Abstract
The influence of muscle length-dependent changes in contractile properties on the mechanomyogram (MMG) was investigated during evoked contractions of the biceps brachii muscle. The biceps of nine healthy subjects was stimulated by single twitches, unfused (10 Hz), and fused (30 Hz) frequencies at elbow joint angles of 75, 90, 105, 120, 135, and 150°. During evoked contractions, the longitudinal movement was estimated by the fluctuation of torque signal, and the lateral movement was detected by MMG. Contraction time and half relaxation time were calculated from the single twitches. For repetitive stimulations, followed by eliminating DC component from torque signals, the root mean square values calculated from the torque and MMG signals, which were torque fluctuation and RMS–MMG, respectively. For the 10 Hz stimulation condition, reductions in the torque fluctuation and RMS–MMG were concomitantly observed with increasing elbow angle, and there was a significant correlation between the torque fluctuation and RMS–MMG. On the other hand, for 30 Hz stimulation, there were no significant differences in the torque fluctuation and RMS–MMG over all elbow angles, and no significant correlation between the two parameters. Moreover, the torque fluctuation and RMS–MMG for 10 Hz stimulation were correlated with the contraction time and half relaxation time obtained at each elbow angle, while there were no correlations for the 30 Hz condition. These data suggest that MMG could be a reliable tool to study the development of fusion and the changes in muscle contractile properties during repetitive unfused contractions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle mechanics are usually investigated using the force parameters, and from the force signal analysis, basic physiological aspects such as length–tension and force–velocity relationships (James et al. 1994; ter Keurs et al. 1978), relationships between the force and muscle fiber types (Baratta and Solomonow 1990; Solomonow et al. 1987), and the activation strategy of the motor units (Baratta and Solomonow 1992; Solomonow 1984) can be obtained. During muscle contraction, another mechanical signal related to the muscle surface oscillations due to pressure waves generated by the dimensional changes of the active muscle fibers (Barry 1987; Frangioni et al. 1987; Gordon and Holbourn 1948) can also be retrieved. Recording of this oscillation was referred to as the mechanomyogram (MMG) because the signal reflects the mechanical activity of contracting muscle (Orizio 1993).
Recently, several studies examined the relationship between MMG and contractile properties of the involved muscle or motor units. Bichler and Celichowske (2001a, b) showed on the gastrocnemius muscle of rats that the amplitude of MMG during repetitive stimulation of isolated motor units was associated with the amplitude of force oscillation. Similar results were also obtained by Yoshitake et al. (2002) in the study that examined on the medial gastrocnemius muscle of humans, indicating that reductions in MMG amplitude with increasing stimulation frequency occurred in parallel with the reductions in force fluctuations. During maximal twitch contractions evoked by direct whole muscle stimulation, the rising time of MMG (from the onset to the highest peak) was similar to the contraction time of the single-twitch force, and was longer in the soleus muscle than in the vastus lateralis muscle (Marchetti et al. 1992). Additionally, the significant reduction in the muscle contractile properties induced by the experimental hypothermia resulted in a significant reduction in force fluctuation, which was mirrored by the reduction in the MMG amplitude (Kimura et al. 2003). On the basis of these results, it is likely that MMG signal could reflect the contractile properties of the activated muscles or motor units.
It is well known that evoked twitch contractile properties are markedly affected by the muscle length and corresponding joint angle (Christova et al. 1998; Edman and Flitney 1982; Ismail and Ranatunga 1978; Jewell and Wilkie 1960; O’Hagan et al. 1993). Given these data, we hypothesized that changes of muscle length and corresponding joint angle affect MMG responses during evoked contractions. However, thus far, there is no direct evidence to support this hypothesis in humans. To test this hypothesis, the present study was undertaken in humans to investigate the effect of joint angle upon contractile properties and the corresponding change in the MMG. Moreover, for the case of repetitive stimulation, there exists a critical fusion frequency at which contractions cause (cease) to be tetanic (Nathan and Tavi 1990), and thus, the question arises whether or not there are differences in the pattern of MMG responses between during unfused and fused tetanic contractions. Therefore, we examined MMG responses to unfused and fused repetitive stimulations at various joint angles.
