Abstract
Cold-induced vasodilatation (CIVD) has been proposed as a potential protective mechanism against cold injuries during exposure of extremities to a cold environment. The purpose of this study was to evaluate the effect of exercise and the associated elevation in core temperature on toe skin temperatures during immersion of the foot in cold (8°C) water. Subjects (N = 8) participated in two trials. In one, they conducted an incremental exercise to exhaustion (exercise) on a cycle ergometer, which was followed by immersion of the right foot in 8°C water. In the second trial (control), immersion of the foot in cold water was not preceded by exercise. Upon completion of the exercise in the exercise trial, and at the onset of the immersion of the foot in cold water, tympanic temperature was 0.6°C (P < 0.01) higher than pre-exercise levels. There was a significant increase (P < 0.05) in the number of CIVD waves, but not their amplitudes, in the exercise trial compared to the control trial. A CIVD response occurred in 57.5% of all toes in the exercise trial, and in only 27.5% in the control trial. Additionally, 50% of subjects exhibited CIVD in at least one toe in the control trial, and 87.5% during the exercise trial. It is concluded that exercise, and particularly the associated elevation in core temperature, enhances the frequency of the toe CIVD responses, and can therefore potentially act as a protective mechanism against cold injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During immersion of feet in cold water, toe temperatures first drop rapidly due to cold-induced vasoconstriction, but may rise again due to cold-induced vasodilatation (CIVD). CIVD has been suggested as a preventative mechanism against cold injuries (Lewis 1930; Yoshimura and Iida 1952; Wilson and Goldman 1970; Daanen 2003).
The extremities are most frequently exposed to severe cold during outdoor activities. The toes are more prone to cold injury than fingers (Juopperi et al. 2002). Constrictive footwear that is usually worn in extremely cold ambient conditions, combined with the position of the feet relative to the heart level, both have a negative influence on the perfusion of the toes (Francis and Golden 1985; Francis and Oakley 1996). CIVD is regularly observed in the hands, and most studies investigating this response have focused particularly on the fingers (Daanen 2003), although most cold injuries occur in the feet, and less in the hands (Daanen and van der Struijs 2005). Results from the few studies that have investigated the CIVD response in toes suggest that the CIVD response in toes is generally poor in comparison to the CIVD response observed in the fingers (Cheung and Mekjavic 2007). The response in the toes does not seem to be a homogenous response, and is not affected in any way by a regime of daily immersions in cold water over a period of 15 days (Reynolds et al. 2007).
It has been demonstrated that moderate hyperthermia can greatly influence the CIVD response in fingers (Daanen et al. 1997). Takano and Kotani (1989) showed that an increase in central body temperature of 0.27°C, caused by ingestion of a meal containing 700 kcal, enhanced the CIVD response in fingers. Flouris et al. (2008) recently reported that the CIVD response of fingers was greater when body temperature was increased either passively using a liquid conditioning garment, or actively using exercise. In contrast, the CIVD response was almost totally abolished during moderate hypothermia (Daanen and Ducharme 1999). Greenfield et al. (1951) showed that during combined general hypothermia and direct exposure of extremities to severe cold, CIVD is abolished, suggesting it loses its role as a cryoprotective mechanism. These findings suggest that as long as internal body temperature is not compromised, the CIVD response can be activated as a local preventative mechanism against cold injury to the fingers. Despite the evidence that hyperthermia enhances CIVD in hands (Flouris et al. 2008), the effect of elevated core temperature on the CIVD responses of toes or feet has not been investigated.
The purpose of this study was to evaluate the influence of exercise on CIVD responses in toes. We hypothesized that the CIVD response will be more prevalent, and its magnitude will be enhanced by exercise-induced elevation in core temperature.
Methods
Subjects
Eight healthy male subjects with an average (SD) age of 25 (5) years, height 180.8 (4.8) cm, and weight 80.4 (5.9) kg participated in the present study. The protocol of the study was approved by the National Medical Ethics Committee of the Republic of Slovenia. Written informed consent was obtained from all subjects before the start of the experiments. A health-screening test was performed before recruiting the subjects to the study. The aim was to exclude subjects with a history of cold injury, Raynaud’s disease, smokers and/or subjects that used any anti-hypertensive medication. Data were collected in the period from September to November. The environmental conditions during this period are moderate, thus any potential acclimatization to either sustained heat or cold due to the micrometeorological conditions was minimal.
