Abstract
Submaximal exercise performance has not previously been assessed in the late follicular phase of the menstrual cycle, which is associated with a pre-ovulatory surge in oestrogen. Therefore, we compared cycling time trial performance during the early follicular (EF), late follicular (LF) and mid-luteal (ML) phase of the menstrual cycle in trained and untrained eumenorrhoeic women who cycled 30 and 15 km, respectively, in a non-fasted state. The women completed the three cycling time trials on a conventional racing bicycle mounted on an air-braked ergometer. We required resting oestrogen to increase by at least twofold above EF phase values in both the LF and ML phases and this resulted in a number of exclusions reducing the sample size of each group. No significant difference was noted in the finishing time between the different menstrual phases in trained (n=5) or untrained (n=8) group, albeit limited by sample size. However, analysis of the combined trained and untrained group data (n=13) revealed a trend for a faster finishing time (P=0.027) in the LF phase compared to the EF phase as 73% of the subjects showed improvements with an average of 5.2±2.9% (or 2.1±1.1 min) in the LF phase (for α=0.05 requires P<0.017). Combined group analysis yielded no difference between performance in the EF and ML phase or between the LF and ML phase. Thus, further research is encouraged to confirm the tendency for a faster time trial in the LF phase, which coincides with the pre-ovulatory surge in oestrogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some studies have observed variations in endurance performance between menstrual phases. One report noted better performance in the follicular phase (Campbell et al. 2001) and others found a marked improvement (Jurkowski et al. 1981) or a trend for improvement (Nicklas et al. 1989) during the luteal phase. Other studies, however, observed no effect of menstrual phase on endurance performance (Bailey et al. 2000; Beidleman et al. 1999).
Recent evidence suggests that the subject’s nutritional status is a large determinant of whether menstrual phase will exert a significant effect on performance (Campbell et al. 2001). Specifically, a difference in exercise performance between menstrual phases is noted only when subjects exercise after an overnight fast. Further, when overnight fasted subjects ingest a carbohydrate supplement during exercise, the difference in performance between phases was obliterated (Campbell et al. 2001). Thus, it appears that the metabolic affects of the ovarian hormones are most pronounced during times of metabolic stress (Campbell et al. 2001; Lavoie et al. 1987) and under such conditions may alter endurance performance.
Previous studies evaluating the influence of menstrual phase on submaximal exercise performance (lasting for 30 min or more) have only made comparisons between the early or mid-follicular phase (when oestrogen and progesterone concentration is low) and the mid-luteal phase (when oestrogen and progesterone concentration is elevated). Thus, all previous studies measuring submaximal exercise performance that we know of have failed to include the period before ovulation (i.e. the late follicular phase) when oestrogen is at it’s highest and progesterone concentration is low (Reilly 2000). However, some studies testing maximal exercise performance (with exercise duration of less than 15 min) have included the late follicular phase (when oestrogen alone is elevated) in their comparison between menstrual phases and have reported no significant difference in maximal exercise capacity between menstrual phases (Bemben et al. 1995; Dean et al. 2003). It is possible that the prominent catecholamine response to maximal exercise may dominate the metabolic response under such conditions and override any ovarian hormone induced differences. In this context, Dean et al. (2003) observed a similar catecholamine response between menstrual phases during maximal exercise.
However, it is important to assess submaximal exercise performance in eumenorrhoeic women during the late follicular phase so that the affects of oestrogen alone (i.e. without the presences of progesterone) can be evaluated on exercise that demands a larger aerobic component. Progesterone may counter the physiological effects that oestrogen may have on performance during the mid-luteal phase when both ovarian hormones are elevated, since progesterone is responsible for the increased body temperature (Hessemer and Brück 1985), respiration rate (Beidleman et al. 1999; Schoene et al. 1981) and heart rate (Hessemer and Brück 1985; Pivarnik et al. 1992; Seebauer et al. 2002) that characterises the luteal phase and is reported to have various anti-oestrogenic affects (Campbell and Febbraio 2001, 2002; D’Eon et al. 2002; Hatta et al. 1988). For example, ovariectomised rats on oestrogen treatment utilised significantly more fatty acids during exercise than those receiving both oestrogen and progesterone (Hatta et al. 1988). Similarly, the positive action of oestrogen to promote plasma glucose availability and uptake by quadriceps muscles, comprising mostly type I fibres, is suppressed by progesterone (Campbell and Febbraio 2002). Progesterone also inhibits the oestrogen-induced upregulation of fatty acid oxidative enzyme activity in skeletal muscle (Campbell and Febbraio 2001). It may not be surprising, therefore, that Hackney et al. (1991) observed greater fat utilisation during exercise in the late follicular phase when oestrogen alone is elevated compared to the early follicular and mid-luteal phase. The potential for oestrogen to promote endurance performance is verified by a study using female ovariectomised rats (Kendrick et al. 1987) in which rats receiving oestrogen supplementation showed massive improvements in endurance capacity (approximately 40% longer time to exhaustion) compared with those receiving placebo treatment.
