Abstract
We used the direct Fick measurements to validate a method for estimating cardiac output by iteratively fitting \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) at the mouth to lung model values. This model was run using a series of 50, 30 and 10 breaths to test sensitivity to number of breaths used for fitting. The lung was treated as a catenary two-compartment lung model consisting of a dead space compartment connected with a single alveolar space compartment, perfused with constant pulmonary blood flow. The implemented mathematical modeling described variations in O2 and CO2 compartmental fractions and alveolar volume. This model also included pulmonary capillary gas exchange. Experimental data were collected from measurements performed on six healthy subjects at rest and during 20, 40, 60 and 85–90% of peak \( \ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }} . \) The correlation between the two methods was highest and the average agreement between the methods was best using 50 breaths \( (\hbox{R} = 0.95;\hbox{P} < 0.0001;\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{{{\text{model}}}} = 1.1\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{{{\text{Fick}}}} - 2.3). \) The mean difference and lower to upper limits of agreement between measured and estimated data were 0.7 l/min (−2.7 to 4.1 l/min) for cardiac output; −0.9 ml/100 ml (−1.3 to −0.5 ml/100 ml) for arterial O2 content; −0.8 ml/100 ml (−3.8 to 2.2 ml/100 ml) for mixed venous O2 content and −0.1 ml/100 ml (−2.9 to 2.7 ml/100 ml) for arteriovenous difference O2 content. The cardiac output estimated by the lung model was in good agreement with the direct Fick measurements in young healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac output measurement \(({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{{{\text{C}}}}})\) is an important feature to assess cardiovascular function in investigations of physiological adaptation and therapeutic trials. However, it is difficult to justify the use of gold standard techniques such as direct Fick method (Fick 1870; Krogh and Lindhard 1912; Werko et al. 1949) or dye/thermo-dilution in healthy subjects because of the inherent risks of catheterization. Therefore, a number of indirect and non-invasive methodologies has been developed. Previous reports (Burma and Saidel 1983; Burma 1985; Saunders and Cummin 1992; Johnson et al. 2000) showed that tidally ventilated homogeneous lung models can be used to correctly estimate \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) at rest and during exercise. These techniques require either rebreathing of expired air or use of inert soluble gases that require specialized equipment for measurement. A technique that does not have either requirement, i.e., requires only normal ventilation and measurement of only the respiratory gases, would be a valuable tool for investigations of the cardio-respiratory responses to exercise.
In a previous investigation, we developed a breathing lung model which was adapted to estimate cardiac output in exercise (Benallal et al. 2002a). To use this method, one needs the breath-by-breath data for VT, breath timing, \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}\) and \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) measured at the mouth. This model includes tidal breathing and gas exchange in the pulmonary capillaries. The main advantages of this method are the minimal specialized equipment needed and the use of the open-circuit with O2 and CO2 respiratory gases, avoiding drawbacks of rebreathing and the use of non-respiratory gases. The tidally ventilated homogeneous lung model has been first implemented to simulate pulmonary gas exchange (Benallal and Busso 2000; Benallal et al. 2002b). Although the simulated difference between end-expiratory and arterial \(\hbox{P}_{{{\text{CO}}_{2} }}\) was in keeping with data published in the literature (Jones et al. 1979), the breathing lung model did not adequately fit \(\hbox{P}_{{{\text{ET,CO}}_{2} }}\) (Benallal et al. 2002b). Then lung model was applied in a different way to derive cardiac output from the data set in VT, breath timing, \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}\) and \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) measured at the mouth (Benallal and Busso 2000). The model was used to iteratively find a solution for mixed venous gases, end-expiratory alveolar volume and cardiac output. These model parameters were estimated when the lung model fits, over 50 breaths, the metabolic CO2 production at the mouth to experimental data. The cardiac output estimated using this lung model was compared with the value measured by the CO2 rebreathing method corrected by the arterial blood data. A high correlation (R2=0.71; P < 0.0001) was observed between the two methods. However, the lung model estimates were significantly lower than the rebreathing data by an average of 2.3 l/min difference. Even if the CO2 rebreathing procedure is corrected by the arterial blood data, this technique does not constitute a gold standard method to measure the cardiac output. Consequently, the cardiac output estimated with the tidally homogeneous lung model must be compared with the value measured using the direct Fick method to conclude definitively about its validity and its reliability.
