Abstract
Acute exercise alters the surface expression of toll-like receptors (TLRs) and HLA.DR on blood monocytes, which could transiently compromise immunity. As serum factors might be responsible, we examined the effects of autologous post-exercise serum exposure on TLR2, TLR4 and HLA.DR expression on resting blood monocytes and their subtypes. Eight trained cyclists completed an ergometer 60 km time trial. PBMCs and serum were obtained before, immediately after and 1 h after exercise. TLR2, TLR4 or HLA.DR expression (gMFI) was determined on blood monocyte subtypes expressing combinations of CD14 and CD16 by flow cytometry, and on resting monocytes exposed to 50% autologous serum (pre, immediately after or 1 h after exercise) for 18 h in culture. Immediately after exercise, total monocyte expression of TLR2 and TLR4 increased by 41 and 27%, respectively, while HLA.DR expression was 39% lower than baseline. TLR2 and TLR4 was 53 and 84% greater 1 h after exercise, respectively, while HLA.DR was 48% lower. Changes in TLR2 and TLR4 expression occurred on the CD14++bright/CD16+dim monocyte subtype only, while HLA.DR expression changed on the CD14+dim/CD16++bright subtype. Serum did not affect monocyte TLR2 or TLR4 expression but 1 h post serum increased expression of HLA.DR on total monocytes and the CD14+dim/CD16++bright subtype, which was in contrast to the change observed at this time after exercise. We conclude that a bout of strenuous aerobic exercise alters the surface expression of TLR2, TLR4 and HLA.DR on blood monocytes and some of their subtypes, but these changes appear to be unrelated to blood serum factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toll-like receptors (TLRs) are Type I trans-membrane glycoproteins that conciliate the recognition of pathogen-associated molecular patterns (PAMPs) and coordinate inflammatory immune responses following pathogen incursion. In particular, TLRs facilitate the recognition of pathogen subtypes, such as Gram-negative and Gram-positive bacteria, DNA and RNA viruses, fungi and protozoa and, therefore, play an important role in innate host immune defense (Francaux 2009; Gleeson et al. 2006; Hemmi et al. 2000); but are also involved in adaptive immune responses including antigen presentation via major histocompatibility complex class II (MHC class II) molecules (Hemmi et al. 2000). TLRs and the MHC class II receptor HLA.DR are typically found on the surface of antigen presenting cells such as monocytes, which are a heterogenous group of phagocytic cells that make up 5–15% of all peripheral blood leukocytes. The levels of TLR (namely TLR2 and TLR4) and HLA.DR expression are known to differ among the monocyte subtypes, with the pro-inflammatory monocytes (CD14+dim/CD16++bright) expressing these receptors at greater levels than the classic monocytes (CD14++bright/CD16−) (Hong and Mills 2008; Passlick et al. 1989; Simpson et al. 2009; Skinner et al. 2005; Ziegler-Heitbrock 2007). An additional monocyte subtype, the so-called IL-10 secreting anti-inflammatory (CD14++bright/CD16+dim) monocytes (Skrzeczynska-Moncznik et al. 2008) also have differing expression levels of these cell surface receptors (Hong and Mills 2008; Simpson et al. 2009; Skrzeczynska-Moncznik et al. 2008). It has been suggested that alterations in monocyte surface expression of TLRs or HLA.DR could compromise host immunity, particularly in relation to PAMP recognition and antigen presentation, and therefore, increase infection risk (Simpson et al. 2009). Moreover, monocyte surface expression of TLRs and HLA.DR appear to have clinical relevance, as they are known to differ and/or predict outcomes in response to sepsis, tuberculosis and chronic liver failure (Armstrong et al. 2004; Hopkins et al. 2008; Monneret et al. 2006; Sanchez et al. 2006; Schaaf et al. 2009; Tsujimoto et al. 2006; Xing et al. 2007).
