Abstract
We have previously shown that vitamin C supplementation affects recovery from an unaccustomed bout of demanding exercise, with the most pronounced effect being that on plasma interleukin-6 concentration. However, because of the proposed role of interleukin-6 in the regulation of metabolism, it was unclear whether this represented a reduced response to muscle damage or some form of interaction with the metabolic demands of the activity. Therefore, the aim of the present study was to investigate the effect of the same form of supplementation on a bout of exercise that initiated similar muscle damage but had a low metabolic cost. Fourteen male subjects were allocated to either a placebo (P) or a vitamin C (VC) group. The VC group consumed 200 mg of ascorbic acid twice a day for 14 days prior to a bout of exercise and for the 3 days after exercise. The P group consumed identical capsules that contained 200 mg lactose. Subjects performed 30 min of downhill running at a gradient of −18% and recovery was monitored for up to 3 days after exercise. Plasma VC concentrations in the VC group increased following supplementation. Nevertheless, downhill running provoked a similar increase in circulating markers of muscle damage (creatine kinase activity and myoglobin concentration) and muscle soreness in P and VC groups. Similarly, although downhill running increased plasma interleukin-6, there was no effect from VC supplementation. These results suggest that vitamin C supplementation does not affect interleukin-6 concentrations following eccentric exercise that has a low metabolic component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unaccustomed demanding exercise has the capacity to produce muscle damage and initiate an acute inflammatory response (MacIntyre et al. 1995; Shephard and Shek 1998). It has been suggested that acute inflammation is probably responsible for the sensation of muscle soreness that develops following such activities (Smith 1991). In support of this hypothesis, investigators have shown increased concentrations of leukocytes in sore and damaged muscles following exercise (Fielding et al. 1993; MacIntyre et al. 1996). In addition to the generation of the sensation of muscle soreness, it appears that this form of inflammatory response has the capacity to lead to secondary muscle damage in certain circumstances (MacIntyre et al. 1996; Lapointe et al. 2002).
The potential negative effects of this acute inflammatory response may be mediated in part by free radicals produced during the phagocytic respiratory burst and xanthine oxidase-mediated superoxide production (Weiss 1989; Duarte et al. 1993; Hellsten et al. 1997). As a corollary, we recently reported that 2 weeks supplementation with an antioxidant (vitamin C) appears to offer some benefits to recovery following unaccustomed demanding exercise (Thompson et al. 2001b). The most striking change following supplementation was a marked reduction in plasma concentrations of interleukin-6 (IL-6) following exercise, although there were also some modest effects on muscle soreness. Interestingly, although post-exercise plasma IL-6 was lower following vitamin C supplementation, there was no effect on circulating markers of muscle damage. It was postulated that vitamin C might affect the response to muscle damage rather than muscle damage per se. However, such a conclusion was limited by the fact that the 90-min intermittent shuttle-running protocol used in this investigation not only produced considerable muscle damage, but also had a very high metabolic cost (Nicholas et al. 1999). In light of the recently proposed role of IL-6 in maintaining metabolic homeostasis (Febbraio and Pedersen 2002), it is possible that the pronounced effect of vitamin C supplementation on plasma IL-6 in our earlier study was more closely related to the metabolic demands of the exercise rather than the extent of muscle damage. There is some support for this contention, since recent investigations have shown mixed antioxidant supplementation to affect the concentrations of various cytokines (including IL-6) following a concentric exercise protocol that is not associated with muscle damage and soreness (Vassilakopoulos et al. 2003). Therefore, it is possible that the effects of vitamin C seen in our earlier investigation were independent of muscle damage and were in some way related to the high metabolic demands of the activity. In order to investigate this phenomenon further, we sought to separate the potential metabolic and muscle damage effects of exercise. In order to facilitate direct comparison with our earlier investigation (Thompson et al. 2001b), we wished to elicit similar muscle damage through a whole-body exercise protocol, whilst at the same time minimising metabolic disturbance. Based on previous investigations (Maughan et al. 1989; Sorichter et al. 1997) and our own pilot work, we established a downhill running protocol that would provoke such a response and could therefore be used with the same supplementation strategy employed in our earlier investigation (Thompson et al. 2001b).
