, however, plasma volume decreased (Maughan et al. 1985; Whithing et al. 1984), as has also been found in an ultra-marathon over 67 km (Rehrer et al. 1992). In longer ultra-endurance races, however, plasma volume increased (Hew-Butler et al. 2007; Stuempfle et al. 2003). Hew-Butler et al. (2007) assumed that the intensity was responsible for these disparate findings since marathoners compete faster compared with ultra-marathoners. Ultra-endurance athletes may preserve a ‘fluid reserve’ in the interstitial fluid of the extracellular fluid compartment. An interesting finding in our subjects was the fact that haematocrit and haemoglobin decreased significantly more in hyponatremic finishers compared to non-hyponatremic ones and that plasma [Na+] decreased in hyponatremic finishers, whereas plasma [Na+] remained stable in non-hyponatremic finishers. Plasma volume increased more in hyponatremic compared with non-hyponatremic finishers. This increase in plasma volume might, however, also be due to an increased activity of aldosterone during a 100 km run (Keul et al. 1981). Fellmann et al. (1999) concluded that the increase in plasma volume after an ultra-endurance race was mainly due to sodium retention. In a 24 h race, they found an increase in plasma volume, aldosterone and vasopressin (Fellmann et al. 1989). Stuempfle et al. (2003) showed an increased activity of both aldosterone and vasopressin after an ultra-endurance race. Presumably, the increase in plasma volume is due to an increased activity of both vasopressin and aldosterone.
Changes in body mass and urinary specific gravity are considered as reliable markers of change in hydration status (Shirreffs 2003; Kavouras, 2002). The post race urinary specific gravity of >1.020 mg/L indicated dehydration; the loss of ~2.4% body mass (~1.8 kg body mass) indicated minimal dehydration (Kavouras 2002). These ultra-runners were dehydrated, with a decrease in body mass and with post race urinary specific gravity >1.020 mg/L, according to the concept of determination of hydration status (Kavouras 2002; Shirreffs 2003). The decrease in body mass might, however, also be due to a decrease in solid mass such as fat mass and skeletal muscle mass, as has been shown in recent studies of ultra-runners (Knechtle et al. 2009c; Knechtle et al. 2010). Considering the associations of Δ body mass with both post race plasma [Na+] and Δ plasma [Na+], and respecting recent literature, body fluid must have been increased. Fluid intake was not related to Δ plasma [Na+] and other factors might be responsible for fluid regulation in ultra-endurance runners. We assume that other factors maintained body fluid homeostasis during this ultra-marathon, such as a hormonal regulation by vasopressin and aldosterone (Fellmann et al. 1989; Stuempfle et al. 2003). In recent studies of marathoners (Siegel et al. 2007) and ultra-marathoners over 56 km (Hew-Butler et al. 2008b), the activity of vasopressin was measured in addition to body mass, plasma [Na+], osmolality and fluid intake. Recent findings suggested that EAH was not only due to fluid overload but also to an increased activity in vasopressin (Hew-Butler 2010; Verbalis 2007). In future studies of 100 km ultra-runners the activity of vasopressin should also be investigated. Since sodium retention was the major factor in the increase in plasma volume (Fellmann et al. 1999), and aldosterone was increased after an ultra-endurance race (Stuempfle et al. 2003), the activity of aldosterone should also be determined. Furthermore, changes in total body water should be determined (Nolte et al. 2010). This might provide more insight into fluid and electrolyte regulation in ultra-marathoners.
Event inexperience is one of the athlete-related risk factors for EAH (Hew-Butler et al. 2005, 2008a; Rosner 2009; Rosner and Kirven 2007). In marathoners, the number of pre race completed marathons varied between one and eight races (Almond et al. 2005; Chorley et al. 2007; Kipps et al. 2009; Mettler et al. 2008) and non-hyponatremic finishers in a marathon had completed more marathons compared to non-hyponatremic finishers (Almond et al. 2005). In these studies, the prevalence rate of EAH amounted to ~22% (Almond et al. 2005; Chorley et al. 2007; Kipps et al. 2009; Mettler et al. 2008). Our 100 km ultra-runners had completed ~30 marathons pre race and 98 athletes (67%) had already completed ~6 100 km ultra-runs. Interestingly, both hyponatremic and non-hyponatremic finishers showed no differences regarding the number and the personal best times in both marathons and 100 km ultra-marathons. Due to the low prevalence of EAH in our ultra-marathoners, we assume that pre race experience is an important determinant in minimising the risk of EAH (Kruseman et al. 2005). The personal best time in a 100 km ultra-run showed about the same correlation coefficient (r = 0.77) compared to a personal best marathon time (r = 0.71) with the 100 km race time. A personal best marathon time might be an independent predictor variable for an ultra-running performance; in a 24 h run, the personal best marathon time was related to total running kilometres (Knechtle et al. 2009b).