Acute bouts of strenuous physical exercise are known to induce profound changes in the composition of monocyte subtypes present in peripheral blood (Hilberg et al. 2004; Hong and Mills 2008; Selkirk et al. 2009; Simpson et al. 2009; Steppich et al. 2000) and alter their expression of specific cell surface receptors including TLRs and HLA.DR (Hong and Mills 2008; Lancaster et al. 2005; Oliveira and Gleeson, 2010; Simpson et al. 2009). In response to acute exercise, there is a preferential mobilization of CD14+/CD16+ monocytes compared to the CD14+/CD16− cells, increasing the ratio of pro-inflammatory to classic monocytes (Hong and Mills 2008; Selkirk et al. 2009; Simpson et al. 2009; Steppich et al. 2000). Although Lancaster et al. (2005) and, more recently, Oliveira and Gleeson (2010) have reported that total monocyte expression of TLR2 and TLR4 is downregulated in response to prolonged cycling exercise, these studies did not take into consideration the altered composition of monocyte subtypes in blood after exercise. Our group was the first to show that changes in TLR2 and TLR4 expression following acute exercise appear to be independent of the altered composition of monocyte subtypes as the changes observed were localized to specific monocyte subtypes, with TLR2 and TLR4 expression decreasing on CD14++bright/CD16+dim and CD14++bright/CD16− monocytes, respectively, after 45-min of intensive treadmill running (Simpson et al. 2009). Two studies have reported that acute aerobic exercise results in a lowered expression of HLA.DR on total blood monocytes (Hong and Mills 2008; Simpson et al. 2009), which appears to affect both the classic (Simpson et al. 2009) and the pro-inflammatory monocyte subtypes (Hong and Mills 2008).
At present, the mechanisms underpinning exercise-induced changes in monocyte TLR and HLA.DR expression, and the potential consequences this has on host immune defense, are largely unknown. While the before and after exercise differences in monocyte TLR and HLA.DR expression could reflect pre-existing differences in expression between blood resident and exercise-mobilized monocytes (Simpson et al. 2009), it is also possible that certain serum factors, which change following exercise, could be responsible. In particular, increasing levels of circulatory cytokines, heat-shock proteins, glucocortocoids, catecholamines, lipopolysaccharide (LPS) concentration and/or acidosis might play a role in modulating the cell signaling pathways that control TLR (i.e. MyD88, p38) and HLA.DR expression (i.e. PKA, PKC) (Gleeson et al. 2006; Oliveira and Gleeson, 2010; Simpson et al. 2009).
The aim of this study was to determine how in vitro exposure of resting monocytes to post-exercise autologous serum affects the cell-surface expression of TLR2, TLR4 and HLA.DR. We used resting blood monocytes only to exclude any differences in TLR expression that might exist between the blood resident and the exercise mobilized cells. We hypothesized that in vitro exposure of resting blood monocytes to autologous serum obtained after an acute bout of strenuous exercise would elicit changes in resting monocyte TLR and HLA.DR expression akin to those seen in vivo in response to exercise.
Methods
Subjects
Eight (3 females) club-level athletes (mean ± SD age: 32.1 ± 4.2 years; height: 176.1 ± 10.7 cm; mass: 69.6 ± 11.7 kg) were identified via non-random methods and invited to participate in this study. All subjects were experienced cyclists currently partaking in structured endurance training regimens. Subjects were non-smokers, in good health and reported no infectious illnesses in the 6 weeks prior to testing (confirmed via health history questionnaire). Subjects were requested to abstain from strenuous physical activity for 24 h prior to testing, which was confirmed via verbal communication on the day of the exercise test. Each subject was supplied with written information describing the purposes and demands of the study prior to giving their written informed consent. A local Institutional Ethics Committee of Edinburgh Napier University granted approval for the study.
Exercise trial and blood sampling
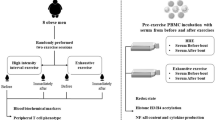
All subjects reported to the laboratory at 0900 h to complete a 60 km time trial on an indoor cycling trainer (Kingcycle, High Wycombe, UK). Prior to the exercise test, subjects were fitted with a heart rate monitor (S610; Polar Electro, Kempele, Finland) and asked to rest in a seated position for 5-min. After this rest period, a pre-exercise blood sample of 60 ml was collected in vacutainers containing either EDTA as an anticoagulant or a serum gel (Becton–Dickinson, Oxford, UK). Subjects used their own personal road bike, which was mounted to the indoor trainer and adjusted to their comfort. The indoor trainer was calibrated with the subject seated in the riding position and was asked to perform a 5–10 min warm-up exercise prior to starting the time trial. After the warm-up period, the subject was asked to cycle the 60 km distance in the fastest possible time. Heart rate (bpm) and power output (watts) were monitored throughout the time trial and mean heart rate and power output were recorded from the test. Subjects consumed water ad libitum throughout the test. Additional blood samples were collected immediately after the exercise test (24 ml), then again 1 h later (24 ml). Total leukocyte, monocyte, lymphocyte, neutrophil, and eosinophil counts were determined at each blood sampling time point using an automated hematology analyzer (Sysmex II, Minnesota, USA). Total numbers of monocyte subtypes were determined by multiplying the percentage of each monocyte subtype within the total CD14+ monocyte population (determined by flow cytometry) by the total monocyte count.