Experimental design
All subjects participated in two trials, which were counterbalanced and conducted at least 2 days apart. In the exercise trial subjects conducted an incremental exercise to exhaustion on a cycle-ergometer (Monark 884E, Sweden), which caused an exercise-induced elevation in core temperature. Immediately upon cessation of the exercise, subjects immersed their right foot in a water bath maintained at 8°C. In the control experiment subjects immersed their right foot in cold water without any prior exercise. All the immersions were conducted at the same time of the day for each subject to avoid any contribution of diurnal variations to the CIVD response. The subjects were asked not to drink coffee or caffeinated beverages, and to abstain from any sport, recreational activity or heavy physical exertion prior to the trials.
Experimental protocol
In each experiment, subjects arrived at the laboratory at least 30 min before the start of the measurements, which allowed adaptation to the controlled thermoneutral (~ 27°C, 35% relative humidity) environment in the laboratory.
In the exercise trial, subjects commenced the exercise with a 2-min period of unloaded pedaling, followed by incremental load exercise to exhaustion. The work rate was increased by 40 W every 2 min until the subjects could no longer maintain a cadence of 60 revolutions per minute. Heart rate and tympanic temperature were recorded at regular intervals (2 min) during the exercise. Upon completion of the exercise subjects were seated in a semi-reclining chair and the CIVD protocol was initiated. In the control trial, the CIVD protocol was not preceded by the exercise.
The CIVD test comprised a 3-min rest period, followed by a 30-min immersion in cold water. Following the rest period, subjects immersed their right foot in a bath of water maintained at 8°C. The foot was immersed 5 cm above the maleolus. After 30 min of immersion the foot was removed from the water bath and dried with a towel.
All parameters (HR, tympanic temperature) were recorded in the pre-immersion phase, and during the cold-water immersion phase. During the cold-water immersion, measurements were made at minutes 1, 3 and 5 and every 5 min thereafter. At the same time periods, subjects were asked to provide a rating of their temperature perception and thermal comfort. All participants were familiarized with the temperature perception and thermal comfort scales to avoid any misinterpretations during the immersion.
Analytical procedure and equipment
Thermocouples were attached to all five toe pads of the right foot and to the dorsal side of the foot. The toe pads proved to be a reliable temperature-measuring site for the evaluation of these changes (Daanen 2003; O’Brien 2005). The toe pad has a rich blood supply and is a site where AV anastomoses are present, and these are responsible for the CIVD response.
Copper-constantan type thermocouples (Concept Engineering, Old Saybrook, CT, USA) were used in all experiments. The probes were attached directly to the skin by thin air-permeable tape (Tegaderm, 3 M; Healthcare, St Paul, MN, USA).
Skin temperatures were measured at eight second intervals by an Almemo data acquisition system (Almemo 5290-9 V5; Ahlborn, Holzkirchen, Germany). The data were first stored in the data logger and then downloaded to a personal computer for further analysis after each experiment.
Beat-by-beat heart rate was measured continuously with a Polar system (Polar Electro Inc., Lake Success, NY, USA) and stored in a Polar wristwatch. Data were downloaded to a desktop computer after each experiment. Tympanic temperature was measured using a commercially available infrared thermometer (ThermoScan IRT 3020; Braun, Kronberg, Germany). Three consecutive measurements were performed each time and the highest value was considered representative of core temperature.
Subjective ratings of thermal perception and thermal comfort were recorded before, during and after the cold-water immersion. The thermal sensation scale ranged from 0 (unbearably cold) to 9 (very hot). The thermal comfort scale ranged from 1 (comfortable) to 5 (extremely uncomfortable).
An insulated metal immersion bath (80 cm × 30 cm × 25 cm) with an active cooling system (Haake, Germany) was used for the cold-water immersions in all the trials. The water was continuously stirred by a small impeller situated in the bath.
Statistical analysis
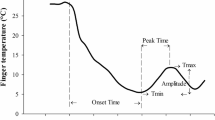
A computer program written in Matlab language (MathWorks Inc., USA) was developed to determine all the CIVD parameters from the raw data. The CIVD response was defined as an increase in toe pad skin temperature > 0.5°C. The results of the computer analysis were also manually checked for any errors.