A limitation of this study is that only 145 out of 5,260 finishers (2.7% of the finishers) were included. Although the number of investigated finishers is relatively high, a selection bias can probably not be excluded. We did not ask about the intake of nonsteroidal anti-inflammatory medication, which is also considered as a risk factor for EAH (Hew-Butler et al. 2008a). The use of nonsteroidal anti-inflammatory drugs might influence renal function (Reid et al. 2004) and increase the risk of exercise-associated hyponatremia (Page et al. 2007). A further limitation is that we did not determine plasma [Na+] in the non-finishers, so if any of these 12 non-finishers did suffer from EAH the prevalence of EAH would have doubled.
Conclusions
To summarise, the prevalence of EAH in 100 km ultra-marathoners was lower compared to reports on marathoners and a recent report on 161 km ultra-marathoners. EAH was asymptomatic and would not have been detected without the measurements of plasma sodium concentrations. Plasma volume increased and plasma [Na+] was maintained although body mass decreased. Fluid intake showed no association with the increase in plasma volume, post race plasma [Na+] and Δ plasma [Na+]. Hydration status was adequately maintained as has been found in other ultra-endurance races (Tam et al. 2009; Hew-Butler et al. 2007; Rose and Peters, 2008). The determination of changes in total body water and of the activity of both aldosterone and vasopressin in ultra-marathoners might give more insight into maintaining body fluid homeostasis in ultra-endurance performances.
References
Almond CS, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, Newburger JW, Greenes DS (2005) Hyponatremia among runners in the Boston Marathon. N Engl J Med 252:1550–1556
Anastasiou CA, Kavouras SA, Arnaoutis G, Gioxari A, Kollia M, Botoula E, Sidossis LS (2009) Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J Athl Train 44:117–123
Barr SI, Costill DL, Fink WJ (1991) Fluid replacement during prolonged exercise: effects of water, saline, or no fluid. Med Sci Sports Exerc 23:811–817
Beltrami FG, Hew-Butler T, Noakes TD (2008) Drinking policies and exercise-associated hyponatremia: is anyone still promoting overdrinking? Br J Sports Med 42:796–501
Chorley J, Cianca J, Divine J (2007) Risk factors for exercise-associated hyponatremia in non-elite marathon-runners. Clin J Sport Med 17:471–477
Davis DP, Videen JS, Marino A, Vilke GM, Dunford JV, Van Camp SP, Maharam LG (2001) Exercise-associated hyponatremia in marathon runners: a two-year experience. J Emerg Med 21:47–57
Fallon KE, Sivyer G, Sivyer K, Dare A (1999) The biochemistry of runners in a 1,600 km ultramarathon. Br J Sports Med 33:264–269
Fellmann N, Bedu M, Giry J, Pharmakis-Amadieu M, Bezou MJ, Barlet JP, Coudert J (1989) Hormonal, fluid, and electrolyte changes during a 72-h recovery from a 24-h endurance run. Int J Sports Med 10:406–412
Fellmann N, Ritz P, Ribeyre J, Beaufrère B, Delaître M, Coudert J (1999) Intracellular hyperhydration induced by a 7-day endurance race. Eur J Appl Physiol 80:353–359
Glace BW, Murphy CA, McHugh MP (2002) Food intake and electrolyte status of ultramarathoners competing in extreme heat. J Am Coll Nutr 21:553–559
Hew TD (2005) Women hydrate more than men during a marathon race: hyponatremia in the Houston marathon: a report on 60 cases. Clin J Sport Med 15:148–153
Hew TD, Chorley JN, Cianca JC, Divine JG (2003) The incidence, risk factors and clinical manifestations of hyponatremia in marathon runners. Clin J Sport Med 13:41–47
Hew-Butler T (2010) Arginine vasopressin, fluid balance and exercise: is exercise-associated hyponatraemia a disorder of arginine vasopressin secretion? Sports Med 40:459–479
Hew-Butler T, Almond C, Ayus JC, Dugas J, Meeuwisse W, Noakes T, Reid S, Siegel A, Speedy D, Stuempfle K, Verbalis J, Weschler L, Exercise-Associated Hyponatremia (EAH) Consensus Panel (2005) Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med 15:208–213