Cell separation
All cell separation and blood-handling techniques were performed under sterile conditions in a laminar flow fume hood. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood via density gradient centrifugation following the methods we have described previously (Simpson et al. 2009). Following separation, PBMCs were aliquoted for direct immunofluorescence assays and cellular analysis by flow cytometry. The remaining resting blood monocytes (i.e. the pre-exercise sample) were prepared for cell culture and serum stimulation assays.
Cell culture and serum stimulation assays
Aliquots of 8 × 106 resting (i.e. the pre-exercise sample) PBMCs were re-suspended in 2 ml RPMI 1640 (supplemented with 1% penicillin/streptomycin and 1% l-Glutamine) cell culture medium and placed into 3 incubation flasks at a final concentration of 2.0 × 106 cells/ml containing 50% of either pre-exercise serum, post-exercise serum, or 1-h post-exercise serum and incubated for 18 h at 37°C in a final volume of 4 ml. We used a final concentration of 2 × 106 cells/ml, as this is a typical resting PBMC count (i.e. combined lymphocyte and monocyte cell count). Similarly, we used 50% serum as this is closely related to typical human plasma volume. These cell concentrations and serum dilutions were intended to simulate resting human physiological conditions. Cell culture flasks remained vertical throughout the incubation period in order to minimize monocyte adherence to flask walls and maximize their recovery following incubation.
Following incubation, the cell culture medium was removed from the flasks, which were flushed thoroughly with PBS-BSA in order to remove and collect any adherent monocytes. The PBMCs then underwent two rounds of washing and centrifugation (room temperature at 350 g) in PBS (supplemented with 1% bovine serum albumin). Following the second centrifugation, the cell supernatant was discarded and the PBMCs were re-suspended in 1 ml of PBS for cell counting, viability determination, direct immunofluorescence assays, and cellular analysis by flow cytometry.
Direct immunofluorescence assays for cell-surface TLR2, TLR4 and HLA.DR detection
All PBMC samples obtained before and after exercise and following the cell culture/serum stimulation assays were labeled with a 0.3 ml cocktail of directly conjugated pre-diluted monoclonal antibodies (mAbs) in a three-color direct immunofluorescence procedure. Aliquots of 1 × 106 PBMCs were labeled with 0.1 ml of anti-CD14 FITC, anti-CD16 PE-Cy7 and either an anti-TLR2, TLR4 or HLA.DR mAb conjugated to PE. The anti-TLR2 and TLR4 mAbs were purchased from ebioscience (San Diego, CA, USA), the anti-CD16 mAb from BD Pharmingen (San Jose, CA, USA) and the anti-CD14 and anti-HLA.DR mAbs from Immunotools (Friesoythe, Germany). All mAbs were pre-titrated to determine optimum dilutions for cellular analysis by flow cytometry. All samples were left to incubate at room temperature and protected from light for 1 h then analyzed by flow cytometry.
Flow cytometry
All flow cytometry analysis was performed using CELLQuest Pro software on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) equipped with a 15 mW argon ion laser emitting light at a fixed wavelength of 488 nm. Blood monocytes were identified and electronically gated using forward light-scatter and side light-scatter modes. CD14+ cells were identified using forward light-scatter against FITC fluorescence. The expression of CD16 was then assessed on the CD14+ cell population and further electronic gates were used to identify the CD14++bright/CD16−, CD14+dim/CD16++bright, CD14++bright/CD16+dim and total CD14+/CD16+ cell populations in accordance with the procedures we have described previously in detail (Simpson et al. 2009). Single parameter histograms were then generated to identify TLR2, TLR4 or HLA.DR expression on total monocytes and their subtypes. Fluorescent signals were collected in logarithmic mode and cell numbers per channel were collected in linear mode. Appropriately conjugated isotype controls were used to eliminate non-specific binding of Ig and set the PMT voltages for each detector filter. Electronic color compensation was used to exclude any overlapping emission spectra, and each directly conjugated mAb was separately analyzed to ensure that the appropriate fluorescent signals appeared in a single detector filter. When all parameters were set, 10,000 of the gated CD14+ events were acquired for analysis. All monocytes expressed TLR2, TLR4 and HLA.DR (>98% positive) and these data were reported as the geometric mean fluorescent intensity (GMFI). Representative flow cytometry dotplots and histograms are presented in Fig. 1. The flow cytometry dotplots show how the monocyte subtype populations were identified using CD14 and CD16 expression. The single parameter flow cytometry histograms have been overlaid to show differences between the pre, immediately after and 1 h after exercise conditions for each cell surface receptor (i.e. TLR2, TLR4 and HLA.DR) in response to exercise or autologous serum exposure. All dotplots and histograms presented were generated on the total CD14+ cell population.