Methods
Subjects
Nine male subjects (24.5±1.1 years, 171.3±2.8 cm, 68.1±2.6 kg, mean ± SE) with no history of neuromuscular disorders volunteered for this investigation. All subjects were instructed about the experimental protocol and their informed consent was obtained before carrying out the experiments. The experimental procedure was approved by the Ethical Committee of Kyoto University Graduate School.
Experimental setup
Each subject was seated in a chair with his right upper and lower arm supported on a horizontal metal plate, and the posture of the subject was held steady by means of shoulder braces and seat belt. The forearm, which was maintained in supinated position, was securely strapped to a rotating metal arm that could be fixed at various joint angles. This posture has no gravitational effect of the forearm on the torque measurements. The axis of the arm was aligned with the elbow axis. With this arrangement, the torque elicited by the electrical stimulations was sensed by a strain gauge (model LUR-A-100NSA1, Kyowa Electronic Instruments, Japan), the signal from which was amplified (model SA-100D, TEAC, Japan) and fed to a computer for analysis.
Carbon-impregnated rubber electrodes (3.5×2 cm) were thinly coated with conductive gel and secured to the right upper arm with adhesive tape. One electrode was placed on the motor point of the biceps brachii, and the other was placed on the distal portion of the biceps brachii. The stimuli were rectangular voltage pulses, 100 μs duration, delivered from an electro-stimulator (model SEN-3301, Nihon Koden, Japan) through a stimulator isolation unit (model SS-104J, Nihon Koden, Japan). A maximal twitch contraction was considered to have been evoked when torque failed to increase with further increases in stimulus intensity.
The method for measuring MMG signals was similar to a method previously employed in our laboratory (Miyamoto and Oda 2003). A microphone sensor (QTEC, Japan; 8-mm diameter, 5-g mass) to detected MMG with a flat frequency response of 5–1,000 Hz was secured vertically over the belly of the biceps brachii with adhesive tape. This sensor was located just medial to the stimulating electrode which was over the motor point. The MMG signal was band-pass filtered (5–100 Hz) and amplified (model MEG-6108, Nihon-Koden; gain 1,000 times, input impedance >100 MΩ, CMRR >80 dB).
Experimental protocol
Elbow flexion torque was measured at six elbow joint angles: 75, 90, 105, 120, 135, and 150° (full extension = 180°). Each subject was instructed to relax during all stimulation trials. If the subject did not appear relaxed during a trial, it was repeated. Based on the results from a pilot study, a frequency of 30 Hz, which was sufficient to produce a fused or nearly fused isometric contraction, was the almost maximum stimulation rate that could be tolerated by the subjects. Thus, the following stimulation protocol was performed for each angle: ten single twitches, a 5-s train at a rate of 10 Hz (unfused tetani), and a 5-s train at 30 Hz (fused tetani). Stimulations were performed in this sequence to allow the subjects to familiarize gradually to pain by tetanic stimulation. At least 2-min rests were given between the stimulations within a given stimulation rate, and at least 5-min rests were given between stimulation rates. For each subject, the order of angles tested was randomized within a given stimulation rate.
Data collection and analysis
The torque and MMG signals were simultaneously and continuously stored on the hard disk of a personal computer for later analysis using a 12-bit analogue-to-digital converter (model PCI-6024E, National Instruments, USA) at a sampling frequency of 1,024 Hz. At first, for twitch data analysis, the torque signals were averaged from 8 to 10 single twitch contractions without artifact for each joint angle. Twitch peak torque (PT), contraction time (CT), and half relaxation time (HRT) were calculated from the averaged twitch torque signals. Contraction time was the interval between the onset of torque signal and the twitch peak torque. The torque onset was defined as the point at which the value exceeded a level of 3% of the PT from the baseline. Half relaxation time was defined as the time taken for the torque signal to decline to one-half of the peak torque value in the relaxation phase. Then, the torque and MMG parameters were analyzed from the middle 2-s segment of the signal during repetitive 10 and 30 Hz stimulations (Fig. 1). Mean torque was calculated, and for the analysis of torque signals the DC component of torque signal was removed. From these signals, the root mean square (RMS) values were calculated for both signals of the MMG (RMS–MMG) and torque fluctuations.