The purpose of this study was to use the direct Fick measurements to validate a method for estimating cardiac output using only breath-by-breath gas exchange and respiratory pattern data in conjunction with a tidally ventilated homogeneous lung model as applied to normal humans at rest and during exercise. The fitted parameters determined with the breathing lung model included mixed venous O2 and CO2 contents and cardiac output. The model parameters were estimated by minimizing the sum-squared differences between model-determined \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) at the mouth and actual data over breaths.
Material and methods
Subjects and exercise protocol
The experimental data were taken from a study described in a previous report (Johnson et al. 2000). The Mayo Clinic Institutional Review Board (ethics committee) approved all aspects of the investigation. Six healthy subjects (five men and one woman) gave their written consent to participate after a full oral explanation of the experiments, though all subjects were well informed of the risk by virtue of being staff clinicians or residents in the Department of Anesthesia. The subjects’ age (year), height (cm), weight (kg) and peak O2 uptake (ml/kg/min) were 36.5±6.1, 185.6±9.0, 85.2±15.3 and 50.2±9.3. Among these subjects, four were former athletes and maintained a moderate level of training during this investigation. The subjects were instructed to avoid exercise and heavy exercise training before the day of experimentation. The experiment was performed early in the morning resulting in a fasting period of at least 8 h.
The exercise studies required two visits to the General Clinical Research Center Exercise Core Laboratory. During the first session, the volunteers were familiarized with the testing apparatus and performed a peak exercise test on cycle ergometry until volitional exhaustion to determine peak O2 uptake \((\hbox{peak}{\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{\text{O}}_{2}}}).\) During this test, the measurements of gas exchange and respiratory pattern were continuously made with a gas exchange system (Medical Graphics CPX/D) on a breath-by-breath basis. For heart rate, the ECG was continuously monitored (Marquette Electronics). Power output (electronically braked cycle ergometer) was incremented between 25 W/min and 50 W/min depending on the subject. During the second session of the experiment, catheters were inserted in the pulmonary (7 Fr, 65 cm, double lumen pulmonary artery catheters; Arrow international, Reading, PA, USA) and radial arteries for blood gas and cardiac output measurements. The correct position of the pulmonary artery catheter was verified by the wedge position and the pressure tracings. Radial artery catheters were inserted percutaneously under local anesthesia into a radial artery to allow repeated measurements. Both catheters were continuously flushed with heparinized saline (3 U/ml of heparin; 3 ml/h) to maintain patency. Data were measured at rest and during constant load exercise at 20, 40, 60 and 85–90% of maximal power output determined from the first visit. This procedure was repeated twice separated by a period of 10-min rest. Each exercise level lasted 7–9 min, the time required to make duplicate measurements after an initial 2–3 min to reach steady state at each intensity.
Blood sample and cardiac output measurements
After 2- or 3-min at constant power to allow steady-state O2 uptake, the arterial and mixed venous blood samples were drawn simultaneously with breath-by-breath \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}},\) \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{\text{CO}}_{2}}}\) and \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{\text{E}}}\) to obtain cardiac output by the direct Fick method. The samples were immediately placed in an ice bath and subsequently analyzed within 30 min of collection. Measurements were obtained at rest and during each submaximal steady-state work intensity at 20, 40, 60 with a final measurement at 85–90% of peak \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{\text{O}}_{2}}}.\) The alveolar-to-arterial oxygen partial pressure difference \((\hbox{A-a}\,\hbox{D}_{{\rm O}_2})\) was measured at rest and during exercise for all subjects. The alveolar O2 partial pressure was calculated using the alveolar gas equation and the arterial O2 partial pressure was measured from collected blood samples.