Representative flow cytometry dotplots and histograms: the two-parameter dotplots (CD14 FITC/CD16 PE-Cy-7) illustrate the effects of exercise on the proportions of monocyte subtypes in blood. The upper left quadrant contains the CD14+dim/CD16++bright pro-inflammatory monocytes; the upper right quadrant contains the CD14++bright/CD16+dim monocytes; and the lower right quadrant contains the CD14++bright/CD16−negative classical monocytes. The histograms illustrate the effects of exercise on total monocyte surface expression of TLR2 (1.a), TLR4 (1.b) and HLA.DR (1.c) and the effects of autologous serum on total resting monocyte surface expression of TLR2 (2.a), TLR4 (2.b) and HLA.DR (2.c): Green pre-exercise condition, Red post-exercise condition, Blue 1 h postexercise condition (color figure online)
Statistical analysis
All statistical analyses were performed using SPSS version 17 for Mac statistical analysis software (Chicago, IL, USA). Data were analyzed using a linear mixed models (LMM) approach to fit residual covariance matrices to account for dependency of the repeated measures, allowing different variances for each time point and different covariances between time points. Initially, separate LMM were used to examine the effects of exercise over three time points [time: pre, immediately after and 1 h after exercise] on monocyte TLR2, TLR4 or HLA.DR expression among the three monocyte subtypes (monocyte subtype: CD14++bright/CD16−negative; CD14+dim/CD16++bright; CD14++bright/CD16+dim). The effects of exercise and the effects of serum were analyzed using separate LMM, as the monocyte cell populations obtained after exercise (i.e. a mixture of both blood resident and exercise-mobilized cells) were different to the resting monocytes (blood resident monocytes only) stimulated with pre and post-exercise serum and it would therefore not be appropriate to include these data in the same LMM. The effects of exercise or serum were also analyzed within each monocyte subtype to examine the effects of exercise or serum on the individual monocyte subtypes over time. When the time main effect was significant, Bonferroni-adjusted paired t tests with were used to test the immediately after and 1 h time points against the pre-exercise value. All data are presented as the mean ± SD. Statistical significance was accepted at P < 0.05.
Results
Exercise performance measures
All subjects successfully completed the 60 km time trial on the indoor cycling ergometer. Mean completion time was 92.1 ± 6.8 min; mean power was 225.3 ± 42.1 watts and mean heart rate was 156 ± 9 bpm. The mean heart rate for the 60 km time trial was equivalent to 83 ± 5% of the age-predicted maximum heart rate (220-age).
The effects of exercise on leukocyte and monocyte subtype populations
Changes in total leukocyte and leukocyte subtypes in response to the exercise challenge are presented in Table 1. Immediately after exercise, blood monocyte concentration was 54% greater than the pre-exercise value (P < 0.01) and remained elevated by 36% 1 h later (P < 0.05). Compared to pre-exercise values, the numbers and proportions of CD14+ monocytes expressing CD16 (CD14+/CD16+ monocytes) was 185 and 57% higher, respectively, immediately after exercise (P < 0.01) (Table 2). The numerical increase was due to a mobilization of both the CD14+dim/CD16++bright (180%) and the CD14++bright/CD16+dim (200%) monocyte subtypes (P < 0.05). All monocyte subtype numbers and proportions were not significantly different from the pre-exercise values at 1 h post-exercise (P > 0.05).