Therefore, we hypothesise that submaximal exercise performance will be optimised during the late follicular phase of the menstrual cycle due to the pre-ovulatory surge in oestrogen that characterises this menstrual phase. And hence, the aim of this study is to assess exercise performance by means of a cycling time trial during the early follicular (EF), late follicular (LF) and mid-luteal (ML) phase of the menstrual cycle in eumenorrhoeic women. As it is not common practice for athletes to compete in a fasted state (i.e. more than 3 h postabsorptive), we chose to study our subjects following their normal dietary routine. Significant findings based on fasted subjects would have little relevance for competitive female athletes.
Furthermore, we chose to include two groups of eumenorrhoeic women that differed according to training status, that is a sedentary group and a trained group. Early studies suggested that endurance training may override any effect that the menstrual cycle may have on exercise performance (De Souza et al. 1990) or metabolism during exercise (Kanaley et al. 1992) as these studies found no change in these variables between menstrual phases in athletic women. Whereas previous studies using less trained individuals had reported menstrual phase differences in exercise performance (Jurkowski et al.1981; Nicklas et al. 1989). A change in exercise performance between menstrual phases may be more noticeable in untrained subjects as they may be more sensitive to discomfort. For example, an increase in the sensation of dyspnea or cardiac strain sometimes associated with the luteal phase may increase perceived effort and hamper performance in untrained subjects (Schoene et al. 1981). Whereas trained athletes are accustomed to physical effort in the face of discomfort and can often perform unimpeded (Schoene et al. 1981). However, a recent study using athletic eumenorrhoeic women have reported metabolic and performance changes in these women between menstrual phases (Campbell et al. 2001). Thus, the finding of no change in metabolism or performance in the athletes between the follicular and luteal phase in those early studies (De Souza et al. 1990; Kanaley et al. 1992) may not have been due to an overriding training response as suggested, but may have been due to a moderate oestrogen to progesterone concentration ratio in the luteal phase where the effects of progesterone cancelled the effects of oestrogen. Therefore, we hypothesise that if the effects of progesterone to increase ventilation rate and cardiac strain predominant in the ML phase, it is possible that exercise performance would be impeded in the ML phase of the untrained group, while this affect should not be evident in the trained group. However, without any noticeable respiratory or cardiac affect we would hypothesise the same response for both groups; an improvement in exercise performance in the LF phase.
Methods
Subjects
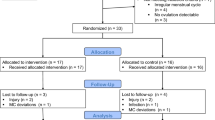
Nine trained and eight untrained healthy eumenorrhoeic females who attended the medical school volunteered to participate in this study (Table 1). All subjects were informed about the nature and purpose of the study before giving written consent to participate. The protocol for this study was granted ethical clearance by the Committee for Research on Human Subjects, University of the Witwatersrand (No. M9806014). The subjects had not taken any oral contraceptive for at least 1 year prior to the study and all were non-smokers. All subjects claimed to experience regular menstrual cycles of between 25 days and 36 days long. The untrained women did not follow any regular exercise programme. The trained women were recreational cyclists and their average weekly exercise training consisted of 5.4±2.3 h per week of a combination of cycling, swimming, jogging, and circuit training. They were required to keep a training logbook for the duration of their participation to ensure a consistent training effort throughout. The average coefficient of variation in weekly training time was 21%, which translates to a possible weekly difference of only one extra or less training session.
Preliminary screening tests
Maximum oxygen consumption
Maximum oxygen consumption (VO2max) was assessed by using a discontinuous incremental protocol on a stationary bicycle ergometer (Excalibur 911 900, Lode, Groningen, The Netherlands) during the follicular phase (day 2–9) of each subject’s menstrual cycle. We chose a discontinuous over a continuous protocol because this method is well tolerated especially in individuals who are not accustomed to regular exercise. In addition, no significant difference in VO2max has been reported when comparing continuous and discontinuous protocols (Duncan et al. 1997).
