Abstract
The use of thermography in the diagnosis and evaluation of complex regional pain syndrome type 1 (CRPS1) is based on the presence of temperature asymmetries between the involved area of the extremity and the corresponding area of the uninvolved extremity. The interpretation of thermographic images is, however, subjective and not validated for routine use. The objective of the present study was to develop a sensitive, specific and reproducible arithmetical model as the result of computer-assisted infrared thermography in patients with early stage CRPS1 in one hand. Eighteen patients with CRPS1 on one hand and 13 healthy volunteers were included in the study. The severity of the disease was determined by means of pain questionnaires [visual analogue scale (VAS) pain and McGill Pain Questionnaire], measurements of mobility (active range of motion) and oedema volume. Asymmetry between the involved and the uninvolved extremities was calculated by means of the asymmetry factor, the ratio and the average temperature differences. The discrimination power of the three methods was determined by the receiver-operating curve (ROC). The regression between the determined temperature distributions of both extremities was plotted. Subsequently the correlation of the data was calculated. In normal healthy individuals the asymmetry factor was 0.91 (0.01) (SD), whereas in CRPS1 patients this factor was 0.45 (0.07) (SD). The performance of the arithmetic model based on the ROC curve was excellent. The area under the curve was 0.97, the P value was <0.001, the sensitivity 92% and specificity 94%. Furthermore, the temperature asymmetry factor was correlated with the duration of the disease and VAS pain. In conclusion, in resting condition, videothermography is a reliable additive diagnostic tool of early stage CRPS1. This objective tool could be used for monitoring purposes during experimental therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex regional pain syndrome type 1 (CRPS1) is a chronic disease which is characterised by severe and constant burning pain, pathological changes in bone and skin, excessive sweating, tissue swelling and allodynia. The syndrome is thought to be a nerve disorder that occurs in patients after trauma or surgery at the site of an injury, most often presented in the extremities. This rare disease could simultaneously or subsequently affect nerves, skin, muscles, blood vessels and bones. The symptoms of CRPS1 vary in severity and duration. There are, however, different stages of the disease that are marked by progressive changes in visible signs. In the first phase, the acute stage of the disease, severe burning pain at the site of the injury is observed. Furthermore, muscle spasm, joint stiffness, restricted mobility, rapid hair and nail growth, and vasospasm that affect colour and temperature of the skin can occur (Schwartzman and Popescu 2002). In the second phase, the dystrophic stage of the disease, which lasts from 3–6 months, the pain intensifies, swelling spreads, joints thicken and muscle atrophy is seen. In the third phase, the chronic/atrophic stage of the disease, changes in the skin and bones become irreversible and pain spreads throughout the entire limb (Bruehl et al. 2002). Although it can occur at any age, the number of CRPS1 cases among adolescents and young adults is increasing. It is more common between the ages of 40 and 60 years and affects mainly women (60–75%). It has been estimated that 7.2% of patients with peripheral nerve injury will suffer from CRPS1 (Sandroni et al. 1998), whereas for wrist fractures the incidence varies from 7 to 37% (Raja and Grabow 2002). How CRPS1 progresses into being such a disabling disorder is not yet known. So far it is poorly understood how a minor injury can result in such a stage with far greater pain and discomfort than was ever observed in the healing period after the initial injury (Veldman et al. 1993). It is quite certain that damaged nerves of the sympathetic nervous system, which are responsible for blood flow and temperature, play an important role in the development of CRPS1 (Huygen et al. 2001). Furthermore, the release of bio-active inflammatory mediators such as neuropeptides, cytokines and eicosanoids also contribute to the acute signs of the disease such as pain, loss of function, redness, swelling and increasing temperature (Birklein et al. 2001a, Huygen et al. 2002). Therefore CRPS1 seems to resemble neurogenic inflammation, sympathetic dysfunction or possibly an interaction between both (Birklein et al. 2001b). Diagnostic criteria for CRPS1 have been extensively described by Bruehl et al. (1999). Diagnostic criteria sets of CRPS1 focus on many different aspects of sensory and autonomic features that generally are described vaguely (Van de Beek et al. 2002). Objective, diagnostic tests that could be performed include a three-phase bone scan, which is sensitive within the first 20–26 weeks of the onset of CRPS1. X-rays usually show osteoporotic changes in chronic stages of CRPS1 (Matsumura et al. 1996). Blood tests will normally demonstrate normal biochemistry estimations (Sandroni et al. 1998). Most oedema volume measurements are not validated and therefore they are open to a question of doubt. Thermographic imaging for measuring skin temperature, which reflects vasomotor activity (Uematsu 1985) and inflammation, could therefore be a worthwhile tool for the diagnosis and monitoring of CRPS1 in the acute stage of the disease.