Statistical analysis
Polynomial contrast was performed to analyze the pattern of the parameters of torque and MMG signals over five elbow angles. For significant differences, the data were further analyzed with Tukey’s post hoc comparisons. To test the significance of the relation for the RMS–MMG and torque fluctuations, these parameters were normalized to the respective maximal value of five elbow angles for each subject, and we calculated Pearson’s correlation coefficient. A probability level of P<0.05 was considered statistically significant. Descriptive statistics include mean and SE.
Results
Table 1 shows the changes in properties of the twitch force. Polynomial contrast analysis yielded significant linear trend from 75° to 150° of elbow angle for all of PT, CT, and HRT. Further analysis showed significant differences in PT, CT, and HRT (Table 1).
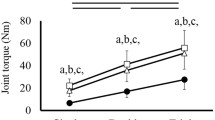
Figure 2 shows typical data derived from 2-s torque fluctuation and MMG recordings during repetitive stimulation at 10 and 30 Hz obtained for two different joint angles. During 10 Hz stimulation, reductions in torque fluctuation and MMG amplitude were concomitantly observed as the elbow joint angle increased, whereas it seems that torque fluctuation and MMG amplitude changed little between 75° and 150° during 30 Hz stimulation. Figures 3 and 4 represent the group data for the changes in the mean torque, torque fluctuation, and RMS–MMG during 10 and 30 Hz stimulation, respectively. It was confirmed by polynomial contrast analysis and Tukey’s post hoc comparisons that there were significant monotonic trend and differences in the mean torque with increase in joint angle during 10 Hz stimulation (75°<105°, 120°, 135°, 150°, and 90°<120°, 135°, 150°), whereas there were significant linear trend and decreases in the torque fluctuation (75°, 90°, 105°, 120°>150°, and 75°>135°). These declines were accompanied by significant linear decreases in the RMS of the MMG amplitude (75°, 90°, 105°>135°, 150°; Fig. 3). Moreover, there was a highly significant correlation between the normalized torque fluctuation and the normalized RMS–MMG (Fig. 5, left). For 30 Hz stimulation, it is revealed that there was a significant monotonic trend and increases in the mean torque as a function of the elbow angle (Fig. 4a). On the contrary, for the torque fluctuation and MMG amplitude, there were no significant differences over all joint angles (Fig. 4b, c), and a significant correlation between the normalized torque fluctuation and the normalized RMS–MMG was not observed (Fig. 5, right). Figure 6 shows the torque fluctuations (a and b) and the RMS–MMG (c and d) in 10 Hz stimulation compared with contraction time and half relaxation time obtained from single twitches. Both the torque fluctuation and the RMS–MMG were significantly related to contraction time and half relaxation time. These results suggest that for unfused 10 Hz stimulation declines in the amplitude of surface MMG signals and fluctuation of torque signals are more pronounced when the contractile properties of the activated muscle are slower. On the other hand, for 30 Hz stimulation, neither the torque fluctuation nor RMS–MMG significantly correlated to both contraction time and half relaxation time (Fig. 7).
Torque fluctuation (a and b) and MMG amplitude (c and d) during repetitive stimulation at 10 Hz compared with contraction time and half relaxation time during twitch contraction. The torque fluctuation and RMS–MMG are normalized to the respective maximal values (% max value) of five elbow angles for each subject
Torque fluctuation (a and b) and MMG amplitude (c and d) during repetitive stimulation at 30 Hz compared with contraction time and half relaxation time during twitch contraction. The torque fluctuation and RMS–MMG are normalized to the respective maximal values (% max value) of five elbow angles for each subject. NS Not significant
Discussion
Major findings of this study were the following: (1) during 10 Hz stimulation, the declines in torque fluctuation and MMG amplitude were concomitantly observed as the elbow joint angle increased; (2) during 30 Hz stimulation, there were no significant differences in torque fluctuation and MMG amplitude between elbow angles. Clearly, force and MMG signals during repetitive stimulation at 10 Hz did not merely change in the same way as during 30 Hz stimulation.