Consequently, the aim of the present investigation was to identify whether vitamin C supplementation would affect plasma IL-6 concentrations during recovery from a bout of downhill running designed to induce similar muscle damage and soreness to our previous investigations (Thompson et al. 1999, 2001b), but that was associated with a much lower metabolic cost.
Methods
Subjects
Fourteen male students volunteered to take part in this study, which had approval from Loughborough University Ethical Advisory Committee. All subjects were informed verbally and in writing about the nature and demands of the study, and subsequently completed a health history questionnaire and gave their written informed consent. The experiments described comply with current UK laws and the declaration of Helsinki. Subjects who smoked or took vitamin supplements were excluded from the study. All participants regularly took part in a variety of activities but were unfamiliar with the exercise protocol used in the present investigation. Subjects were allocated to either a vitamin C (VC) or a placebo (P) group in a double-blind design (Table 1). Physical characteristics, self-reported levels of habitual activity and skinfold measurements taken from four sites (biceps, triceps, subscapular and suprailiac) were similar in the two groups (Table 1).
Preliminary measurements
Subjects performed two preliminary treadmill-based tests at least 7 days prior to the main trial using methods described previously (Williams et al. 1990). Briefly, subjects performed an incremental submaximal running test to determine the relationship between running speed and oxygen uptake, and also an incremental running test to determine maximal oxygen uptake (V̇O2max).
During the main test, subjects performed a muscle function test on an isokinetic dynamometer as described previously (Thompson et al. 2001b). Subjects were familiarised with this test on three occasions prior to the main trial.
In order to assess their normal dietary intake of vitamin C, subjects weighed and recorded their food and fluid intake for 3 days prior to any other testing. Weighed food records were analysed by a dietician for energy and composition using the software COMP-EAT 4.0 (Nutrition Systems, UK). Normal dietary vitamin C intake was similar in VC and P groups (Table 1).
Experimental design and procedures
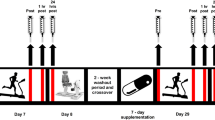
Subjects reported to the laboratory 14 days prior to performance of the main trial following an overnight fast of between 10 and 12 h. A venous blood sample was taken and subjects began the course of supplementation. The supplements were given to subjects in the form of gelatin capsules each containing 200 mg of vitamin C (ascorbic acid) or 200 mg lactose (Nova Laboratories, UK). Subjects consumed capsules in the morning and evening of each day, and therefore the total amount of vitamin C consumed above that obtained in the normal diet was 400 mg day−1. Subjects continued to take supplements for 2 days after the main exercise trial. This supplementation strategy is associated with optimal bioavailability (Levine et al. 1996) and has had various beneficial effects in previous studies (Jakeman and Maxwell 1993; Thompson et al. 2001b).
On the day of the main trial (day 15) subjects arrived in the laboratory after an overnight fast of 10–12 h. Subjects were instructed to abstain from strenuous exercise for at least 2 days prior to the main trial. An 11-ml resting venous blood sample was taken from a forearm vein after subjects had been standing for at least 15 min. Subjects subsequently rated soreness as described previously (Thompson et al. 2001b). Briefly, subjects assessed soreness in the quadriceps using a 0–100 mm scale that had no adjectives or divisions with the exception of normal on the left and very very sore on the right. In order to activate the sensation of soreness, subjects assessed soreness whilst actively contracting the leg extensors against a resistance equivalent to 75% of each individual’s one-repetition maximum. Subsequently, subjects performed the muscle function test described above.