After a 10-min warm up, subjects exercised for 3 min bouts at fixed workloads that started between 100 W and 200 W depending on the subjects’ ability and increased in 20-W increments. Each bout was interspersed with passive recovery periods (~10–20 min) during which heart rate had to return to at least 100 beats min−1. Oxygen consumption (VO2) was recorded at 30 s intervals during each 3 min exercise bout using open circuit spirometry (Oxycon-4, Mijnhardt, Bunnik, Netherlands). The metabolic anaylser was zeroed using 100% nitrogen gas and calibrated using room air and a known mixture of gas (16% oxygen and 5% carbon dioxide). VO2max was considered to have been attained when VO2 varied by less than 1.5 ml kg−1 min−1 between workloads.
Familiarisation trial
All sedentary subjects completed a familiarisation trial during which they were required to spend 20 min familiarising themselves with the bicycle to be used in the performance trials. The trained subjects did not perform an acclimation session as they were all cyclists and most used their own bicycle in the performance trials and therefore, were accustomed to the gear settings and bicycle set up. Two previous studies have found no significant time difference between a set of repeated cycling time trials in trained cyclists (average coefficient of variation of 1 and 3.4%) (Jeukendrup et al. 1996; Palmer et al. 1996). Therefore, a learning effect does not occur in cyclists performing the test on their own bicycle and hence, a familiarisation trial was not necessary for the trained group.
Menstrual cycle
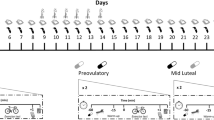
All subjects recorded their oral temperature using a digital thermometer (Vital sign VS-10, Soar Corporation, Japan) on waking each morning for one complete menstrual cycle before participation, and for the two to three cycles during which they participated in the study. A biphasic temperature pattern is characteristic of a normal ovulatory cycle i.e. body temperature is elevated by approximately 0.3°C post ovulation due to elevated progesterone levels. The day of ovulation was estimated by measuring urine luteinising hormone (LH) concentration using the Home Ovulation Prediction Kit (Clearplan, Unipath Ltd., Bedford, England). A positive result is achieved from the time corresponding to the onset of the LH surge, which begins approximately 36 h before ovulation. Subjects tested their first morning urine sample each day starting three days before the expected day of ovulation.
Each subject completed three performance trials with each trial occurring during a different menstrual phase. Timing of the menstrual phases was based on temperature and urine LH measurements taken during the previous and current menstrual cycle. The three different menstrual phases included: early follicular (EF), 2–7 days after the onset of menses; late follicular (LF), 2 days before a positive LH result to the day of a positive LH reading; and mid-luteal (ML), 4–10 days post a positive LH reading. The order of these performance trials was randomised and all three trials were conducted over the course of at least two menstrual cycles. The subjects completed the three trials at a similar time of day to eliminate any possible variation that may be attributed to circadian rhythm. A resting blood sample was taken to confirm the subjects’ menstrual phase by measuring serum oestrogen and progesterone concentration by radioimmunoassay (Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA, USA). All samples from each subject were tested in the same assay and the intra-assay coefficient of variation for oestrogen was 5.3 and 4.7% for progesterone. The accepted concentration range for the ovarian hormones during each menstrual phase was: 37–220 pmol l−1 for oestrogen and <3 nmol l−1 for progesterone during the EF phase; 360–1,377 pmol l−1 for oestrogen and <5 nmol l−1 for progesterone during the LF phase; and 220–955 pmol l−1 for oestrogen and >10 nmol l−1 for progesterone during the ML phase. In addition a minimum twofold increase in oestrogen from EF to LF or EF to ML was also required for inclusion of the subject’s data in the analysis since D’Eon et al. (2002) suggests that oestrogen must differ by approximately twofold between menstrual phases in order to produce a significant impact on substrate metabolism during exercise.
Performance trials
For 2 days before the first trial, the subjects kept a record of timing and content of their meals and followed this record before their subsequent trials. All caffeine-containing foods and drinks were prohibited on the day of the trial. The subjects kept to a similar training programme for the 24 h before all three trials and were not permitted to include exhaustive exercise during this 24-h period.