Lung model and cardiac output estimations
A catenary two-compartment lung model previously described elsewhere in simulation and estimation studies (Benallal and Busso 2000; Benallal et al. 2002a, 2002b) was employed to estimate cardiac output at rest and in exercise at the same time as the direct Fick measurements. The breathing lung model, which included alveolar gas exchange of CO2 and O2 was applied to interpret changes in gas fractions at the mouth and alveolar compartments during the respiratory cycle. The lung model consisted of an anatomical dead space compartment connected in series with a single alveolar compartment whose volume varies during the breaths. The alveolar space is perfused with constant pulmonary blood flow with no diffusion limitation for O2 and CO2 (see Fig. 1). When the model is run, a sine-wave input function for gas flow generates a new data point every 10 ms. In each 10 ms interval, values for gas concentrations in the dead space and alveolar compartments are solved using standard mass balance equations. Before each estimation, the variables of the model were first initialized using the mass balance equations for a steadily ventilated homogeneous lung. The initialization process was appropriate to the level of exercise. Tidal volume and respiratory rate were averaged from experimental measurement and used to compute alveolar ventilation. \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}\) and \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) were averaged from measurements at the mouth and cardiac output in liters per minute was calculated from \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}\) by the mean of the equation: \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}(\hbox{l}/\hbox{min})=5\times \ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}(\hbox{l}/\hbox{min}) + 5.\) Initial gas fractions within the alveoli and the dead space were estimated from the alveolar gas equations using average alveolar ventilation, O2 uptake and metabolic CO2 production. Arterial O2 and CO2 partial pressures were initialized using an alveolar composition given by the alveolar gas equations. Gas contents in arterial blood were initialized from Pa,O2 and Pa,CO2 and mixed venous contents were calculated using the Fick principle. These different steps allowed to initialize all the variables before model iterations according to the metabolic rate at each level of exercise. The O2 dissociation curve was taken from the data of Lobdell (1981) based on the equation of Kelman (1966). The arterial content in CO2 was calculated from the equation of Douglas et al. (1988).
Tidally ventilated homogeneous lung model with the mass balance equations for gas. \({\ifmmode\expandafter\dot\else\expandafter\. \fi{\hbox{V}}_{\rm IE}(\hbox{t})}\)and \({\ifmmode\expandafter\dot\else\expandafter\. \fi{\hbox{V}}_{\rm gas}(\hbox{t})}\)represent the tidal airflow and the pulmonary gas exchange respectively during a breath. \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) indicates the pulmonary blood flow, index of cardiac output. Ca, gas (t) and \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{\hbox{v}},\hbox{gas}}}}} (\hbox{t})\) indicate the arterial and mixed venous gas content during a breath. VD and VA(t) designate the constant anatomical dead space and alveolar volume varying with breath respectively. FD, gas(t) and FA, gas(t) represent the gas fractions in anatomical dead space and alveolar spaces during a breath. Fα, gas represents the inspired gas fraction in inspiration and the gas fraction in anatomical dead space compartment in expiration. Fβ, gas indicates the gas fraction in anatomical dead space compartment in inspiration and the gas fraction in alveolar compartment in expiration
The physiological assumptions of the model were: (1) an equilibration persists over the breath between the alveolar gas and the capillary blood; (2) ventilation and perfusion inhomogeneities are assumed to be negligible; (3) pulmonary blood flow was constant during the series of breaths fitted to the model and (4) the tidal volume and respiratory rate are constant, set equal to the mean values, over the period of measurement.
To implement the modeling, the values for measured tidal volume (VT) and respiratory frequency were used to generate a sine-wave of changes in flow and volume during the breaths with 10 ms time resolution. During inspiration, gas from air entered the anatomical dead space compartment. The inspired gas flowed through dead space by plug flow into the perfectly mixed alveolar compartment perfused by pulmonary capillaries. A pair of differential equations described gas exchange at the alveolar level assuming complete equilibrium between alveolar air and blood. On expiration, the process was reversed. Under these conditions, the two-compartment lung model generated, by classic compartmental analysis, five non-linear first-order differential equations describing the dynamics in O2 and CO2 fractions in compartments during inspiration and expiration, and two equations for pulmonary capillary gas exchange. The mathematical modeling of the mass transfer in gas during breaths between the anatomical dead space and the alveolar compartments is also given in Fig. 1.