The effects of exercise on blood monocyte TLR2, TLR4 and HLA.DR expression
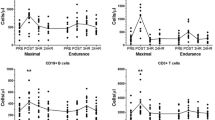
The effects of exercise on blood monocyte TLR2, TLR4 and HLA.DR expression are shown in Fig. 2. Significant effects of exercise were observed for TLR2, TLR4 and HLA.DR expression when all monocyte subtypes were considered collectively (P < 0.05). Immediately after exercise TLR2 and HLA.DR expression was, on average, 41% greater and 39% less compared to the respective pre-exercise values (P < 0.05); and at 1 h post-exercise, TLR2 and TLR4 was 53 and 84% greater than the respective pre-exercise values (P < 0.05), while HLA.DR expression was 48% lower (P < 0.01). Within individual monocyte subtypes, TLR2 expression on CD14++bright/CD16+dim cells was found to be 21.3% greater than pre-exercise values immediately after exercise (P < 0.05). No other significant exercise-by-monocyte effects for TLR2 expression were found (P > 0.05). At 1 h post-exercise, TLR4 expression on CD14++bright/CD16+dim monocytes was 90% higher than pre-exercise values (P < 0.05). No other exercise effects were found for TLR4 expression on monocyte subtypes (P > 0.05). Compared to the pre-exercise values, HLA.DR expression on CD14+dim/CD16++bright monocytes was 43% lower immediately after exercise (P < 0.05) and 35% lower 1 h later (P < 0.01). On the CD14++bright/CD16−negative classical monocytes, HLA.DR expression was 49% lower than the pre-exercise values at 1 h post-exercise (P < 0.05).
The effects of exercise and autologous serum on the cell surface expression of TLR2, TLR4 and HLA.DR (GMFI) on all CD16+ monocytes (CD14+positive/CD16+positive), CD14++bright/CD16+dim monocytes, CD14+dim/CD16++bright monocytes and CD14++bright/CD16−negative monocytes. The graphs on the left hand side show the effects of exercise on monocyte subtype expression of TLR2, TLR4 and HLA.DR, while the graphs on the right hand side show the effects of autologous serum exposure (serum obtained before, immediately after and 1 h after exercise) on resting blood monocyte subtype expression of TLR2, TLR4 and HLA.DR. Statistically significant differences from the pre-exercise values for all cell types combined: # P < 0.05, ## P < 0.01. Statistically significant differences from pre-exercise values among monocyte subtypes: *P < 0.05, **P < 0.01
The effects of autologous serum exposure on resting blood monocyte expression of TLR2, TLR4 and HLA.DR
The effects of autologous serum exposure on resting blood monocyte expression of TLR2, TLR4 and HLA.DR are shown in Fig. 2. Exposing resting blood monocytes to autologous serum obtained from the subjects immediately and 1 h after exercise did not significantly alter TLR2 or TLR4 expression in comparison to resting serum exposure (P > 0.05). A significant effect of serum exposure was observed for HLA.DR expression, with the level of expression on resting monocytes exposed to 1 h post-exercise serum being 50% greater than the resting monocytes exposed to resting serum (P < 0.01). Serum obtained immediately after exercise did not alter HLA.DR expression on resting monocytes (P > 0.05). Within monocyte subtypes, the serum obtained at 1 h post-exercise was found to increase HLA.DR expression on resting CD14+dim/CD16++bright monocytes by 33% compared to the pre-exercise serum (P < 0.05). HLA.DR expression on the other monocyte subtypes was not significantly altered by serum (P > 0.05).
Discussion
This study examined the effects of autologous serum obtained after an acute bout of strenuous cycling exercise on resting blood monocyte surface expression of TLR2, TLR4 and HLA.DR. Despite the changes in monocyte TLR2 and TLR4 expression that occurred with exercise, exposing resting blood monocytes to serum obtained after exercise had no effect on the surface expression of these pathogen recognition receptors. Consistent with our previous study (Simpson et al. 2009), we found that exercise resulted in a lowered expression of HLA.DR immediately after and 1 h after exercise, particularly in the pro-inflammatory monocyte subtype (CD14+dim/CD16++bright). Although autologous serum obtained at 1 h post-exercise altered HLA.DR expression on resting CD14+dim/CD16++bright monocytes, the levels of HLA.DR expression were actually found to increase with serum exposure, which was in contrast to what was observed in response to exercise. We conclude that exposure of resting blood monocytes to post-exercise serum does not elicit the same changes as those observed in vivo with regard to cell-surface expression of TLR2, TLR4 and HLA.DR. Thus, it is reasonable to speculate that exercise induced changes in monocyte TLR2, TLR4, and HLA.DR cell-surface expression are not caused by soluble serum factors.