The aim of the present study was to assess the sensitivity and specificity of computer-assisted infrared thermography in patients with early stage CRPS1 in one hand. As a result, an asymmetric temperature factor to characterise the presence and severity of CRPS1 has been calculated. This paper describes a reliable computer-calculated method of collecting thermographic data that eliminates subjective biases and is suitable for longitudinal follow-up measurements during experimental treatment of CRPS1.
Methods
Patients and controls
The study was approved by the local medical ethical committee of the Erasmus MC (MEC no. 198.780/2001/24). All patients gave their informed consent. The study was performed on 18 patients, 16 women and 2 men (mean age 44.8 years, range 22–68), with the diagnosis of unilateral CRPS1 in the hand or wrist, who were referred to the Pain Treatment Centre of the Erasmus MC of Rotterdam from spring 2002 to autumn 2002 (Table 1). CRPS1 was diagnosed according to the criteria defined by Bruehl et al. (1999). Only patients defined as phase 1 or 2, and therefore expected to exert a significant increase in temperature in the affected hand, were included in the present study.
Thirteen healthy volunteers, without any history of neurotrauma or vasculair disease, (10 women and 3 men) served as a control group (mean age 33.3 years, range 23–53).
Observations and measurements
Questionnaires were used to describe pain, physical limitations and quality of life. In our study two sets of parameters to represent pain were used, the visual analogue scale (VAS) and the McGill Pain Questionnaire (MPQ, Dutch version). The VAS is a reliable and valid instrument for the measurement of pain intensity (Carlsson 1983). It is used to measure the momentary pain, the worst pain, the least pain and how much pain was tolerated in the previous 24 h. Scores range from 0 (no pain) to 10 (most intense pain). The MPQ is a reliable and valid tool for measuring the amount of pain in a variety of complaints (Lowe et al. 1999); from the list of adjectives, the total number of words chosen was used in the present study; a maximum of 20 words can be marked in the list. The active range of motion (AROM) was used to reflect physical dysfunction. Scores from both the unaffected hand and the affected hand were measured, and the differences in range of motion from five joints were recorded (range 1–5 points per joint, 5 points for maximal limitation, Oerlemans et al. 1999b). The presence of oedema in the affected limb was measured in comparison with the unaffected hand. The percentage differences in volume were determined after successive immersion of both hands in a tube containing water of approximately 30°C. The amount of displaced water was weighed on-line through a laboratory balance (Sartorius, Breukelen, The Netherlands; accuracy 1 g), based on the method described by Fereidoni et al. (2000).
Skin temperature measurement with videothermography
Skin temperature of both hands was measured with a computer-assisted infrared thermograph (ThermaCAM SC2000, Flir Systems).
The thermal sensitivity of the thermograph is 0.05°C at 30°C, the spectral range is 7.5–13 μm and the built-in digital video is 320×240 pixels (total 76,800 pixels). Data were obtained through a high-speed (50 Hz) analysis and recording system coupled with a desktop PC (ThermaCAM Researcher 2001 HS). Thermograms were stored on a hard disk (14-bit resolution) for further analysis. With an interval of −40°C to 120°C this results in a resolution of 9.8×10−3°C per bit, which fits well in the range of the thermal sensitivity. The thermograph camera produces a matrix of temperature values. These temperature values each represent a pixel in the image measured. The distance between the objective and the hand being measured was set at 68 cm. Thereby the resolution on the hand was 0.8×0.8 mm2. To obtain only those pixels that represented the extremity, the data were filtered by a threshold. On average one extremity is represented by 23,540 pixels (approximately 32.5% of total pixels recorded; Table 2). For further analyses a frequency table was calculated; the classes consist of temperatures with an interval of 0.1°C. Because the range of temperature is different between both hands, the maximum temperature is defined as the highest temperature in the involved hand, whereas the minimum temperature is defined as the lowest temperature in the uninvolved hand. The temperatures that do not occur in a hand are given a class value of zero. In this way both the frequency tables represent the same range of temperature. Both hands were recorded in a predefined position with the aid of a Plexiglas curved frame with positioning points between digit 1 and digit 2, and between digit 3 and digit 4. Plexiglas was used because of the high resistance to conduct heat and the inability to conduct infrared. Furthermore, the curved frame was suspended by a box of the same material to minimise the influence of airflow. The emissive factor of the skin was predefined to be 0.98.