The unknown model parameters were the end-expiratory alveolar volume (EEAV), mixed venous O2 and CO2 contents and cardiac output. In the previous study (Benallal et al. 2002a), model-estimated EEAV decreased from 2.9 l at rest to 1.7 l at 75% of maximal O2 uptake. However, in this investigation, EEAV estimated by the model, surprisingly, was found to be low and non-realistic (<1.5 l). Since this parameter has little effect on model estimations, EEAV was fixed at 2,500 ml to avoid non-realistic values. The unknown model parameters were estimated by minimizing the sum-squared differences between model-determined \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) at the mouth and actual data over breaths. The Euclidean distance between experimental data and model outputs for minimization was given by Eq. 1:
where
- f::
-
distance between experimental data and model outputs;
- V A (0)::
-
the end-expiratory alveolar volume;
- \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{v},CO}}_{{{2}}} }} \)::
-
mixed venous blood CO2 content;
- \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{v},O}}_{{{2}}} }} \)::
-
mixed venous blood oxygen content;
- \( {\ifmmode\expandafter\dot\else\expandafter\.\fi{Q}_{\text{C}}}\)::
-
cardiac output;
- \({{\rm{\ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{\text{CO2exp}}}}}\)::
-
metabolic CO2 production measured at the mouth
- \(\ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{{\text{CO}}_{2} {\text{mod}}}} \)::
-
metabolic CO2 production estimated at the mouth;
- Nd::
-
number of breaths.
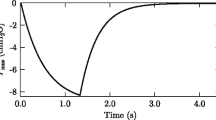
The algorithm proceeds by first adjusting one of the parameters of the model to minimize Eq. 1, then adjust the next parameter to find its minimum, until all parameters are adjusted. It then starts over with the first parameter and repeats the cycle until parameter adjustments result in little change in the sum-squared errors (Eq. 1). The fit of model parameters was done for each subject at rest and in exercise so that the square root of the sum of squared differences of breath-by-breath metabolic CO2 production between actual and model data was < 5 ml min−1. At rest, the model parameters calculated from five subjects were only estimated over 20 breaths because of the lack of enough breaths during this period. The parameters of the lung model in exercise were estimated using 50, 30 and 10 breaths to analyze the effect of the breath number on model-estimated parameters and particularly on cardiac output. Figure 2 shows an example of the fit of \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) over 50 breaths for one subject during exercise.
Breath-by-breath metabolic CO2 production in exercise for one subject. This figure illustrates the adjustment of model— \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) at the mouth (dashed line) to experimental data (solid line) using Eq. 1. The fit of model— \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) at the mouth to experimental data yields the estimation of the model parameters, O2 and CO2, in mixed venous blood and cardiac output
Data analysis
Because of the repeated for repeated measurements of cardiac output during the second session at the lower work of intensities, each subject had two data points per work except at the highest work intensity where only one point was available. A linear regression was employed during exercise to test the correlation between the measured and the model-estimated data in arterial and mixed venous O2 content, arteriovenous difference O2 content and cardiac output. A linear regression was used during exercise to test the relationship between the error in cardiac output and the alveolar-to-arterial O2 partial pressure difference. Linear regression was also employed between the direct Fick method and the estimation from the equation \({\ifmmode\expandafter\dot\else\expandafter\.\fi{Q}_{\text{C}}}=5\times \ifmmode\expandafter\dot\else\expandafter\.\fi{V}_{{{\text{O}}_{2} }}+5.\) Two-way ANOVA was used to study the effect of workload and treatment (measures versus estimates) on model-parameters. When the results were significant, the F-test was followed by a post-hoc procedure (Scheffé’s test) to locate the differences. Statistical analysis was performed using STATVIEW-PC statistical software (SAS Institute Inc. 1998, version 5.0). Bland and Altman analysis (Altman and Bland 1983; Bland and Altman 1986) was applied only during exercise for model parameters to determine the difference between the model-estimated values and direct Fick measurements against the reference method. The reliability of the method for estimating model parameters was assessed using the mean difference between the two methods (bias) and the limits of agreement which was computed from ±2 SD above and below the mean value of the difference (bias±2 SD). Twenty-five data sets were implemented for each model estimation by adding a 5% random experimental noise on \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) measured at the mouth. The sensitivity of the model to this experimental noise was assessed from the SD of \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}},\) \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{\hbox{v}},O}}_{{{2}}} }} \) and \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{\hbox{v}},CO}}_{{{2}}} }} \) computed from the data sets. The sensitivity of the model to the starting point of the iterative process for assessing \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) was also evaluated. \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) for each level of exercise was estimated for one subject starting the computations with values for \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) 10% lower and higher than those given by the equation \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}=5 \times \ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }}+5.\)
Results
Model-estimated parameters
The model-determined values for mixed venous O2 and CO2 contents and cardiac output at rest and for exercising conditions are recapitulated in Table 1. These parameters were estimated using the objective function (Eq. 1) over 20 breaths for rest and over 50 breaths for exercise conditions before the Fick measurements. The cardiac output significantly increased from 4.7±0.6 l/min at rest to 23.9±3.2 l/min at 85–90% of peak power. Mixed venous O2 content significantly decreased from 14.1±1.7 ml/100 ml at rest to 7.2±1.5 ml/100 ml at 85–90% of peak power. Mixed venous CO2 content significantly increased from 50.3±2.4 ml/100 ml at rest to 54.8±4.3 ml/100 ml at the first level of exercise (20% of peak power). However, the differences in mixed venous CO2 content between subsequent levels of exercise were not significant.
Comparison between model-estimated and actual parameters
Table 2 includes the comparison between actual data and the values estimated with the lung model for arterial and mixed venous O2 contents, arteriovenous difference O2 contents and the cardiac output at rest and during exercise. ANOVA showed that exercise level acted significantly on cardiac output, mixed venous O2 content and arteriovenous difference O2 content (P<0.0001 to P<0.05). On the contrary, exercise level did not affect the content of O2 in arterial blood significantly. No significant difference was observed between the lung model estimates and the actual data for cardiac output, mixed venous O2 content and arteriovenous difference O2 content. Unlike the others, arterial O2 content from the lung model was significantly higher than actual data (P < 0.001). Figs. 3, 4, 5 and 6 represent the results of Bland and Altman analysis during exercise for cardiac output, mixed venous and arterial O2 contents and arteriovenous difference O2 content over 50 breaths. Bland and Altman analysis showed that none of the parameter differences (model-observed) correlated with parameter values, indicating that the model fits the data well across all levels of exercise. Bland and Altman analysis for all parameters estimated at rest over 20 breaths is shown in table 3, and indicates the model slightly underestimated cardiac output while overestimating the O2 content. Bland and Altman findings for exercise data are listed in Table 4. The significant underestimate of cardiac output was also evident during exercise for models using 50 and 30 breaths. The cardiac output for the 10-breath fit was not significantly different from the direct Fick value, though the range of agreement for the 10-breath fit was nearly 3× than for the 50-breath fit. Figure 7 shows the correlation between the measured and the model-estimated data in cardiac output. There was a significant negative correlation between the error in cardiac output (actual-model) and the alveolar-to-arterial O2 partial pressure difference (Fig. 8), suggesting a possible relationship to \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{A} /\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}}\) mismatching.
Difference between Fick and model cardiac output relative to the measured alveolar-to-arterial oxygen partial pressure difference \((\hbox{A}-\hbox{a} \hbox{D}_{{\text{O}}_{2}})\) during exercise in all subjects. The difference between Fick and model was negatively correlated to the A–a \(\hbox{D}_{{\text{O}}_{2}},\) suggesting ventilation and perfusion inhomogeneities or diffusion limitation contribute to the observed difference in cardiac output. CO, cardiac output
Sensitivity analysis
For exercise, the model parameters were estimated with the breathing lung model over 50, 30 and 10 breaths to analyze the effect of the breath number on the mixed venous and arterial O2 contents, arteriovenous difference O2 content and cardiac output. The Bland and Altman analysis showed that the range in the limits of agreement for 30 and 10 breaths were progressively more extended than for 50 breaths, indicating reduced reliability of the method using fewer breaths. However, no significant difference was observed for mean bias between these three conditions. For cardiac output and arterial O2 content, the limits of agreement observed in exercise over 50 breaths were in accordance with the limits of agreement at rest over 20 breaths.