Since mood changes may alter the subjects’ motivation to put in their best performance, we assessed the subjects’ mood as they arrived at the laboratory by asking them to mark a visual analogue scale that was bordered by the terms “utterly calm and peaceful” and “highly agitated”. They were asked to give a written comment on any special or distressing event that had occurred during the day. This method of mood assessment has been used previously in premenopausal women (Baker et al. 1999). Certain individuals are susceptible to mood changes that are dependent on variations in the concentration of ovarian hormones (Reilly 2000). For this reason, we compared the subjects mood between menstrual phases to ensure that any difference noted in time trial performance was not related to altered mood. Finally the subjects’ height and body mass were recorded.
To date, most studies assessing exercise performance at various phases of the menstrual cycle have compared time to exhaustion at relative work intensities (Bailey et al. 2000; Bemben et al. 1995; Jurkowski et al. 1981; Nicklas et al. 1989). Jeukendrup et al. (1996) suggested that trials of time to exhaustion do not have a definite end point and are therefore highly affected by psychological variables (e.g. motivation, monotony and boredom) whereas a time trial has a fixed end point and thus reduces the impact of the psychological factors. Thus, time to exhaustion trials have poor test–retest reliability (coefficient of variation of ~27%) (Jeukendrup et al. 1996) compared to time trial protocols, which have a greater reproducibility (coefficient of variation of ~1–3.4%) (Bishop 1997; Hickey et al. 1992; Jeukendrup et al. 1996; Palmer et al. 1996). Hence, in this study we have chosen to measure performance by means of a cycling time trial. Thus, our subjects were told to cycle a given distance as fast as they could. The untrained group were required to cycle 15 km, whereas the trained group cycled 30 km.
The time trials were performed on a conventional racing bicycle set up on an air-braked ergometer (Cateye cyclosimulator CS-1000, Cateye CO., LTD., Osaka, Japan). The front wheel is removed so that the front forks can be secured to the ergometer by means of a quick release system and the rear wheel rests on a roller connected to a turbo fan. Thus, the subject can change their exercise intensity by altering pedalling cadence and gear ratios according to their ability and how much effort they wished to exert. Each subject used the same bicycle for all three of the performance trials. All adjustable features on the bicycle were kept constant between trials for each subject e.g. saddle height, type of pedal etc.. The distance of the rear wheel hub from the roller was maintained constant to ensure a consistent pedalling resistance between trials. During the trial the subjects could monitor the distance they had covered, however the speed and time elapsed were concealed. The subjects were allowed to drink water ad libitum and were cooled by a fan. Before starting each time trial the subjects were reminded that the objective of the trial was to complete the distance as fast as possible. They did not receive any further verbal encouragement.
Heart rate (HR) readings were recorded at rest and at 10 min intervals throughout exercise (Polar Edge, Polar Electro OY, Kempele, Finland). VO2 and respiratory exchange ratio (RER) were recorded at rest and at 10 min intervals during the 15 km time trial and at 8 km intervals during the 30 km time trial. Using these recording intervals at least three measurements were taken per trial for both trained and untrained groups. In the trained group we chose to take measurements at fixed distances rather than time intervals as these subjects may be more experienced at tracking their own performance and therefore, may have been able to use the recording intervals as a means of comparing their progress between time trials. At these respective recording times, the ventilatory equivalent (Ve-Eq) was calculated as the dividend of minute ventilation rate (l min−1) and VO2 (l min−1).
Statistical analysis
A paired t-test with Bonferroni correction was used to analyse variation between any two menstrual phases for all variables (for significance at the 5% level with the Bonferroni correction for three multiple comparisons, P must be less than 0.017). Repeated measures analysis of variance (ANOVA) could not be performed as a few subjects had one of their three trials excluded (due to measured ovarian hormones being out of range for the particular menstrual phase) and thus resulted in some missing data points, i.e. a total of four missing points out of 39. In order to perform repeated measures ANOVA under such conditions where the number of observations per treatment is uneven requires estimating the missing data points by interpolation. In this particular sample of subjects the estimated data points will dilute the real data particularly as the inter-individual variability is quite high and the estimated points are dependent on not only the subject’s other real data points but also on all the remaining subjects’ data in the treatment group. Thus, estimated data points tend to be very different from the subject’s real data points and thereby reduce the statistical power of the repeated measures design. Nonetheless, the Bonferroni test is the most suitable multiple comparison procedure for adequately controlling the chance of error in repeated measure design studies (Curran-Everett 2000) and there is very little chance of making a type I error (Curran-Everett 2000). The visual analogue scale measurements for mood perception were normalised using the arcsine transformation. All data are presented as mean ± standard deviation (SD).