Following a 10-min warm-up consisting of running at 50% V̇O2max (5 min) and stretching (5 min) subjects began the main trial. Subjects ran on the treadmill at a speed corresponding to 60% V̇O2max (0% gradient) for 15 min after which the treadmill gradient was decreased to −18% and subjects ran for a further 30 min at the same speed. This protocol was designed to elicit similar muscle damage and soreness as our previous investigations (Thompson et al. 1999, 2001b), but with a lower metabolic cost. One-minute expired air samples were taken at 6, 14, 24, 34 and 44 min, along with perceived ratings of exertion (Borg 1982) and heart rate (Sports Tester, Polar Electro, Finland). Subjects were allowed to consume water ad libitum throughout exercise, although the volume of water was monitored and used in the subsequent calculation of sweat loss. Nude body mass was determined before and after exercise, and a venous blood sample was taken immediately after exercise and 1 h later.
Subjects returned to the laboratory in the morning of the 3 days after the main trial following an overnight fast. A blood sample was taken from a forearm vein after subjects had been standing for at least 15 min. Subsequently, subjects rated the intensity of soreness and performed the muscle function test outlined above.
For the 2 days before the main trial and the following 3 days, subjects weighed and recorded their food and fluid intake. Weighed records were analysed as described above. Subjects were also asked to refrain from participation in exercise during this period.
Blood analysis
Aliquots of whole blood were used to determine lactate concentration and changes in plasma volume as described previously (Thompson et al. 1999). Plasma vitamin C was analysed using high-performance liquid chromatography as described previously (Thompson et al. 2001b). Serum creatine kinase (CK) and myoglobin were analysed using commercially available methods (Roche Products, UK) and an automated system (COBAS Mira Plus, Roche Diagnostic Systems, Switzerland). Serum IL-6 was analysed using a commercially available solid-phase high-sensitivity ELISA (Quantikine, R and D Systems, UK).
Statistical analysis
An independent two-way analysis of variance (ANOVA) with repeated measures was used to compare results between treatments and over time. Where significant F ratios were found, a Tukey honest significant difference test was used to determine the location of the variance. When there were only single comparisons, an independent Student’s t-test was used to determine whether any differences between treatments existed. Certain results were not normally distributed (CK and myoglobin), and therefore these values were log transformed prior to ANOVA. Log transformation always resulted in a normal distribution, and therefore these ANOVA results are reported. Significance was accepted at the 5% level, and values are presented as means (SEM). Effect size (ES) is also presented as suggested by Thomas et al. (1991) and was calculated at the time point after supplementation that was associated with the maximum difference between VC and P groups (treatment−control). These results were treated according to convention where >0.8 equals a large effect.
Results
The responses to exercise were very similar in both groups. Mean oxygen uptake during the 15 min of level running was 60 (1)% V̇O2max in the VC group and 59 (2)% V̇O2max in the P group. During the subsequent 30 min of downhill running, oxygen uptake was 40 (2)% V̇O2max in the VC group and 43 (3)% V̇O2max in the P group. The running speeds required to elicit this exercise intensity in VC and P groups were 9.4 (0.7) and 9.8 (0.7) km h−1, respectively. Total estimated energy expenditures during both level and downhill running components were 1,801 (191) kJ and 1,712 (108) kJ in VC and P groups, respectively. Heart rate was also similar in both groups, being 147 (3) beats min−1 and 145 (2) beats min−1 during the 15 min of level running, and 141 (1) beats min−1 and 139 (1) beats min−1 during 30 min of downhill running, for VC and P groups respectively. Blood lactate concentrations at the end of exercise were 1.4 (0.1) mmol l−1 in the VC group and 1.1 (0.1) mmol l−1 in the P group. Mean ambient temperature was similar in VC and P groups [21.9 (0.7) vs. 22.3 (0.3)°C], as was relative humidity [73 (6) vs. 72 (3)%]. The VC group consumed 0.6 (0.1) l of water during exercise and lost 1.1 (0.1) kg in body mass (after correction for fluid intake), which was not different to the P group, which consumed 0.8 (0.1) l of water and lost 1.4 (0.1) kg in body mass. Plasma volume changes were not different between VC and P groups at any point over the period of testing (data not shown).