Our data showed that in the unfused 10 Hz stimulation condition, there were significant reductions in the fluctuations of torque signal with increasing elbow angle, which were approximately accompanied by similar and significant reductions in the amplitude of MMG signal. Kimura et al. (2003) studied the relationship between the force fluctuation and the MMG amplitude during unfused repetitive stimulation (10 Hz) under experimentally induced hypothermia condition. They showed that the reduction in MMG amplitude in cooling condition was concomitantly occurred with a reduction in force fluctuation, which was due to a slowing of contractile speed. Yoshitake et al. (2002) also indicated that when the stimulation frequency was increased from 5 Hz to 20 Hz in micro-stimulation in human medial gastrocnemius, the reduction in MMG amplitude was accompanied by a reduction in force fluctuation, and that the rate of declines in these parameters were greater for slower motor units than for faster motor units. Collectively, MMG amplitude during repetitive stimulation might depend largely on the contractile properties of the muscle and motor units evoked. Together with the present findings that systematic reductions in torque fluctuation and MMG amplitude were concomitantly observed with increasing elbow angle during repetitive stimulation at 10 Hz, it is likely that during unfused repetitive evoked contractions the MMG amplitude reflects length-dependent muscle contractile properties. On the contrary, for 30 Hz stimulation, there were no significant differences in the torque fluctuation and MMG amplitude.
Fluctuations of torque during repetitive evoked contractions are generated by the oscillation in tension during a series of contraction and relaxation. Also, the slower the twitch of the muscle, the lower the fusion frequency (Burke et al. 1973; Steg 1964). As a corollary, torque fluctuations during unfused tetanic stimulation would be smaller at longer muscle length that has slower contractile properties. In fact, the amplitude of torque fluctuation during 10 Hz stimulation, which was below the critical fusion frequency previously reported for the biceps (15.4 Hz; Nathan and Tavi 1990), decreased with increasing elbow angle (Fig. 3b). This is further supported by significant correlations between the normalized torque fluctuation and contraction time as well as half relaxation time (Fig. 6a, b). On the other hand, as a rule, the relative contribution of the relaxation phase to the fluctuations becomes smaller as the stimulation frequency increases. Since the interpulse interval of 30 Hz stimulation (approximately 33 ms) is shorter than the smallest CT value observed in this study, interference would occur in the process of torque fluctuation, resulting in a reduction in the force ripple determining a fusion-like situation. Thus, it seems that torque fluctuations during fused tetanic stimulation would be independent of the contractile properties of the muscle. In this study, the torque fluctuations for 30 Hz stimulation were much smaller than those for 10 Hz stimulation, and there were no significant differences in the amplitude of torque fluctuation for 30 Hz stimulation among all elbow angles (Fig. 4b). Furthermore, the torque fluctuation was found not to be related to CT and HRT (Fig. 7a, b).
Again, similar to the case for torque fluctuation, the RMS of MMG was significantly correlated with CT and HRT in the 10 Hz stimulation condition (Fig. 6c, d), while there were no correlations in the 30 Hz condition (Fig. 7c, d). During muscle contractions, longitudinal shortening and lateral expansion of the muscle fibers almost simultaneously occur (Walker et al. 1990; Orizio et al. 1999). The longitudinal movement can be estimated by measuring the force (torque) fluctuation, and the lateral movement can be detected using a vibration transducer. If the two signals are concurrently generated from a common source (Barry 1987; Frangioni et al. 1987; Orizio 1993; Orizio et al. 1999; 2000), features which influence the fusion development of force could affect the lateral movement of the muscle fibers and characteristics of surface MMG as a consequence.
In this study, a condenser microphone was used as a MMG sensor. It has been shown that during twitch contraction, MMG signals detected by microphones lasted for the entire duration of the twitch force (Bichler 2000; Yoshitake et al. 2002). The MMG recorded during unfused tetanus, which is a result of the summation of a series of successive contractions, is formed by successive MMG components; because the duration of successive MMG signals derived by a microphone sensor is longer than the interpulse intervals, interference occurs in the process of MMG generation (Bichler and Celichowske 2001a). Yoshitake and Moritani (1999) reported that when the posterior tibial nerve was supramaximally stimulated at 5 and 20 Hz, the MMG power spectral peak frequency matched the stimulation frequency at 5 Hz for both medial gastrocnemius and soleus muscles. However, at 20 Hz, the peak frequency was synchronized only in the medial gastrocnemius with the stimulation frequency; the soleus did not show such synchronization. Their results imply that during stimulation at 20 Hz, the slow twitch soleus muscle would become fully tetanized, resulting in complete fusion and consequently a reduction in oscillations of the longitudinal and lateral movement. Moreover, according to a study in cat gastrocnemius (Orizio et al. 2000), when motor units were stimulated at 30 Hz, no ripple was observed in the force and MMG signals, in which case the tendon behaved as a “rigid force transmission element without contributing any of its viscoelastic properties to the model” (Baratta and Solomonow 1990). In fact, in 10 Hz stimulation condition of the present study, a highly significant correlation was found between the normalized torque fluctuation and the normalized RMS–MMG (Fig. 5a). On the other hand, there was no significant correlation in the 30 Hz condition (Fig. 5b), which may be attributed to the possibility that repetitive stimulations at high frequency cause the biceps brachii fully tetanized to such an extent that the signal to noise ratio for proper analysis might not be possible.