Three previous studies, to our knowledge, have reported changes in monocyte TLR expression in response to acute aerobic exercise (Lancaster et al. 2005; Oliveira and Gleeson 2010; Simpson et al. 2009). All of these papers reported decreases in total monocyte TLR4 expression immediately after (Lancaster et al. 2005; Oliveira and Gleeson 2010) and/or during the recovery phase of exercise (Lancaster et al. 2005; Oliveira and Gleeson 2010; Simpson et al. 2009). In contrast, we found in the present study that a single bout of strenuous cycling exercise caused monocyte TLR2 and TLR4 expression to increase; TLR2 was elevated immediately after exercise and remained elevated 1 h later, whereas TLR4 expression was only elevated 1 h after the exercise challenge. These discrepancies could be due to experimental differences. For instance, in the present study, we examined competitive athletes performing an “all out” 60 km cycling time trial that ranged in duration from 83 to 103 min. This type of exercise is likely to be more intensive than other studies that controlled the intensity of exercise at 70 or 75% VO2max for durations ranging from 45 min to 2.5 h. Differences in exercise modality also exist. The investigation by Oliveira and Gleeson (2010) and the present study used an indoor cycling protocol and, although Lancaster et al. (2005) also used a cycling protocol, this was conducted in a hot environment, while our previous study (Simpson et al. 2009) used a treadmill-running protocol. Due to these equivocal data and the variation in experimental design among these studies, a future study comparing changes in blood monocyte TLR expression in response to acute exercise of differing intensities, durations, and modality would be illuminating.
Some studies failed to examine TLR expression on monocyte subtypes, which also complicates the interpretation of the data (Lancaster et al. 2005; Oliveira and Gleeson, 2010). For instance, in our previous paper (Simpson et al. 2009), we found total monocyte TLR4 expression to be lower during the recovery phase of exercise, an effect that was consistent with the findings of Lancaster et al. (2005) and Oliveira and Gleeson (2010); however, TLR4 expression on CD14+dim/CD16++bright pro-inflammatory monocytes was elevated despite the lowered expression on the total monocyte population (Simpson et al. 2009). This is more in corroboration with the present study as, although not statistically significant, there was a trend for elevated TLR4 expression on pro-inflammatory monocytes 1 h after exercise. TLR2 expression on total CD14+ monocytes has been reported to remain unchanged in response to acute exercise (Lancaster et al. 2005; Oliveira and Gleeson 2010; Simpson et al. 2009); however, when monocyte subtypes are examined, exercise appears to independently alter the expression of TLR2 on the CD14++bright/CD16+dim IL-10 secreting monocytes. We previously reported that TLR2 on CD14++bright/CD16+dim was reduced immediately after 45-min of treadmill running (Simpson et al. 2009), while the present study, in contrast, found TLR2 expression to increase on this monocyte subtype immediately after the “all-out” cycling time trial; an effect that could be influenced by the intensity, duration and/or mode of exercise. Moreover, given that monocyte subtypes have a heterogeneous expression of TLRs, inconsistent reports on monocyte TLR expression following exercise (particularly when only the total monocyte cell population has been examined) are probably due to differences in recirculation between the blood and tissues among monocyte subtypes in response to exercise of differing intensities and durations.
Monocyte HLA.DR expression was lowered immediately after and during the recovery phase of exercise, which is consistent with our previous report (Simpson et al. 2009). Although the potential consequence of these changes on host immune defense are largely unknown; it is important to note that in chronic inflammatory conditions such as sepsis, blood monocyte expression of TLR2 and TLR4 tends to be elevated (Armstrong et al. 2004; Hopkins et al. 2008; Schaaf et al. 2009; Tsujimoto et al. 2006), while HLA.DR expression tends to be lowered, particularly in the CD14+positive/CD16+positive monocyte subset population (Tsujimoto et al. 2006). Moreover, HLA.DR expression on blood monocytes has been shown to predict mortality from sepsis upon hospital admittance (Monneret et al. 2006) and is also lowered in other conditions, such as tuberculosis (Sanchez et al. 2006) and chronic liver failure (Xing et al. 2007). Interestingly, the patterns of change observed in monocyte TLR2, TLR4 and HLA.DR expression with this bout of strenuous exercise appear to mirror the changes in expression seen with sepsis, indicating that acute strenuous exercise might elicit inflammatory-like immune perturbations that could have clinical relevance. Indeed, participants performing acute bouts of strenuous exercise are known to experience endotoxemia, evident by increases in plasma lipopolysaccharide (LPS) concentration (Ashton et al. 2003; Ng et al. 2008; Nieman et al. 2006). Moreover, prolonged endurance exercise is known to alter monocyte production of the pro-inflammatory cytokines IL-1, IL-6 and TNF when challenged with LPS in vitro (Starkie et al. 2001). Future studies should attempt to examine the link between changes in TLR expression at the cell surface, plasma LPS concentration and monocyte inflammatory responses following acute exercise.