There were no differences between groups in terms of dietary composition over the 5-day period of the test (Table 2).
Plasma vitamin C
Vitamin C supplementation increased plasma vitamin C concentrations above those of the P group (Fig. 1) (P<0.05). Unfortunately, one post-supplementation plasma vitamin C sample was lost due to technical problems during HPLC analysis in both VC and P groups and therefore these results are based on n=6. Plasma vitamin C concentrations on the day of exercise (day 15) were 67.9 (5.1) μmol l−1 in the VC group and 50.8 (5.2) μmol l−1 in the P group (ES=0.93).
Interleukin-6
Plasma IL-6 concentrations increased immediately after exercise and remained above pre-exercise values 1 h after exercise (Fig. 2). There was no difference between VC and P groups at any time point (ES=0.12).
CK and myoglobin
Serum CK activity was increased above pre-exercise values 24 h after exercise in both VC and P groups (P<0.05), although there was no difference between groups (Fig. 3; ES=−0.10). Serum myoglobin concentrations increased immediately after exercise in both groups (P<0.05), and continued to increase over the 1 h following exercise (Fig. 3). There was no difference between groups at any time point (ES=0.20).
Muscle soreness and muscle function
Muscle soreness in the leg extensors increased in both VC and P groups 24–72 h after exercise (Fig. 4). There was no difference between groups at any time (ES=0.56). There was also no difference in leg extensor muscle function between VC and P groups (data not shown). Muscle function was impaired up to 48 h after exercise in both groups.
Discussion
The aim of the present investigation was to examine the impact of prolonged vitamin C supplementation on plasma IL-6 and other aspects of recovery following a bout downhill running. This exercise model was designed to elicit comparable muscle damage and soreness to our earlier investigation where vitamin C supplementation had demonstrated beneficial effects (Thompson et al. 2001b), whilst at the same time limiting the metabolic demands of the activity. Interestingly, in the present investigation, vitamin C supplementation had no effect on plasma concentrations of IL-6, muscle soreness, circulating markers of muscle damage, or muscle function.
The extent of muscle damage and soreness induced in the present investigation was very similar to that seen following our earlier investigations that employed prolonged intermittent shuttle-running (Thompson et al. 1999, 2001b). Mean serum CK activity peaked 24 h after exercise (956 U l-1) and myoglobin concentrations 1 h after exercise (243 μg l−1). Mean soreness in the leg extensors peaked 48 h after exercise (57 mm). Therefore, the exercise model employed in the present investigation successfully produced similar muscle damage and soreness to our previous investigation where vitamin C had shown beneficial effects (Thompson et al. 2001b). These responses were also similar to other studies that employed downhill running (Schwane et al. 1983; Maughan et al. 1989; Sorichter et al. 1997), although it should be pointed out that the extent of changes in markers of muscle damage was much lower than following repeated eccentric contractions of a single muscle group (Sorichter et al. 1997; Croisier et al. 1999). Importantly, we observed the well-characterised fall in oxygen uptake and heart rate that has been reported when running downhill (Schwane et al. 1983), and the metabolic cost of the exercise bout in the present study was relatively low (<45% V̇O2max during the downhill running). Indeed, estimated energy expenditure during the protocol used in the present study was approximately one-third of energy expenditure during the intermittent shuttle running used in our previous investigation (Nicholas et al. 2000). Therefore, the downhill running protocol employed in the present investigation successfully initiated the desired changes in muscle damage and soreness in the absence of a pronounced metabolic cost.