Lastly, we discuss why we targeted the biceps brachii for evoked contractions and MMG recordings. For pennate muscles such as the quadriceps and triceps surae muscles, the recorded MMG signal is considered not only to reflect the pressure wave, but also to include the vibration (rippling movement) due to the longitudinal shortening of the muscle fibers (Akataki et al. 1999). In addition, the pennation angle of the triceps surae increases with increasing ankle angle at rest and during MVC (Maganaris 2003; Maganaris et al. 1998), suggesting that the relative position between the muscle fiber and the MMG transducer attached at the skin surface could change in relation to the joint angle. On the other hand, since the biceps brachii is fusiform muscle (Kawakami et al. 1994; Murray et al. 1995, 2000), the MMG transducer might detect only the pressure wave due to the lateral expansion of the contracting fibers, irrespective of the elbow angle. Moreover, the muscle has a defined motor point which is readily accessible for electrical stimulation of the whole muscle. Therefore, we targeted the biceps brachii for the present investigation using MMG recording and electrical stimulation.
In conclusion, our data showed that MMG amplitude during unfused tetanic contractions was correlated to both the contraction time and half relaxation time, and that it reflected changes in the torque fluctuation with varying muscle length. These data support that MMG during unfused evoked contractions could be a reliable tool to study the development of fusion and changes in muscle contractile properties.
References
Akataki K, Mita K, Itoh Y (1999) Relationship between mechanomyogram and force during voluntary contractions reinvestigated using spectral decomposition. Eur J Appl Physiol 80:173–179
Baratta R, Solomonow M (1990) The dynamic responses model of nine different skeletal muscles. IEEE Trans Biomed Eng 37:243–251
Baratta R, Solomonow M (1992) The dynamic performance model of the skeletal muscle. Crit Rev Biomed Eng 19:419–459
Barry DT (1987) Acoustic signals from frog skeletal muscle. Biophys J 51:769–773
Bichler E (2000) Mechanomyograms recorded during evoked contractions of single motor units in the rat medial gastrocnemius muscle. Eur J Appl Physiol 83:310–319
Bichler E, Celichowski J (2001a) Mechanomyographic signals generated during unfused tetani of single motor units in the rat medial gastrocnemius muscle. Eur J Appl Physiol 85:513–520
Bichler E, Celichowski J (2001b) Changes in the properties of mechanomyographic signals and in the tension during the fatigue test of rat medial gastrocnemius muscle motor units. J Electromyogr Kinesiol 11:387–394
Burke RE, Levine DN, Tsairis P, Zajac FE (1973) Physiological types and histochemical profiles I motor units of the cat gastrocnemius. J Physiol (Lond) 234:723–748
Christova P, Kossev A, Radicheva N (1998) Discharge rate of selected motor units in human biceps brachii at different muscle lengths. J Electromyogr Kinesiol 9:287–294
Edman KAP, Flitney FW (1982) Laser diffraction studies of sarcomere dynamics during “isometric” relaxation in isolated muscle fibres of the frog. J Physiol (Lond) 329:1–20
Frangioni JV, Kwan-Gett TS, Dobrunz LE, Mcmahon TA (1987) The mechanism of low-frequency sound production in muscle. Biophys J 51:775–783
Gordon G, Holbourn HS (1948) The sounds from single motor units in a contracting muscle. J Physiol (Lond) 107:456–464
Ismail HM, Ranatunga KW (1978) Isometric tension development in a human skeletal muscle in relation to its working range of movement:the length-tension relation of biceps brachii muscle. Exp Neurol 62:595–604