Hew-Butler TD, Sharwood K, Collins M, Speedy D, Noakes T (2006) Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon. Br J Sports Med 40:255–259
Hew-Butler T, Collins M, Bosch A, Sharwood K, Wilson G, Armstrong M, Jennings C, Swart J, Noakes T (2007) Maintenance of plasma volume and serum sodium concentration despite body weight loss in ironman triathletes. Clin J Sport Med 17:116–122
Hew-Butler T, Ayus JC, Kipps C, Maughan RJ, Mettler S, Meeuwisse WH, Page AJ, Reid SA, Rehrer NJ, Roberts WO, Rogers IR, Rosner MH, Siegel AJ, Speedy DB, Stuempfle KJ, Verbalis JG, Weschler LB, Wharam P (2008a) Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med 18:111–121
Hew-Butler T, Jordaan E, Stuempfle KJ, Speedy DB, Siegel AJ, Noakes TD, Soldin SJ, Verbalis JG (2008b) Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab 93:2072–2078
Irving RA, Noakes TD, Buck R, van Zyl Smit R, Raine E, Godlonton J, Norman RJ (1991) Evaluation of renal function and fluid homeostasis during recovery from exercise-induced hyponatremia. J Appl Physiol 70:342–348
Kavouras SA (2002) Assessing hydration status. Curr Opin Clin Nutr Metab Care 5:519–524
Keul J, Kohler B, von Glutz G, Lüthi U, Berg A, Howald H (1981) Biochemical changes in a 100 km run: carbohydrates, lipds, and hormones in serum. Eur J Appl Physiol 47:181–189
Kipps C, Sharma S, Tunstall Pedoe D (2009) The incidence of exercise-associated hyponatremia in the London Marathon. Br J Sports Med. doi:10.1136/bjsm.2009.059535
Knechtle B, Duff B, Schulze I, Rosemann T, Senn O (2009a) Anthropometry and pre-race experience of finishers and non-finishers in a multistage ultra-endurance run–Deutschlandlauf 2007. Percept Mot Skills 109:105–118
Knechtle B, Wirth A, Knechtle P, Zimmermann K, Kohler G (2009b) Personal best marathon performance is associated with performance in a 24-h run and not anthropometry or training volume. Br J Sports Med 43:836–839
Knechtle B, Wirth A, Knechtle P, Rosemann T (2009c) Increase of total body water with decrease of body mass while running 100 km nonstop–formation of edema? Res Q Exerc Sport 80:593–603
Knechtle B, Wirth A, Knechtle P, Rosemann T, Senn O (2010) Do ultra-runners in a 24-h run really dehydrate? Ir J Med Sci [Epub ahead of print]
Kruseman M, Bucher S, Bovard M, Kayser B, Bovier PA (2005) Nutrient intake and performance during a mountain marathon: an observational study. Eur J Appl Physiol 94:151–157
Lebus DK, Casazza GA, Hoffman MD, Van Loan MD (2010) Can changes in body mass and total body water accurately predict hyponatremia after a 161-km running race? Clin J Sport Med 20:193–199
Maughan RJ, Whiting PH, Davidson RJ (1985) Estimation of plasma volume changes during marathon running. Br J Sports Med 19:138–141
Maughan RJ, Shirreffs SM, Leiper JB (2007) Errors in the estimation of hydration status from changes in body mass. J Sports Sci 25:797–804
Mettler S, Rusch C, Frey WO, Bestmann L, Wenk C, Colombani PC (2008) Hyponatremia among runners in the Zurich Marathon. Clin J Sport Med 18:344–349
Noakes T (2003) Fluid replacement during marathon running. Clin J Sport Med 13:309–319
Noakes TD, Carter JW (1976) Biochemical parameters in athletes before and after having run 160 kilometres. S Afr Med J 50:1562–1566
Noakes TD, Goodwin N, Rayner BL, Branken T, Taylor RK (1985) Water intoxication: a possible complication during endurance exercise. Med Sci Sports Exerc 17:370–375
Noakes TD, Norman RJ, Buck RH, Godlonton J, Stevenson K, Pittaway D (1990) The incidence of hyponatremia during prolonged ultraendurance exercise. Med Sci Sports Exerc 22:165–170
Noakes TD, Sharwood K, Speedy D, Hew T, Reid S, Dugas J, Almond C, Wharam P, Weschler L (2005) Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2, 135 weighed competitive athletic performances. Proc Natl Acad Sci USA 102:18550–18555
Nolte H, Noakes TD, van Vuuren B (2010) Ad libitum fluid replacement in military personnel during a 4 hour route march. Med Sci Sports Exerc 42(9):1675–1680
Page AJ, Reid SA, Speedy DB, Mulligan GP, Thompson J (2007) Exercise-associated hyponatremia, renal function, and nonsteroidal anti-inflammatory drug use in an ultraendurance mountain run. Clin J Sport Med 17:43–48