Results
Menstrual cycle characteristics
All subjects were able to successfully determine their day of ovulation using body temperature fluctuations and the urine LH test (Table 1). The day of a positive LH surge tended to occur later in the menstrual cycle of the trained as compared to the untrained group (P<0.05). Thus, the trained subjects have a longer follicular phase than the untrained group.
The resting oestrogen and progesterone concentration on the day of the trial confirmed the subjects’ menstrual phase (Table 2). However, four trained subjects were excluded as their oestrogen concentration did not increase by the minimum required twofold in their LF or ML phase compared to their EF phase, thus reducing the sample size of the trained group to five. In a few cases, we excluded only one of the three time trials (see Table 2) since during that particular trial the ovarian hormone profile was not indicative of the subject being in the correct phase. The hormone profile fell within the expected range for all three time trials in the remaining nine subjects. The magnitude of the increase in oestrogen concentration in the LF phase above the EF phase values was on average 4.5±2.1-fold (range 2.5–8.5-fold). The magnitude of the increase in oestrogen above EF phase values in the ML phase was 4.7±2.9-fold (range 2–11.5-fold). In the ML phase the ratio of oestrogen to progesterone (pmol/nmol) was 21±10.8 and ranged between 5.6 and 40.8.
On arrival at the laboratory the subjects recorded their mood on a 100 mm VAS by marking 54±21 mm in the EF phase, 62±23 mm in the LF phase and 49±19 mm in the ML phase from the “highly agitated” anchor. No significant difference was noted in mood state in the subjects between the three menstrual phases.
Time trial performance
Test for an order-effect
Despite randomising the order of the time trials with regard to menstrual phase, we tested for the possible occurrence of an order or learning effect to satisfy concerns of adequate familiarisation. Repeated measures ANOVA revealed that no significant order-effect was evident for the finishing time in the first, second and third time trial (P=0.56).
Individual group results
When the trained and untrained performance data was analysed separately, we found no significant difference in the time to complete the 15 km or 30 km time trials between menstrual phases (Fig. 1a, b).
Time to complete the a 15 km (untrained group; n=8) and b 30 km (trained group; n=5) cycling time trial. The data for each individual (presented as solid circles) has been superimposed on the group mean for each menstrual phase (represented as bar graphs). The menstrual phases are abbreviated as EF early follicular, LF late follicular and ML mid-luteal
In addition, analysis of the separate groups revealed no significant difference in exercise intensity (%VO2max), RER, HR or ventilatory equivalent between menstrual phases when these variables were averaged over the full duration of each time trial (Table 3).
However, due to the exclusion of subjects or individual trials, the analysis based on the individual groups is limited by small sample sizes that reduced the statistical power to between 60% and 70% for detecting a significant difference of 2 min between time trials with the current within subject variability (SD) of ±1.4 min. However, if the data for the trained and untrained groups are combined the statistical power increases to 78% for LF versus ML phase comparison with n=9 and 82% for both the EF versus LF phase and EF versus ML phase comparison with n=11. The repeated measures design of the study allows use of the combined data despite the distinct differences in training status and time trial distance between groups.
Combined group results
Combined group (trained plus untrained group) mean overall times for the EF, LF and ML phase time trials was 42.4±10.8, 40.8±11.4 and 42.1±11.6 min, respectively (Fig. 2). The pooled data reveal a trend for a faster time trial in the LF phase compared to the EF phase (P=0.0265, Bonferroni correction for three multiple comparisons requires P<0.017 for significance). This trend was identified in eight out of the eleven subjects (or 73%) included in this analysis, who demonstrated a mean improvement of a 2.12±1.13 min (range 0.8–4.14 min) faster finishing time in the LF phase compared to the EF phase (Fig. 2). The faster time trial during the LF phase in these subjects translates to a 5.2±2.9% (range 1.7–10.2%) improvement over their EF phase finishing time.