Measurement with tympanic thermometer
Skin temperature of both hands was measured by a tympanic thermometer (M3000A, First Temp Genius). The thermal sensitivity of the thermometer is 0.05°C at 30°C. Five measuring points on both extremities were marked with a predefined matrix, which was based on the method described by Oerlemans et al. (1999a).
Calculation methods for thermography
Method 1: asymmetry factor
This method determines the asymmetry factor (correlation) between the two calculated frequency tables derived from the images, with the following equation:
where ρ is the asymmetry factor (0<ρ<1), L i represents class values (temperature) left extremity (number of pixels), R i represents class values (temperature) right extremity (number of pixels), i is the total number of classes and N is the total number of pixels that represent an extremity.
Method 2: ratio
This method calculates a weight factor for each class by means of the product of each class with this frequency. The product is then summed from the first to the last class.
This method was performed for the data of both extremities. The values of the healthy side and the CRPS1 side were then divided. A cold CRPS1 will be represented by a ratio <1 and a warm CRPS1 by a ratio >1.
Method 3: mean difference in temperature
The mean temperature recorded by the videothermograph of each extremity was calculated. The absolute difference was determined by subtracting the mean temperature of the uninvolved extremity from the mean temperature of the involved extremity.
Calculation methods for the tympanic thermometer
The mean of five standard points measured on each extremity was calculated. Thereafter the difference between the means was determined.
According to Oerlemans et al. (1999a), impairment level sumscores from 1–10 were given for the increase of mean surface temperatures.
Reproducibility
The following observations in the development of standardised thermographs were performed. From five healthy controls during 5 days, thermographs of both hands were obtained once a day under standardised conditions. One further measurement was made 6 weeks later. Room temperature was maintained between 22 and 24°C. Prior to investigations no cold or hot drinks were taken. Subjects were kept in the observation room for at least 15 min before temperature was measured. Prior to measurements, both hands were positioned on a curved Plexiglas frame, which was fixed in an open tray of the same material. The fingers were positioned divergent in order to detect temperatures in between them. Asymmetry calculations on the distribution of surface temperatures were made and the deviation within a series of five consecutive measurements in 1 week and with an interval of 6 weeks was determined.
Statistical analysis
For comparison of non-parametric data between CRPS1 patients and healthy controls, the Mann-Whitney U and Spearman tests were used. Data are given as means (SD) and medians. P values of <0.05 were considered as statistically significant. The reproducibility is calculated by intraclass correlation (two-way mixed absolute agreement), P<0.05 was considered as statistically significant. The resolving capacity of the different methods were analysed with the aid of a receiver-operating curve (ROC).
Results
Pain questionnaires, mobility and oedema, and sickness impact profile
The individual patient scores of pain, expressed by both VAS and MPQ, are presented in Table 1. The set of data to indicate the severity of the disease in each patient was completed by the AROM score as a measure of physical dysfunction, the hand volume difference, as indication for inflammation-related oedema and the difference in the mean surface skin temperature of the hand (Table 1)
Reproducibility of temperature measurements in healthy controls
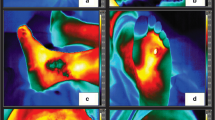
In order to determine the asymmetry factor between a single measurement of the palmar sites of both hands, videothermographs were obtained under standardised conditions as described in Methods. In Fig. 1 examples of a healthy control (upper panels) and a CRPS1 patient (lower panels) are given. Each pixel indicated by a chosen colour depicts the corresponding temperature. Thereafter the distribution of all surface temperatures found in the restricted area of both hands was plotted as shown in the corresponding histograms. Further analysis of this set of data is shown in an asymmetry (correlation) curve (coloured regression plots right panels of Fig. 1). Repeated measurements of five healthy controls, which were obtained during 5 consecutive days (reliability) revealed an intraclass correlation of 0.78 (P<0.02) for the asymmetry factor. An intraclass correlation of 0.86 (P<0.01) was obtained with an interval of 6 weeks for the determination of the asymmetry factor (stability).