James C, Sacco P, Hurley MV, Jones DA (1994) An evaluation of different protocols for measuring the force-velocity relationship of the human quadriceps muscles. Eur J Appl Physiol 68:41–47
Jewell BR, Wilkie DR (1960) The mechanical properties of relaxing muscle. J Physiol (Lond) 152:30–47
Kawakami Y, Nakazawa K, Fujimoto T, Nozaki D, Miyashita M, Fukunaga T (1994) Specific tension of elbow flexor and extensor muscles based on magnetic resonance imaging. Eur J Appl Physiol 68:139–147
Kimura T, Hamada T, Ueno LM, Moritani T (2003) Changes in contractile properties and neuromuscular propagation evaluated by simultaneous mechanomyogram and electromyogram during experimentally induced hypothermia. J Electromyogr Kinesiol 13:433–440
Maganaris CN (2003) Force-length characteristics of the in vivo human gastrocnemius muscle. Clin Anat 16:215–223
Maganaris CN, Baltzopoulos V, Sargeant AJ (1998) In vivo measurement of the triceps surae complex architecture in man; implications for muscle function. J Physiol (Lond) 512:603–614
Marchitti M, Felici F, Bernardi M, Minasi P, Di Filippo L (1992) Can evoked phonomyography be used to recognize fast and slow muscle in man?. Int J Sports Med 13:65–68
Miyamoto N, Oda S (2003) Mechanomyographic and electromyographic responses of the triceps surae during maximal voluntary contractions. J Electromyogr Kinesiolo 12:451–459
Murray WM, Delp SL, Buchanan TS (1995) Bariation of muscle moment arms with elbow ad forearm position. J Biomech 28:513–525
Murray WM, Buchanan TS, Delp SL (2000) The isometric functional capacity of muscles that cross the elbow. J Biomech 33:943–952
Nathan R, Tavi M (1990) The influence of stimulation pulse frequency on the generation of joint moments in the upper limb. IEEE Trans Biomed Eng 37:317–322
O’Hagan F, Tsunoda N, Sale DG, MacDougall JD (1993) Elbow flexor evoked twitch contractile properties in untrained men and women and male bodybuilders. Eur J Appl Physiol 66:240–245
Orizio C (1993) Muscle sound: bases for the introduction of mechanomyographic signal in muscle studies. Crit Rev Biomed Eng 21:201–243
Orizio C, Baratta RV, Zhou BH, Solomonow M, Veicsteinas A (1999) Force and surface mechanomyogram relationship in cat gastrocnemius. J Electromyogr Kinesiol 9:131–140
Orizio C, Baratta RV, Zhou BH, Solomonow M, Veicsteinas A (2000) Force and surface mechanomyogram frequency responses in cat gastrocnemius. J Biomech 33:427–433
Solomonow M (1984) External control of the neuromuscular system. IEEE Trans Biomed Eng 31:752–763
Solomonow M, Baratta R, Zhou B, Shoji H, D’Ambrosia D (1987) The EMG-force model of skeletal muscle: dependence on control strategy and predominant fiber type. IEEE Trans Biomed Eng 34:692–703
Steg G (1964) Efferent muscle innervation and rigidity. Acta Physiol Scand 61:1–53
ter Keurs HEDJ, Iwazumi T, Pollack GH (1978) The sarcomere length-tension relation in skeletal muscle. J Gen Physiol 72:565–592
Walker FO, Donofrio PD, Harpold GJ, Ferrell WG (1990) Sonographic imaging of muscle contraction and fasciculations: a correlation with electromyography. Muscle Nerve 13:33–39
Yoshitake Y, Moritani T (1999) The muscle sound properties of different muscle fiber types during voluntary and electrically induced contractions. J Electromyogr Kinesiol 9:209–217
Yoshitake Y, Shinohara M, Ue H, Moritani T (2002) Characteristics of surface mechanomyogram are dependent on development of fusion of motor units in humans. J Appl Physiol 93:1744–1752
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyamoto, N., Oda, S. Effect of joint angle on mechanomyographic amplitude during unfused and fused tetani in the human biceps brachii muscle. Eur J Appl Physiol 95, 221–228 (2005). https://doi.org/10.1007/s00421-005-1359-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-1359-7