A two-way analysis of variance (ANOVA; Statistica 5.0, USA) was used to evaluate the differences between temperature parameters of all five toes and between the control and exercise trials. The independent variables for comparison were toes (five digits) and condition (two trials: control and exercise). A one-way ANOVA was used to define the differences in HR between exercise and control trials. A Tukey post hoc test was used to test specific differences of mean values. The comparison between the number of waves and their respective magnitudes observed in the control and exercise trials was evaluated with a Wilcoxon’s matched pairs non-parametric test. This test was also used to evaluate the differences in thermal perception (TSS) and comfort (TCS) between control and exercise trials. TSS and TCS are presented as median (range), whereas the remaining data are presented as mean (SD). The significance level was set at 0.05.
Results
The average duration of the exercise was 9.2 (2.3) min, during which the subjects attained an average (SD) maximal work rate of 221.2 (45.2) W. Upon completion of the exercise in the exercise trial, tympanic temperature was 37.4 (0.4)°C. This was 0.6°C higher than the observed pre-exercise value of 36.8 (0.3)°C, and the level observed in the control trial. Tympanic temperature remained elevated above pre-exercise levels throughout the 30 min of immersion (Fig. 1). A CIVD response was observed in 57.5% of all toes in the exercise trial, and in only 27.5% in the control trial. Additionally, 50% of subjects exhibited CIVD in at least one toe in the control trial and 87.5% in the exercise trial (Table 1). In toes that exhibited a CIVD response, the number of hunting waves was greater in the exercise trial (Table 1). No CIVD responses were observed at the dorsum.
Most CIVD responses in the toes during both trials were atypical (Fig. 2). During the exercise trial, three subjects showed a strong atypical CIVD response, whereas the remaining four subjects showed weak responses (Table 2). In one subject, one big long period hunting wave was found in all toes. Another subject exhibited a plateau response in all toes with toe temperatures fluctuating between 13 and 17°C. The third subject showed a big wave in the first two toes, a normal CIVD response with two waves in the third toe and no CIVD response in the fourth and fifth toes. During the control trial, a strong CIVD response was observed only in one subject in just two toes. The remaining CIVD responses were weak.
Pre-immersion HR values were 105.6 (7.6) min−1 in the exercise trial, and 74.4 (8.3) min−1 in the control trial. During the first minute of immersion, HR was 104.0 (6.9), and 77.4 (8.6) min−1 in the exercise and control trials, respectively. HR attained end-immersion values of 93.4 (5.4) min−1 and 77.0 (11.9) min−1 in the exercise and control trials, respectively (P < 0.01). No statistically significant differences were observed between thermal sensation and thermal comfort scores between the two trials (Fig. 3).
The average (SD) values for pre-immersion skin temperatures (T pre) were similar in all five toes and dorsum of the foot in both trials. T pre ranged from 30.9 (2.5)°C in the fifth toe to 30.2 (2.7)°C in the third toe in the exercise trial. In the control trial, T pre ranged from 28.3 (4.5)°C in toe 5 to 27.6 (5.1)°C in toe 2. Values were similar on the dorsum of the foot in both trials and were 30.5 (2.0)°C and 31.5 (2.0)°C in the exercise and control trials, respectively.
The average CIVD amplitude for all toes during the immersion was 0.5 (0.6)°C in the control and 2.2 (2.3)°C in the exercise trial (Table 2); the difference was not statistically significant. Minimum temperature (T min) was similar in all five toes in the exercise trial and ranged between 8.3 (0.6)°C in 5th toe and 8.5 (0.8)°C in the 2nd toe. In the control trial, T min ranged between 8.0 (0.6)°C in the 1st toe and 8.5 (0.2)°C in the 3rd toe. There were no statistically significant differences in T min between the toes or between the two trials.
Discussion
The principal finding of this study is that exercise, and the consequent rise in tympanic temperature, enhances the frequency of the CIVD response in the toes, which usually show poor CIVD responses (Cheung and Mekjavic 2007).
Significant differences in CIVD responses were found between exercise and control trials. During the exercise trial, the percentage of CIVD occurrence in at least one toe was 30% higher. However, a CIVD response rarely occurred in all five toes (only in two subjects during the exercise trial). In toes that showed a CIVD response, the number of hunting waves was greater in the exercise trial. Our findings are in agreement with those of Greenfield et al. (1951) who showed that higher ambient temperatures, which probably caused a slight increased core temperature, caused an increase in cyclic vasodilatations in toes during cold-water immersion.