As demonstrated in our previous investigation, this form of supplementation led to a small increase in plasma vitamin C concentrations (Thompson et al. 2001b). This small increase may reflect the relatively high habitual dietary intake of vitamin C in these subjects prior to and during testing. Indeed, Levine et al. (1996) showed that plasma vitamin C concentration only increases modestly (~10 μmol l−1) when dietary vitamin C intake is increased from 100 to 200 mg per day. These authors also showed that leukocyte vitamin C is maximum at an intake of 100 mg per day, although it is unclear whether this is also the case for concentrations of vitamin C in skeletal muscle. Although the high vitamin C intake may ostensibly explain the lack of effect from vitamin C supplementation, subjects in our previous study using an exercise protocol with a high metabolic cost and where we had observed an effect from vitamin C supplementation also had a high habitual vitamin C intake (Thompson et al. 2001b).
Downhill running increased plasma IL-6 concentrations approximately threefold in both VC and P groups. Interestingly, in spite of muscle damage and soreness being similar to our earlier investigations (Thompson et al. 1999, 2001a, 2001b, 2003), vitamin C supplementation had no effect on this or any other parameter. Consequently, it appears that when vitamin C supplementation precedes a bout of exercise that initiates muscle damage but has a low metabolic cost, it is unable to affect plasma IL-6. Based on this finding and our earlier observations (Thompson et al. 2001b), it is tempting to suggest that vitamin C supplementation has the capacity to affect plasma IL-6 only when the metabolic demands of the activity are high. This is supported by other investigators who have shown vitamin C or mixed antioxidant supplementation (including vitamin C) to affect plasma IL-6 concentrations following demanding exercise (Nieman et al. 2000; Vassilakopoulos et al. 2003), although this finding has not been entirely consistent (Petersen et al. 2001). The potential mechanisms that might explain this finding are not clear, although it is quite possible that it reflects an antioxidant effect of vitamin C and other antioxidants on IL-6 transcription (Grimble 1996; Vassilakopoulos et al. 2003). Since exercising skeletal muscle has been identified as the primary source of plasma IL-6 (Febbraio and Pedersen 2002), such an effect would almost certainly have to be operating in this compartment.
Much of the preceding discussion is speculative at the present time. In particular, it should be noted that the increase in plasma IL-6 concentrations in the present investigation was much lower than in our earlier investigation where vitamin C had demonstrated an effect (Thompson et al. 2001b). As a result, an alternative explanation for the present finding is that vitamin C was unable to exert an effect because the absolute change in plasma IL-6 was too low. It is possible that vitamin C was unable to modulate IL-6 because the increase did not achieve some form of critical threshold level. Interestingly, the present study does lend some support to the suggestion that the greatest stimulus for IL-6 release during exercise is not muscle damage but some other factor (Croisier et al. 1999; Febbraio and Pedersen 2002; Willoughby et al. 2003). Given the likely role for muscle glycogen depletion in IL-6 release (Febbraio and Pedersen 2002), it is possible that vitamin C supplementation had no effect in the present study because the downhill running protocol employed did not produce a significant challenge to glycogen stores.
In summary, the results of the present study suggest that vitamin C does not affect IL-6 concentrations following eccentric exercise that has a low metabolic component. It is possible that the previously reported beneficial effect of vitamin C supplementation is explained by some form of interaction with the high metabolic demands of the activity used in this earlier investigation (Thompson et al. 2001b), although the mechanisms behind such a proposed effect remain to be elucidated.