The 5% random experimental noise added to \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2} }}\) measured at the mouth provided intra-subject SD ranging from 0.035±0.001 l/min to 0.048±0.001 l/min for \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) according to exercise level. SD for mixed venous blood content ranged from 0.041±0.001 ml/100 ml to 0.045±0.001 ml/100 ml for O2 and from 0.036±0.001 ml/100 ml to 0.047±0.001 ml/100 ml for CO2. These low SD values for model parameters would attest the little sensitivity of the model to experimental errors. Since the starting point of the computation for assessing \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\)depended on the level of exercise, the sensitivity to the initial value was assessed. Model-estimated \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\)varied from 5 to 10% according to exercise level with 10% change in initial value. Nevertheless, the correlation between \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) given by the equation used to initialize the computation \(({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}=5\times \ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }} +5)\) and the direct Fick method (R=0.50, P<0.0001) was much lower than those observed between model-estimated \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}_{\text{C}}}\) and the direct Fick method (R=0.95, P<0.0001; Fig. 7).
Discussion
The major findings of this investigation showed that the cardiac output estimated using the tidally ventilated homogeneous lung model was in accordance with the direct Fick measurements at rest and during exercise in healthy young subjects. The other parameters such as the arterial and mixed venous O2 contents and the arteriovenous difference O2 content were also in agreement with the actual data. Estimation of the cardiac output using this model requires only breath-by-breath data for \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{O}}_{2} }},\) \(\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{{{\text{CO}}_{2}}}\) and the data for ventilation. The model uses primarily the data for CO2 exchange to solve for cardiac output along with mixed venous values of CO2 contents.
The findings showed a significant and reasonably high correlation between direct measurements and model results for arterial and mixed venous O2 contents, arteriovenous difference O2 content as well as cardiac output (Table 4 and Fig. 7). Differences in the variations for \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{\hbox{v}},O}}_{{{2}}} }} \) according to exercise intensity appeared however between the model and actual data. Differences in variations for \(C_{{{\text{\ifmmode\expandafter\bar\else\expandafter\=\fi{\hbox{v}},O}}_{{{2}}} }} \) were also observed between our previous report (Benallal et al. 2002a) and the present investigation. These discrepancies could arise from the use of CO2 gas exchange to fit the model parameters (Eq. 1) which would affect the precision for mixed venous O2 content. Cardiac output from the lung model was slightly but significantly less than values obtained by direct Fick measurements at rest and during exercise (Figs. 3, 4, 5, 6, Tables 3, 4; bias <1.1 l/min). Although ANOVA indicated no relationship of this bias to level of exercise, correlation analysis indicated that the bias becomes progressively larger with wider A–a gradient for O2. This suggests that the underestimate may be due to \({\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{V}}_{\rm A} /\ifmmode\expandafter\dot\else\expandafter\.\fi{\hbox{Q}}}\) mismatch, which was not included in the model. Other methodologies for measuring cardiac output have been published in the literature (Triebwasser et al. 1977; Zenger et al. 1993; Barker et al. 1999; Johnson et al. 2000). The comparison between the direct Fick method and these non-invasive methods was generally done using regression analysis rather than the Bland and Altman method. The correlation coefficient obtained at rest and during exercise in this study was 0.95, which is in line with the coefficients obtained for other indirect methods. The comparison with the direct Fick or dye dilution measurements at rest and exercise showed similar correlations in healthy subjects: R=0.94 and 0.95 for two different open-circuit acetylene techniques (Johnson et al. 2000), R=0.87 (Smyth et al. 1984) and R=0.94 (Triebwasser et al. 1977) for modified acetylene rebreathing procedure, R=0.96 for CO2 rebreathing method (Reybrouck and Fagard 1990), R=0.94 for modified impedance cardiography (Richard et al. 2001) and R higher than 0.90 for Doppler echocardiography (Vinet et al. 2001).