The incidence of toe CIVD response in the control trial was 27.5%, which made it difficult to conduct comparisons of the CIVD characteristics between the two trials. Interestingly, in seven toes (from five subjects) that showed weak CIVD responses with low amplitudes and short periods, the CIVD responses were not enhanced in the exercise trial. It is generally believed that high body temperature increases local CIVD responses through a central mechanism, most likely sympathetic withdrawal (Daanen et al. 1997). Our findings are not in agreement with this suggestion, since during sympathetic withdrawal, enhancement of pre-existing hunting waves would be expected. However, although it is possible that a release of sympathetic tone can trigger a CIVD response, the possibility that moderate hyperthermia could stimulate local mechanisms that trigger a CIVD response cannot be excluded (Flouris et al. 2008).
That exercise per se does not affect CIVD is supported by findings of O’Brien and Montain (2003), who used exercise in a hot and humid environment to assess the effect of hypohydration on the CIVD response. The CIVD response was determined approximately 2 h after cessation of exercise and following a recovery period to allow core temperature to return to resting pre-exercise levels, and to allow equilibration of body fluid spaces. Despite the recovery period, not all subjects’ core temperature returned to resting levels, and the authors divided the subjects into two groups: a group who had similar core temperatures in the control and hypohydrated trials, and another that did not. There were no significant differences in any of the reported CIVD characteristics between the euhydrated and hypohydrated trials for the group that had similar core temperatures, whereas in the group with significantly higher core temperatures, both in the euhydrated and hypohydrated trials there was a significant enhancement of the CIVD response: higher apex and mean finger temperatures measured at the finger nail bed, and significantly higher nadir and mean finger temperatures measured at the finger pad. These results therefore confirm that the enhancement of the CIVD response following exercise is most likely due to the elevation in core temperature resulting from the exercise, rather than from some other exercise-related factor. In contrast, Geurts et al. (2006) reported no effect of exercise, combined with repeated cold exposure of the hand, on the CIVD response in the index finger. The CIVD response was examined daily for 10 days in two groups of subjects: one group of subjects conducted daily training immersion of the hand in cold water (8°C) for 30 min, concomitant with exercise on a cycle ergometer, whilst the other group conducted the daily hand immersions without any exercise training. No differences were observed in any of the characteristics of the index finger CIVD response, despite a 0.6°C exercise-induced elevation in rectal temperature. That repeated daily immersions of the hand or foot in cold water does not affect the CIVD response has been confirmed by Reynolds et al. (2007) and Mekjavic et al. (2008), respectively. The lack of observed enhancement of CIVD during exercise (Geurts et al. 2006) is possibly due to the experimental design used. Namely, due to the substantial individual variability observed in the CIVD responses (Geurts et al. 2006; Mekjavic et al. 2008), comparison of the CIVD response only in one finger between two subject groups, as conducted by Geurts et al. (2006), may not have captured the enhancement observed when the same group of subjects is tested on repeated occasions. The responses observed in one finger on one experimental day may not be present on another day (Mekjavic et al. 2008). It is therefore warranted to measure the CIVD response in all digits of a test limb.
We have recently provided evidence (Flouris et al. 2008) that during warming of clothed subjects exposed to subzero ambient temperatures, CIVD onset coincided with a threshold mean body temperature (T b). Once CIVD was initiated, T b began to decrease, and eventually ceased when T b fell below the threshold value. Based on these observations we proposed (Flouris et al. 2008) that the CIVD response could also be considered a heat dissipating mechanism, preventing excessive increases in T b. Although defining the skin temperature variations in the study of Flouris et al. (2008) as CIVD responses has recently been challenged (Daanen 2009), and that the role of CIVD in the regulation of T b is speculative, the observed enhancement of CIVD above a T b threshold value further supports the notion of a cold protective mechanism. Namely, to prevent cold injury of peripheral tissue, heat must be transferred, from the core region to the excessively cooled peripheral tissue. As demonstrated by the results of Flouris et al. (2008), such CIVD-induced transfer of heat from the core to the peripheral tissues causes significant reductions in core temperature, thus enhancing the risk of hypothermia. It is therefore plausible, that CIVD as a cryoprotective response is most efficient when T b is elevated above normal resting values, and that the cessation of the response once T b falls below a threshold values prevents further decreases of T b below the normal resting value. There would be no survival value in continuing to initiate the CIVD response to maintain peripheral tissue temperatures elevated at the expense of risking general hypothermia.