References
Borg G (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Croisier J, Camus G, Venneman I, Deby-Dupont G, Juchmes-Ferir A, Lamy M, Crielaard J, Deby C, Duchateau J (1999) Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle Nerve 22:208–212
Duarte J, Appell H, Carvalho F, Bastos M, Soares J (1993) Endothelium-derived oxidative stress may contribute to exercise-induced muscle damage. Int J Sports Med 14:440–443
Febbraio MA, Pedersen BK (2002) Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16:1335–1347
Fielding R, Manfredi T, Ding W, Fiatarone M, Evans W, Cannon J (1993) Acute phase response in exercise III. Neutrophil and IL-1β accumulation in skeletal muscle. Am J Physiol 265:R166–R172
Grimble R (1996) Interaction between nutrients, pro-inflammatory cytokines and inflammation. Clin Sci 91:121–130
Hellsten Y, Frandsen U, Ørthenblad N, Sjødin B, Richter E (1997) Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol (Lond) 498:239–248
Jakeman P, Maxwell S (1993) Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur J Appl Physiol 67:426–430
Lapointe BM, Frenette J, Cote CH (2002) Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. J Appl Physiol 92:1995–2004
Levine M, Conry-Cantilena C, Wang Y, Welch R, Washko P, Dhariwal K, Park J, Lazarev A, Graumlich J, King J, Cantilena LR (1996) Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93:3704–3709
MacIntyre D, Reid WD, McKenzie DC (1995) Delayed muscle soreness: the inflammatory response to muscle injury and its clinical implications. Sports Med 20:24–40
MacIntyre D, Reid W, Lyster D, Szasz I, McKenzie D (1996) Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol 80:1006–1013
Maughan RJ, Donnelly AE, Gleeson M, Whiting PH, Walker KA, Clough PJ (1989) Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve 12:332–336
Nicholas CW, Tsintzas K, Boobis L, Williams C (1999) Carbohydrate-electrolyte ingestion during intermittent high- intensity running. Med Sci Sports Exerc 31:1280–1286
Nicholas CW, Nuttall FE, Williams C (2000) The Loughborough Intermittent Shuttle Test: A field test that simulates the activity pattern of soccer. J Sports Sci 18:97–104
Nieman DC, Peters EM, Henson DA, Nevines EI, Thompson MM (2000) Influence of vitamin C supplementation on cytokine changes following an ultramarathon. J Interferon Cytokine Res 20:1029–1035
Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J, Pedersen BK (2001) Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol 280:C1570–C1575
Schwane J, Johnson S, Vandenakker C, Armstrong R (1983) Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc 15:51–56
Shephard RJ, Shek PN (1998) Immune responses to inflammation and trauma: a physical training model. Can J Physiol Pharmacol 76:469–472
Smith L (1991) Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc 23:542–551
Sorichter S, Mair J, Koller A, Gebert W, Rama D, Calzolari C, Artner-Dworzak E, Puschendorf B (1997) Skeletal troponin I as a marker of exercise-induced muscle damage. J Appl Physiol 83:1076–1082
Thomas JR, Salazar W, Landers DM (1991) What is missing in p<0.05? Effect Size. Res Q Exerc Sport 62:344–348
Thompson D, Nicholas CW, Williams C (1999) Muscle soreness following prolonged intermittent high-intensity shuttle running. J Sports Sci 17:387–395
Thompson D, Williams C, Kingsley M, Nicholas CW, Lakomy HKA, McArdle F, Jackson MJ (2001a) Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. Int J Sports Med 22:68–75
Thompson D, Williams C, McGregor SJ, Nicholas CW, McArdle F, Jackson MJ, Powell JR (2001b) Prolonged vitamin C supplementation and recovery from demanding exercise. Int J Sport Nutr Exerc Metab 11:466–481
Thompson D, Williams C, Garcia-Roves P, McGregor S, McArdle F, Jackson MJ (2003) Post-exercise vitamin C supplementation and recovery from demanding exercise. Eur J Appl Physiol 89:393–400
Vassilakopoulos T, Karatza M-H, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C (2003) Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol 94:1025–1032
Weiss S (1989) Tissue destruction by neutrophils. N Engl J Med 320:365–376
Williams C, Nute M, Broadbank L, Vinall S (1990) Influence of fluid intake on endurance running performance. Eur J Appl Physiol 60:112–119
Willoughby DS, McFarlin B, Bois C (2003) Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med 24:15–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, D., Bailey, D.M., Hill, J. et al. Prolonged vitamin C supplementation and recovery from eccentric exercise. Eur J Appl Physiol 92, 133–138 (2004). https://doi.org/10.1007/s00421-004-1064-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-004-1064-y