In this study, the model parameters were estimated with the objective function (Eq. 1) over 50, 30 and 10 breaths during exercise in order to analyze the influence of the breath number on model parameters and particularly on the cardiac output. The parameters were determined using sinusoidal respiratory flow profiles adjusted to tidal volumes and respiratory frequencies experimentally measured. During the fit of the model with the local variation method used to find a local minima, there is a trade-off between the model parameters. The estimated values for cardiac output and mixed-venous blood contents could compensate for each other. However, in this study, the model parameters were in a range which was physiologically acceptable. Consequently, no restriction was added to the model computations. The values used to start the iterations were chosen according to the exercise conditions because of the influence of the initialization on the model-estimated values. The sensitivity of the model to the starting point of the computation could be a limitation to the use of this method. It is however noteworthy that the proposed method provided a much better correlation with direct Fick method than the equation used to initialize the computation (R=0.95 vs 0.50). This improvement in the correlation showed that the precision in model-estimated \({\ifmmode\expandafter\dot\else\expandafter\. \fi{\hbox{Q}}_{\text{C}}}\) did not result only on its initialization but also on the relevance of this model.
Bland and Altman statistical analysis showed that the precision over 50 breaths was better than over 30 and 10 breaths (Table 4). However, no significant difference was observed in bias between these three conditions. In addition, the limits of agreement in exercise over 50 breaths were close to the limits of agreement at rest over 20 breaths, indicating the model fits the data well across rest and all levels of exercise. A high correlation was observed between model-determined cardiac output and Fick data for each condition, although the correlation for 50 breaths was better than at 30 and 10 breaths. Thus, in practice, during exercise, there will be a trade-off between better temporal resolution using fewer breaths in the model and better reproducibility and accuracy using more breaths. The breath number to obtain an agreement seems to be greater for exercise than for rest although our limited data set obtained at rest (no more than 20 breaths) did not allow us to conclude this issue. The 50 breaths necessary to reach a correct accuracy for cardiac output estimation forced to take the measurement during exercise at a constant load for a number of minutes. It would be thus difficult to use the model in standard protocol in a exercise testing.
It is known that the ventilation-to-perfusion inhomogeneities increase with exercise (Gledhill et al. 1978). In our study, however, the model consisting of a tidally ventilated homogeneous lung did not account for these inhomogeneities and diffusion limitation. Considering this, the question is, how sensitive the estimates of cardiac output would be to an error in the underlying assumptions (e.g., ventilation-to-perfusion mismatching and/or significant diffusion limitation). In this investigation, most subjects presented a progressive increase in alveolar-to-arterial partial pressure difference in O2 consistent with an increase in ventilation-to-perfusion inhomogeneities or diffusion limitation. There was a significant negative correlation between A–a DO2 and the Fick-model difference averaged over the 2 measurements at each level of exercise (Fig. 8). The parameters increasing the A–a \(D_{{\text{O}}_{2}}\) as ventilation-to-perfusion inhomogeneities and diffusion limitation could induce an overestimation in cardiac output with the model. Figure 8 did not allow us to make a distinction between the respective effect of ventilation-to-perfusion mismatch and diffusion limitation on the model-estimation of cardiac output. Based on these findings, the model estimation of cardiac output could be thus less reliable in old subjects or patients with COPD where inhomogeneities increase a lot either physiologically or pathologically. Furthermore, it could be difficult to generalize the results of the present investigation to patients which would be at the lower end of the normal range for cardiac output.
In conclusion, a non-rebreathing and non-invasive method based on a tidally ventilated homogeneous lung model has been devised to estimate cardiac output. The values obtained with the lung model were in good agreement with the direct Fick measurements in healthy young subjects at rest and in exercise. However, the estimation of cardiac output could be affected by the initial value used in model computation and by large inhomogeneities of ventilation-to-volume and ventilation-to-perfusion ratios or diffusion limitation. Further, validation studies should be made in patients with large inhomogeneities or small cardiac output during exercise.