The cell shifts that occur within the peripheral blood compartment in response to acute exercise complicate the attempts to ascertain if cell surface protein expression is altered at the individual cell level due to exercise. For instance, when cell populations are isolated from blood immediately after exercise, they comprise a mixture of monocytes that were already present in the blood with monocytes that entered the blood due to exercise. It is difficult, therefore, to determine if exercise alters TLR and HLA.DR expression at the individual cell level, or whether these changes are due to pre-existing differences in TLR and HLA.DR expression between blood resident monocytes and monocytes that are mobilized with exercise. To address this, we considered the effects of blood serum factors that could potentially alter the expression of TLRs and HLA.DR at the cellular level in response to exercise, as previous work has shown post-exercise serum to alter chemokine receptors on monocytes (Okutsu et al. 2008) and IL-2 and TNF-α secretion in a Jurkat T-cell line (Radom-Aizik et al. 2007). Despite exposing resting blood monocytes to post-exercise autologous serum in vitro at concentrations typical of resting blood (i.e. 50% serum), TLR expression on the resting monocytes were unaffected by serum exposure. It is likely, therefore, that the exercise-induced changes in monocyte TLR expression seen in the present study are not due to serum factors such as glucocorticoids, stress hormones, heat-shock proteins or acidosis, which have been cited as possible physiological stimuli (Gleeson et al. 2006). Rather, this might reflect pre-existing differences in TLR expression between blood resident monocytes and comparable cells mobilized by exercise. It is acknowledged, however, that some other in vivo factors that have not been mimicked in the present in vitro cell culture model, such as mechanical and circulatory stress, could, alternatively, be responsible for altering TLR surface expression at the cellular level. Moreover, the kinetics of certain soluble factors that change with exercise might also influence this response, as these are not always accurately reflected when serum samples are taken at a defined time-point.
Exercise induced a lower HLA.DR expression on total monocytes and the pro-inflammatory monocyte subtype immediately after the exercise protocol, which remained lower than baseline levels during exercise recovery. Exposing the resting monocytes to serum obtained 1 h after exercise also altered their expression of HLA.DR compared to pre-exercise serum; however, HLA.DR expression increased on the pro-inflammatory monocyte subtype, which was opposite to that observed in monocytes obtained 1 h after exercise. While the lowered expression of HLA.DR after exercise could compromise antigen presentation capabilities to CD4+ T-cells (Simpson et al. 2009), the heightened expression of HLA.DR on the resting monocytes by serum might indicate that certain serum factors present in blood after exercise are capable of inducing monocyte activation, facilitating diapedesis and migration into the tissues. Moreover, as the total blood monocyte and CD14+dim/CD16++bright subtype count was lowered at 1 h post-exercise, it is likely that any monocytes activated by exercise-induced serum components will have already extravasated the blood compartment in vivo. Essentially, the serum-induced increase in HLA.DR expression in vitro might identify a population of cells that would have otherwise vacated the blood compartment in response to exercise.
In conclusion, although an acute bout of strenuous cycling exercise altered the overall surface expression of TLR2, TLR4 and HLA.DR on blood monocytes, exposing resting monocytes to post-exercise serum did not elicit a similar response. Exercise-induced changes in monocyte TLR and HLA.DR expression might, therefore, be unrelated to soluble serum factors that are known to change in response to exercise (i.e. glucocorticoids, stress hormones, acidosis, etc.); but could merely be a result of different expression levels that already exist between monocytes resident in blood and those that are mobilized in/out blood with exercise. Limitations of the present study include the relatively small sample size, which might explain why we observed very few changes in expression among the monocyte subtypes, and the responses of cultured cells to the circulating factors in post-exercise sera may not reflect those normally occurring in vivo. Future research should attempt to (1) explore the link between monocyte surface expression of TLRs and the responsiveness of these cells to pathogen challenge following an acute bout of exercise; and (2) examine the effects of different intensities, durations and forms of exercise on blood monocyte subtype trafficking and their expression of TLRs and other cell surface receptors.