Rehrer NJ, Brouns F, Beckers EJ, Frey WO, Villiger B, Riddoch CJ, Menheere PP, Saris WH (1992) Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. Eur J Appl Physiol 64:1–8
Reid SA, King MJ (2007) Serum biochemistry and morbidity among runners presenting for medical care after an Australian mountain ultramarathon. Clin J Sport Med 17:307–310
Reid SA, Speedy DB, Thompson JM, Noakes TD, Mulligan G, Page T, Campbell RG, Milne C (2004) Study of haematological and biochemical parameters in runners completing a standard marathon. Clin J Sport Med 14:344–353
Rose SC, Peters EM (2008) Ad libitum adjustments to fluid intake in cool environmental conditions maintain hydration status in a three-day mountain bike race. Br J Sports Med. doi:10.1136/bjsm.2008.049551
Rosner MH (2009) Exercise-associated hyponatremia. Semin Nephrol 29:271–281
Rosner MH, Kirven J (2007) Exercise-associated hyponatremia. Clin J Am Soc Nephrol 2:151–161
Sanders B, Noakes TD, Dennis SC (1999) Water and electrolyte shifts with partial fluid replacement during exercise. Eur J Appl Physiol Occup Physiol 80:318–323
Sharwood K, Collins M, Goedecke J, Wilson G, Noakes T (2002) Weight changes, sodium levels, and performance in the South African Ironman triathlon. Clin J Sport Med 12:391–399
Sharwood KA, Collins M, Goedecke JH, Wilson G, Noakes TD (2004) Weight changes, medical complications, and performance during an Ironman triathlon. Br J Sports Med 38:718–724
Shirreffs SM (2003) Markers of hydration status. Eur J Clin Nutr 57:S6–S9
Siegel AJ, Verbalis JG, Clement S, Mendelson JH, Mello NK, Adner M, Shirey T, Glowacki J, Lee-Lewandrowski E, Lewandrowski KB (2007) Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am J Med 120:461–467
Speedy DB, Rogers IR, Noakes TD, Thompson JM, Guirey J, Safih S, Boswell DR (2000) Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clin J Sport Med 10:52–58
Speedy DB, Thompson JMD, Rodgers I, Collins M, Sharwood K (2002) Oral salt Supplementation during ultradistance exercise. Clin J Sport Med 12:279–284
Strauss MB, Davies RK, Rosenbaum JD, Rossmeisl EC (1951) Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest 30:862–868
Stuempfle KJ, Lehmann DR, Case HS, Bailey S, Hughes SL, McKenzie J, Evans D (2002) Hyponatremia in a cold weather ultraendurance race. Alaska Med 44:51–55
Stuempfle KJ, Lehmann DR, Case HS, Hughes SL, Evans D (2003) Change in serum sodium concentration during a cold weather ultradistance race. Clin J Sport Med 13:171–175
Tam N, Hew-Butler T, Papadopoulou E, Nolte H, Noakes TD (2009) Fluid intake and changes in blood chemistry, running speed and body mass during an 80 kg mountain trail race. Med Sport 13:108–115
Twerenbold R, Knechtle B, Kakebeeke TH, Eser P, Müller G, von Arx P, Knecht H (2003) Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br J Sports Med 37:300–303
van Beaumont W (1972) Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol 32(5):712–713
Verbalis JG (2007) Renal function and vasopressin during marathon running. Sports Med 37:455–458
Vrijens DM, Rehrer NJ (1999) Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol 86:1847–1851
Whithing PH, Maughan RJ, Miller JD (1984) Dehydration and serum biochemical changes in marathon runners. Eur J Appl Physiol 52:183–187
Acknowledgments
We thank the crew of the ‘100-km Biel’ for their generous support and the athletes for their promptness in the collection of data during the race. For her help in translation, we thank Mary Miller from Stockton-on-Tees, Cleveland, England.
Conflict of interest
The authors have no conflict of interest and received no external funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
Rights and permissions
About this article
Cite this article
Knechtle, B., Knechtle, P. & Rosemann, T. Low prevalence of exercise-associated hyponatremia in male 100 km ultra-marathon runners in Switzerland. Eur J Appl Physiol 111, 1007–1016 (2011). https://doi.org/10.1007/s00421-010-1729-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1729-7