Unlike CIVD responses in hands, the CIVD responses of toes in this study were mostly atypical (Fig. 2). Atypical CIVD waves have been described and characterized by Mekjavic et al. (2008) and have been termed as no response, small wave, big wave and plateau response. During the hyperthermic trial, only three subjects showed a strong CIVD response, whereas the remaining four subjects showed weak low amplitude (0.5–1°C) responses, with short periods, which usually appeared in the first minute of the immersion. The strong responses were also atypical and appeared either as one big hunting wave, or as a plateau response. During the normothermic experiment, a strong CIVD response was observed only in one subject in just two toes. The rest of the CIVD responses were weak responses. The incidence of CIVD in at least one toe in the control trial was 50% in this study. This is not in agreement with the findings of Cheung and Mekjavic (2007), and Reynolds et al. (2007) who found that CIVD is a rare response in toes. The reason could be in the criteria to define a CIVD response. Both studies defined a CIVD to have an amplitude of 1°C or higher, whereas we defined 0.5°C to be the minimum amplitude of a hunting wave. If we defined 1°C to be minimum hunting wave amplitude, the incidence of CIVD would be 35% in the exercise trial and 10% in the control trial. Another possible explanation could be that in the study of Cheung and Mekjavic (2007), and Reynolds et al. (2007), the toe temperature was measured at the nail bed site in contrast to the pad site used for toe skin temperature recording in the present study. Although it has been demonstrated that the reproducibility of the temperature measurements at the nail bed is better than at the pad site in fingers during cold-water immersion (O’Brien 2005), the same comparison has not been made for toes. Toe skin temperature responses varied greatly within one foot in the present study. This supports the idea of measuring skin temperatures of all digits to correctly represent overall thermal responses of the extremities to cold exposure (Mekjavic et al. 2008; Cheung and Mekjavic 2007).
Core temperature in the present study was assessed with an infrared tympanic thermometer. Since the aim of the study was to determine whether the frequency and magnitude of the CIVD response are enhanced at elevated core temperature, and not to correlate findings with absolute core temperature, infrared thermometry was considered an adequate index of core temperature. Despite reported concerns regarding the use of such measurements, especially in children suffering seizures triggered by fever (Romanovsky et al. 1997), Mekjavic et al. (1992) reported good correlation of T ty with oesophageal temperature during cooling of subjects to hypothermic levels, and also during the subsequent rewarming. The correlation was not as good with rectal temperature. Daanen (2006) also compared T ty measured with an infrared thermometer and T es during passive heating of subjects, and reported that T ty underestimated T es by about 0.4°C. Thus, for the purpose of the present study, we can assume that the tympanic temperature measured with an infrared tympanic thermometer reflected the change in core temperature. In the event that our measurements may have been lower than T es, as suggested by Daanen (2006), this would not alter the main finding of the present study.
In conclusion, mild hyperthermia enhances prevalence of CIVD in toes. However, the incidence of the CIVD response was rare and atypical. It remains to be investigated whether greater changes in core temperature would trigger stronger CIVD responses in all toes. The results of the present study and those of Flouris et al. (2008) clearly demonstrate the benefit of elevated core temperature in enhancing the CIVD response, and thus minimizing the risk of cold injury. Flouris et al. (2008) achieved an elevation in core temperature with passive peripheral heating using a water-perfused garment, whereas in the present study exercise provided active core warming. In a cold environment, minimizing heat loss or rather passive heating may not be sufficient to prevent cold injury of the feet in individuals that are inactive (Mekjavic et al. 2005). In such scenarios, a regime of continuous or intermittent exercise may be necessary to maintain core temperature elevated, maintaining not only better perfusion of the toes, but also maintaining body temperature above the threshold temperature at which CIVD is initiated (Flouris et al. 2008), should toe skin temperatures decrease towards unacceptable levels.