References
Armstrong L, Medford AR, Hunter KJ, Uppington KM, Millar AB (2004) Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol 136:312–319
Ashton T, Young IS, Davison GW, Rowlands CC, McEneny J, Van Blerk C, Jones E, Peters JR, Jackson SK (2003) Exercise-induced endotoxemia: the effect of ascorbic acid supplementation. Free Radic Biol Med 35:284–291
Francaux M (2009) Toll-like receptor signalling induced by endurance exercise. Appl Physiol Nutr Metab 34:454–458
Gleeson M, McFarlin B, Flynn M (2006) Exercise and Toll-like receptors. Exerc Immunol Rev 12:34–53
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745
Hilberg T, Glaser D, Koksch M, Schmidt V, Sossdorf M, Gabriel HH (2004) Differentiation of platelet-leukocyte conjugate formation by short term exercise. Clin Hemorheol Microcirc 31:217–226
Hong S, Mills PJ (2008) Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs elevated blood pressure. Brain Behav Immun 22:590–599
Hopkins PA, Pridmore AC, Ellmerich S, Fraser JD, Russell HH, Read RC, Sriskandan S (2008) Increased surface toll-like receptor 2 expression in superantigen shock. Crit Care Med 36:1267–1276
Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M (2005) The physiological regulation of toll-like receptor expression and function in humans. J Physiol 563:945–955
Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P (2006) Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32:1175–1183
Ng QY, Lee KW, Byrne C, Ho TF, Lim CL (2008) Plasma endotoxin and immune responses during a 21-km road race under a warm and humid environment. Ann Acad Med Singapore 37:307–314
Nieman DC, Henson DA, Dumke CL, Oley K, McAnulty SR, Davis JM, Murphy EA, Utter AC, Lind RH, McAnulty LS, Morrow JD (2006) Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav Immun 20:578–584
Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M (2008) The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav Immun 22:1066–1071
Oliveira M, Gleeson M (2010) The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur J Appl Physiol 109:251–257
Passlick B, Flieger D, Ziegler-Heitbrock HW (1989) Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74:2527–2534
Radom-Aizik S, Leu SY, Cooper DM, Zaldivar F Jr (2007) Serum from exercising humans suppresses T-cell cytokine production. Cytokine 40:75–81
Sanchez MD, Garcia Y, Montes C, Paris SC, Rojas M, Barrera LF, Arias MA, Garcia LF (2006) Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect 8:2492–2500
Schaaf B, Luitjens K, Goldmann T, van Bremen T, Sayk F, Dodt C, Dalhoff K, Droemann D (2009) Mortality in human sepsis is associated with downregulation of Toll-like receptor 2 and CD14 expression on blood monocytes. Diagn Pathol 4:12
Selkirk GA, McLellan TM, Wright HE, Rhind SG (2009) Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol 296:R575–R586
Simpson RJ, McFarlin BK, McSporran C, Spielmann G, o Hartaigh B, Guy K (2009) Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun 23:232–239
Skinner NA, MacIsaac CM, Hamilton JA, Visvanathan K (2005) Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin Exp Immunol 141:270–278
Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J (2008) Peripheral blood CD14 high CD16+ monocytes are main producers of IL-10. Scand J Immunol 67:152–159
Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA (2001) Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Physiol Cell Physiol 280:C769–C774
Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW (2000) Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol 279:C578–C586
Tsujimoto H, Ono S, Majima T, Efron PA, Kinoshita M, Hiraide H, Moldawer LL, Mochizuki H (2006) Differential toll-like receptor expression after ex vivo lipopolysaccharide exposure in patients with sepsis and following surgical stress. Clin Immunol 119:180–187
Xing T, Li L, Cao H, Huang J (2007) Altered immune function of monocytes in different stages of patients with acute or chronic liver failure. Clin Exp Immunol 147:184–188
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81:584–592
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Ward.
Rights and permissions
About this article
Cite this article
Booth, S., Florida-James, G.D., McFarlin, B.K. et al. The impact of acute strenuous exercise on TLR2, TLR4 and HLA.DR expression on human blood monocytes induced by autologous serum. Eur J Appl Physiol 110, 1259–1268 (2010). https://doi.org/10.1007/s00421-010-1616-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1616-2