Combined trained and untrained (n=13) finishing time during each menstrual phase. The data for each untrained individual over 15 km is presented as open circles and each of the trained subjects’ data for 30 km is presented as closed circles. The pooled means for each menstrual phase is presented as bar graphs. The menstrual phases are abbreviated as EF early follicular, LF late follicular and ML mid-luteal. * Denotes a trend for a faster finishing time (in 8 out of 11 subjects) during the LF phase compared to the EF phase
No difference was noted in the overall trial time between EF and ML phase (P=0.5). Thus, a trend for a better performance in the LF compared to the ML phase may be expected; however, this comparison was limited by a sample size of only nine subjects. Of the nine subjects five displayed improvements of between 5.1% and 12.8% in finishing time in LF phase compared to the ML phase (corresponding to 3.0±0.81 min faster time in LF versus ML phase). However, the remaining four subjects had a 0.02–5.6% poorer performance in the LF phase compared to the ML phase (corresponding to a 1.0±0.81 min slower time in the LF versus ML phase). Thus, no definite trend was evident between performances in the LF and ML phases (P=0.14).
However, the combined group analysis still revealed no significant difference in the exercise intensity (%VO2max), respiratory exchange ratio, heart rate or ventilatory equivalent when the averages for these variables over the time trial were compared between menstrual phases (Table 3).
Discussion
The inclusion of the LF phase in the current study is a novel contribution to the current literature on the influence of the menstrual cycle on submaximal exercise performance in women who exercised postprandially. We hypothesised that performance in endurance events might be optimised in the LF phase, as oestrogen is present in high concentrations without the antagonistic affects of progesterone. Thus, the first important finding of this study was a trend for an improved time trial performance in the LF phase compared to the EF phase based on finishing time (P=0.0265). Although not quite reaching significance, this observation of a better performance in the LF phase in 73% of our subjects justifies further research in this area using a larger sample size. The second finding was that cycling time trial performance was similar in the EF and ML phases of the menstrual cycle. Likewise some previous studies have observed no difference in endurance performance between the follicular and luteal phases of the menstrual cycle when subjects were approximately 3 h post absorptive (Bailey et al. 2000; Beidleman et al. 1999). Finally, we found no difference between the LF and ML phase performances; however, this comparison is based on a smaller sample size (n=9) than the EF and ML phase (n=11) or EF and LF phase (n=11) comparisons. Furthermore, the oestrogen to progesterone ratio (E/P) in the ML phase may be an important determinant of the overall impact of the ovarian hormone milieu during this phase (D’Eon et al. 2002; Hatta et al. 1988). A high E/P could allow oestrogen to mitigate the anti-oestrogenic actions of progesterone and thus produce a similar response to the LF phase when oestrogen alone is elevated. Interestingly, three out of the four subjects who performed slightly better in the ML phase compared to the LF phase also recorded the highest E/P ratio of >30 (the remaining six subjects recorded E/P ratios of <21 in their ML phase).
The exercise protocol used to measure submaximal exercise performance (i.e. either time to exhaustion at a fixed workload or time trial performance) may be an important variable determining whether performance is optimised in a particular menstrual phase. For example, Jurkowski et al. (1981) and Nicklas et al. (1989) observed improvements in the ML phase compared to the EF phase during a submaximal time to exhaustion test, while Campbell et al. (2001) reported a faster cycling time trial in the EF compared to during the ML phase. The reason for the discrepancy may lie in the different fuel sources needed to optimise performance in the respective protocols. While the luteal phase promotes fat oxidation, carbohydrate sparing and reduced glucose kinetics and hepatic glucose production, the follicular phase favours carbohydrate utilisation (Campbell et al. 2001; Zderic et al. 2001). However, these significant changes to substrate metabolism in the luteal phase depend on the ratio of oestrogen to progesterone and the magnitude of the increase in oestrogen from follicular to luteal phase (D’Eon et al. 2002), as well as nutritional status (Campbell et al. 2001). Nonetheless, studies using time to exhaustion at a submaximal intensity are measuring endurance capacity (Bishop 1997) and this favours the metabolic profile associated with the luteal phase as suggested by the findings of some previous studies (Jurkowski et al. 1981; Nicklas et al. 1989), although not all (Biedleman et al. 1999; Bailey et al. 2000). Time trials are a better measure of exercise performance (Hickey et al. 1992; Jeukendrup et al. 1996) and demand greater proportions of carbohydrate oxidation to sustain the high intensity effort. Thus, the follicular phase would promote optimal performance in such an event as observed in the study of Campbell et al. (2001) when subjects exercised following an overnight fast. Nutritional status may, however, override the ovarian hormone-induced variation in substrate oxidation in the luteal phase (Campbell et al. 2001). Thus, during high intensity exercise in the non-fasted state, glucose availability is not limited and therefore, carbohydrate oxidation can be maintained equally in the ML phase as it is in the EF phase, resulting in a similar time trial performance as observed in the current study and in the study of Campbell et al. (2001) (when their overnight fasted subjects ingested carbohydrate supplements during exercise).