References
Altman DG, Bland JM (1983) Measurement in medicine: the analysis of method comparison studies. Statistician 32:307–317
Barker RC, Hopkins SR, Kellogg N, Olfert IM, Brutsaert TD, Gavin TP, Entin PL, Rice AJ, Wagner PD (1999) Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol 87:1506–1512
Benallal H, Busso T (2000) Analysis of end-tidal and arterial PCO2 gradient using a breathing model. Eur J Appl Physiol 83:402–408
Benallal H, Denis C, Prieur F, Busso T (2002a) Assessment of arterial gas pressures and cardiac output using a breathing lung model. Med Sci Sports Exercise 34:1750–1756
Benallal H, Denis C, Prieur F, Busso T (2002b) Modeling of end-tidal and arterial PCO2 gradient: comparison with experimental data. Med Sci Sports Exercise 34:622–629
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 8:307–310
Burma GM (1985) Multitracer gas transport in the lung. Dissertation
Burma GM and Saidel GM (1983) Pulmonary blood flow and tissue volume: model analysis of rebreathing estimation methods. J Appl Physiol 55:205–211
Douglas AR, Jones NL, Reed JL (1988) Calculation of whole blood CO2 content. J Appl Physiol 65:473–477
Fick A (1870) Uber die blutquantums in den Herzventrikeln. Sitzung Phys Med Gesell Würzburg 16
Gledhill N, Froese AB, Buik FJ, Bryan AC (1978) VA/Q inhomogeneity and A–a DO2 in man during exercise: effect of SF6 breathing. J Appl Physiol 45:512–515
Johnson BD, Beck KC, Proctor DN, Miller J, Dietz M, Joyner MJ (2000) Cardiac output during exercise by open circuit acetylene washing method: comparison with direct Fick. J Appl Physiol 88:1650–1658
Jones NL, Robertson DG, Kane JW (1979) Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol 47:954–960
Kelman GR (1966) Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol 21:1375–1376
Krogh A, Lindhard J (1912) Measurement of the blood flow through the lungs of man. Scand Arch Physiol 27:100–125
Lobdell DD (1981) An invertible simple equation for computation of blood O2 dissociation relations. J Appl Physiol 50:971–973
Reybrouck T, Fagard R (1990) Assessment of cardiac output at rest and during exercise by a carbon dioxide rebreathing method. Eur Heart J 11:21–25
Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J (2001) Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85:202–207
Saunders KB, Cummin AR (1992) Estimates of mean alveolar PCO2 during steady-state exercise in man: a theoretical study. J Theor Biol 159:307–327
Smyth RJ, Gledhill N, Froese AB, Jamnik VK (1984) Validation of non-invasive maximal cardiac output measurement. Med Sci Sports Exercise 16:512–515
Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG (1977) Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med 48:203–209
Vinet A, Nottin S, Lecoq AM, Guenon P, Obert P (2001) Reproducibility of cardiac output measurements by Doppler echocardiography in prepubertal children and adults. Int J Sports Med 22:437–441
Werko L, Lagerlof H, Bucht H (1949) Comparison of the Fick and Hamilton methods for the determination of cardiac output in man. Scan J Clin Invest 1:109–113
Zenger MR, Brenner M, Haruno M, Mahon D, Wilson AF (1993) Measurement of cardiac output by automated single-breath technique and comparison with thermodilution and Fick methods in patients with cardiac disease. Am J Cardiol 71:105–109
Acknowledgements
The investigators thank Kathy O‘Malley, Cathy Swee and Darrell Loeffler for technical help during the study, Drs. Bradley Narr, Michael Joyner, Nicki Dietz and David Seamans for expertise in placing catheters and blood-gas sampling. This work was supported by National Institutes of Health grants HL 52230 and the General Clinic Research Center grant M01 RR00585 and the grant NHLBI HL 71478.
Conflict of interest:
No information supplied
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benallal, H., Beck, K.C., Johnson, B.D. et al. Evaluation of cardiac output from a tidally ventilated homogeneous lung model. Eur J Appl Physiol 95, 153–162 (2005). https://doi.org/10.1007/s00421-005-1376-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-1376-6