References
Cheung SS, Mekjavic IB (2007) Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. Eur J Appl Physiol 99:701–705. doi:10.1007/s00421-006-0383-6
Daanen HA (2003) Finger cold-induced vasodilation: a review. Eur J Appl Physiol 89:411–426. doi:10.1007/s00421-003-0818-2
Daanen HA (2006) Infrared tympanic temperature and ear canal morphology. J Med Eng Technol 30:224–234. doi:10.1080/03091900600711613
Daanen HA (2009) Cold-induced vasodilatation. Eur J Appl Physiol 105:663–664. doi:10.1007/s00421-008-0958-5
Daanen HA, Ducharme MB (1999) Finger cold-induced vasodilation during mild hypothermia, hyperthermia and at thermoneutrality. Aviat Space Environ Med 70:1206–1210
Daanen HA, van der Struijs NR (2005) Resistance index of frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med 76:1119–1122
Daanen HA, Van de Linde FJ, Romet TT, Ducharme MB (1997) The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol Occup Physiol 76:538–543. doi:10.1007/s004210050287
Francis TJ, Golden FS (1985) Non-freezing cold injury: the pathogenesis. J R Nav Med Serv 71:3–8
Francis TJR, Oakley EHN (1996) Cold injury. In: Tooke JE, Lowe GDO (eds) A textbook of vascular medicine. Arnold, London, pp 353–370
Flouris AD, Westwood DA, Mekjavic IB, Cheung SS (2008) Effect of body temperature on cold induced vasodilation. Eur J Appl Physiol 104:491–499. doi:10.1007/s00421-008-0798-3
Geurts CL, Sleivert GG, Cheung SS (2006) Local cold acclimation during exercise and its effect on neuromuscular function of the hand. Appl Physiol Nutr Metab 31:717–725. doi:10.1139/H06-076
Greenfield AD, Kernohan GA, Marshall RJ, Shepherd JT, Whelan RF (1951) Heat loss from toes and fore-feet during immersion in cold water. J Appl Physiol 4:37–45
Juopperi K, Hassi J, Ervasti O, Drebs A, Näyha S (2002) Incidence of frostbite and ambient temperature in Finland, 1986–1995. A national study based on hospital admissions. Int J Circumpolar Health 61:352–362
Lewis T (1930) Observations upon the reactions of the vessels of the human skin to cold. Heart 15:177–208
Mekjavic IB, Sun J, Lun V, Giesbrecht G (1992) Evaluation of an infra-red tympanic thermometer during cold water immersion and rewarming. In: Lotens W, Havenith G (eds) Proceedings of the 5th international conference on environmental ergonomics. Maastricht, The Netherlands, pp 42–43
Mekjavic IB, Kocjan N, Vrhovec M, Golja P, House C, Eiken O (2005) Foot temperatures and toe blood flow during a 12 km winter hike and guard duty. In: Prevention of cold injuries (pp. 5-1–5-4). Meeting proceedings RTO-MP-HFM-126, paper 5. RTO, Neuilly-sur-Seine. Available from: http://www.rto.nato.int/abstracts.asp
Mekjavic IB, Dobnikar U, Kounalakis SN, Musizza B, Cheung SS (2008) The trainability and contralateral response of cold-induced vasodilatation in the fingers following repeated cold exposure. Eur J Appl Physiol 104:193–199. doi:10.1007/s00421-008-0727-5
O’Brien C (2005) Reproducibility of the cold-induced vasodilation response in the human finger. J Appl Physiol 98:1334–1340. doi:10.1152/japplphysiol.00859.2004
O’Brien C, Montain SJ (2003) Hypohydration effect on finger skin temperature ad blood flow during cold-water finger immersion. J Appl Physiol 94:598–603. doi:10.1063/1.1574179
Romanovsky AA, Quint PA, Benikova Y, Kiesow LA (1997) A difference of 5°C between ear and rectal temperatures in febrile patient. Am J Emerg Med 15:383–385. doi:10.1016/S0735-6757(97)90133-9
Reynolds LF, Mekjavic IB, Cheung SS (2007) Cold-induced vasodilatation in the foot is not homogenous or trainable over repeated cold exposure. Eur J Appl Physiol 102:73–78. doi:10.1007/s00421-007-0566-9
Takano N, Kotani M (1989) Influence of food intake on cold-induced vasodilatation of finger. Jpn J Physiol 39:755–765. doi:10.2170/jjphysiol.39.755
Wilson O, Goldman RF (1970) Role of air temperature and wind in the time necessary for a finger to freeze. J Appl Physiol 29:658–664
Yoshimura H, Iida T (1952) Studies on the reactivity of skin vessels to extreme cold II. Factors governing the individual difference of the reactivity, or the resistance against frostbite. Jpn J Physiol 2:177–185
Acknowledgments
The authors wish to express their gratitude to the subjects for their participation. The study was supported, in part, by Knowledge for Security and Peace grant from the Ministries of Defense, and of Science (Republic of Slovenia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dobnikar, U., Kounalakis, S.N. & Mekjavic, I.B. The effect of exercise-induced elevation in core temperature on cold-induced vasodilatation response in toes. Eur J Appl Physiol 106, 457–464 (2009). https://doi.org/10.1007/s00421-009-1035-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-009-1035-4