Although the faster time trial performance in the LF phase, when oestrogen alone was elevated, compared to the EF phase can only be reported as a trend, it is nonetheless an interesting observation and thus warrants an explanation. Possible improvements to time trial performance in the LF phase cannot be simply related to a lower progesterone concentration, as then we would also expect to find performance to be greater in the EF phase compared to the ML phase. However, a combination of the low progesterone and very high oestrogen that characterises the LF phase could result in oestrogen promoting performance without the anti-oestrogenic effects of progesterone as may occur in the ML phase. A recent study measured glucose uptake, GLUT 4 content, and glycogen storage and utilisation in various tissues during exercise in ovariectomised rats receiving different combinations of ovarian hormones (Campbell and Febbraio 2002). Oestrogen alone promoted glucose uptake and muscle glycogen sparing during exercise in quadriceps muscle fibres (predominantly type I fibres) and hepatic glycogen storage at rest and hepatic glycogenolysis during exercise, i.e. improved plasma glucose availability to oxidative skeletal muscle fibres. Progesterone demonstrated an inhibitory effect on all of these variables and antagonised the positive effects of oestrogen when both progesterone and oestrogen were administered together in physiological doses (Campbell and Febbraio 2002). Subjects in the current study chose to exercise at an intensity of 70–80% VO2max. At such intensities carbohydrate oxidation becomes increasingly more important to maintain the energy supply (Achten et al. 2002). Therefore, even though previous studies (Carter et al. 2001; Ruby et al. 1997) have observed whole body glucose uptake to be reduced when subjects receive oestrogen supplements, an oestrogen-induced increase in glucose uptake into specifically oxidative muscle fibres (Campbell and Febbraio 2002) could be beneficial in optimising high intensity submaximal activity as suggested by the trend noted in the LF phase of the current study. Further investigations using human subjects are necessary to elucidate the possible metabolic modifications associated with the surge in oestrogen before ovulation.
Some studies have reported lower RER at rest in the luteal phase (Nicklas et al. 1989) or during exercise at ovulation (Hackney et al. 1991) and in the luteal phase (Campbell et al. 2001; Dombovy et al. 1987; Zderic et al. 2001), suggesting that the ovarian hormones optimise free fatty acid oxidation. We found no change in the RER across menstrual phases possibly, due to the nature of the exercise trial employed in the current study that allowed subjects to vary their intensity at any time and therefore prevented us from obtaining readings of VO2 and VCO2 during a fixed workload for all three menstrual phases.
In the current study, heart rate and ventilation rate during exercise remained unaltered by menstrual phase as is also reported by most previous studies (Beidleman et al. 1999; Bemben et al. 1995; Hackney et al. 1991; Jurkowski et al. 1981; Lebrun et al. 1995; Nicklas et al. 1989).
Conclusion
This study confirms previous findings that in non-fasted subjects cycling time trial performance is not altered during the EF and ML phase of the menstrual cycle. However, we observed a trend for cycling time trial performance to be optimised during the LF phase associated with the pre-ovulation surge of oestrogen and thus further studies are necessary to verify whether menstrual phase needs to be considered in events such as a cycling time trial.
References
Achten J, Gleeson M, Jeukendrup AE (2002) Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc 34:92–97
Bailey SP, Zacher CM, Mittleman KD (2000) Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J Appl Physiol 88:690–697
Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D (1999) High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol 277:E1013–E1021
Beidleman BA, Rock PB, Muza SR, Fulco CS, Forte VA Jr, Cymerman A (1999) Exercise V E and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol 86:1519–1526
Bemben DA, Salm PC, Salm AJ (1995) Ventilatory and blood lactate responses to maximal treadmill exercise during the menstrual cycle. J Sports Med Phys Fitness 35:257–262
Bishop D (1997) Reliability of a 1-h endurance performance test in trained female cyclists. Med Sci Sports Exerc 29:554–559
Campbell SE, Febbraio MA (2001) Effect of ovarian hormones on mitochondrial enzyme activity in fat oxidation pathway of skeletal muscle. Am J Physiol 281:E803–E808
Campbell SE, Febbraio MA (2002) Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol 282:E1139–E1146
Campbell SE, Angus DJ, Febbraio MA (2001) Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion. Am J Physiol 281:E817–E825
Carter S, McKenzie S, Mourtzakis M, Mahoney DJ, Tarnopolsky MA (2001) Short-term 17β-estradiol decreases glucose R a but not whole body metabolism during endurance exercise. J Appl Physiol 90:139–146
Curran-Everett D (2000) Multiple comparisons: philosophies and illustrations. Am J Physiol 279:R1–R8
De Souza MJ, Maguire MS, Rubin KR, Maresh CM (1990) Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc 22:575–580
Dean TM, Perreault L, Mazzeo RS, Horton TJ (2003) No effect of menstrual cycle phase on lactate threshold. J Appl Physiol 95:2537–2543
D’Eon TM, Sharoff C, Chipkin SR, Grow D, Ruby BC, Braun B (2002) Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am J Physiol 283:E1046–E1055
Dombovy ML, Bonekat HW, Williams TJ, Staats BA (1987) Exercise performance and ventilatory response in the menstrual cycle. Med Sci Sport Exerc 19:111–117
Duncan GE, Howley ET, Johson BN (1997) Applicability of VO2max criteria: discontinuous versus continuous protocols. Med Sci Sports Exerc 19:111–117
Hackney AC, Curley CS, Nicklas BJ (1991) Physiological responses to submaximal exercise at the mid-follicular, ovulatory and mid-luteal phases of the menstrual cycle. Scand J Med Sci Sports 1:94–98
Hatta H, Atomi Y, Shinohara S, Yamamoto Y, Yamada S (1988) The effects of ovarian hormones on glucose and fatty acid oxidation during exercise in female ovariectomized rats. Horm Metab Res 20: 609–611
Hessemer V, Brück K (1985) Influence of menstrual cycle on thermoregulatory, metabolic, and heart rate responses to exercise at night. J Appl Physiol 59:1911–1917
Hickey MS, Costill DL, McConell GK, Widrick JJ, Tanaka H (1992) Day to day variation in time trial cycling performance. Int J Sports Med 13:467–670
Jeukendrup A, Wim HM, Brouns F, Kester AD (1996) A new validated endurance performance test. Med Sci Sports Exerc 28:266–270
Jurkowski Hall JE, Jones NL, Toews CJ, Sutton JR (1981) Effects of the menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol 51:1493–1499
Kanaley JA, Boileau RA, Bahr JA, Misner JE, Nelson RA (1992) Substrate oxidation and GH responses to exercise are independent of menstrual phase and status. Med Sci Sports Exerc 24:873–880
Kendrick ZV, Steffen CA, Rumsey WL, Goldberg DI (1987) Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol 63:492–496
Lavoie J-M, Dionne N, Helie R, Brisson GR (1987) Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol 62:1084–1089
Lebrun CM, McKenzie DC, Prior JC, Taunton JE (1995) Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc 27:437–444
Nicklas BJ, Hackney AC, Sharp RL (1989) The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int J Sports Med 10:264–269
Palmer GS, Dennis SC, Noakes TD, Hawley JA (1996) Assessment of the reproducibility of performance testing on an air-braked cycle ergometer. Int J Sports Med 17:293–298
Pivarnik JM, Marichal CJ, Spillman T, Morrow JR Jr (1992) Menstrual cycle phase affects temperature regulation during endurance exercise. J Appl Physiol 72:543–548
Reilly T (2000) The menstrual cycle and human performance: an overview. Biol Rhythm Res 31:29–40
Ruby BC, Robergs RA, Waters DL, Burge M, Mermier C, Stolarczyk L (1997) Effects of estradiol on substrate turnover during exercise in amenorrheic females. Med Sci Sports Exerc 29:1160–1169
Schoene RB, Robertson HT, Pierson DJ, Peterson AP (1981) Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J Appl Physiol 50:1300–1305
Seebauer M, Frühwirth M, Moser M (2002) Changes of respiratory sinus arrhythmia during the menstrual cycle depend on average heart rate. Eur JAppl Physiol 87:309–314
Zderic TW, Coggan AR, Ruby BC (2001) Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol 90:447–453
Acknowledgements
We would like to thank Sr. F. Koetle for her medical assistance. This study was funded by the Medical Research Council of South Africa and the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oosthuyse, T., Bosch, A.N. & Jackson, S. Cycling time trial performance during different phases of the menstrual cycle. Eur J Appl Physiol 94, 268–276 (2005). https://doi.org/10.1007/s00421-005-1324-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-005-1324-5