Left panels Representative example of a single measurement of skin temperatures by a computer-assisted infrared thermograph in a healthy volunteer (upper sections) and a complex regional pain syndrome type 1 (CRPS1) patient (lower sections). In these thermographs each pixel indicated by a designated colour depicts the corresponding temperature; the distribution of all surface temperatures found in the restricted area of both hands were plotted and are shown in the corresponding histograms (middle panels). Further analysis of this set of data resulted in a correlation curve, in which the number of pixels of each existing temperature at the skin surface of the right hand was plotted against the left hand (coloured regression plots, right panels). In fact these plots are three-dimensional, the number of pixels per temperature in the right hand being the first parameter, the number of pixels per temperature in the left hand being the second parameter, and the corresponding temperature being the third parameter. The calculated asymmetry factor in the healthy volunteer was 0.96, compared with that of 0.37 in the CRPS1 patient due to the right-shifted histogram of the CRPS1 hand
In Table 2, the ratios for the healthy/CRPS1 areas under the temperature histogram as presented in Fig. 1 (middle panels) are given. Furthermore, the asymmetry factors derived from the number of pixels per temperature as plotted in the right panels in Fig. 1 were calculated. In order to evaluate the asymmetry factor as a prognostic value, it is plotted against the results derived from the methods (as presented in the section ‘Observations and measurements’) during the development of the disease. Six patients (patient numbers 2, 4, 7, 9, 12 and 17 in Table 1), who used DMSO (dimethyl sulfoxide) cream, which is a standard therapy in The Netherlands (Zuurmond et al. 1996), were monitored during 12 consecutive weeks. A representative graph of patient no. 9 is displayed in Fig. 2. In order to investigate a relationship between temperature asymmetry and clinical parameters, correlations were calculated from data from all patients as listed in Table 1. In Table 3 the significant correlations are given. The momentary VAS pain correlated with the asymmetry factor (r=0.40 and P=0.04). The duration of the disease correlated negatively with the asymmetry factor (r=−0.45 and P=0.03). Other clinical parameters were not correlated with the asymmetry factor. The number of pixels per paired sample selected from the right and the left hands showed excellent correlation in all 31 subjects (correlation 0.991, P=0.000). Thus, the temperature asymmetry factor is not influenced by differences in the number of pixels recorded from the left extremity and the right extremity.
Representative example of the successful treatment of early stage CRPS1 by topical application of 50% dimethylsulfoxide cream during a follow-up period of 12 weeks (week 0: prior to treatment; week 12: end of treatment). Dashed line and ◆ For direct comparison in this figure, the calculated temperature asymmetry factor, derived from the left- and right-hand plotted number of pixels per corresponding temperature, was expressed as [(1−factor)×100%]. Bold continuous line and ■Total scores (precentages of total) as a sum of visual analogue score pain, McGill Pain Questionnaire, oedema volume and EuroQol. The correlation between these two trend analyses was 0.76 (P<0.01)
Calculated ratios are dependent on the increase of skin temperature in the CRPS1 hand, whereas the asymmetry factors are independent of differences in temperature but reflect distribution of common temperatures.
To determine which method has the highest sensitivity and specificity, the ROC curves were calculated and plotted as presented in Fig. 3. Based on interpretation of the curves, the average video thermography values have no discriminating power. The average tympanic temperature has some, but not significant, power. The ratio method based on video thermography has a useful and significant power. The most discriminating power is, however, reached by the calculation of the asymmetry factor derived from video thermographs.
Graphics of the calculated receiver-operating curve curves comparing different methods. ■ Asymmetry factor calculated as described in Methods and presented in Fig. 1, ▲ ratio of area under histograms as plotted in Fig. 1, ● difference of mean left and mean right temperatures as determined by tympanic thermometer, ◆ difference of mean left and mean right temperatures as determined by videothermography. An area larger then 0.81 is statistically significant. The following intervals indicate guidelines of the accuracy of the tests described in this paper: (sensitivity–specificity) : 1–0.9=excellent, 0.9–0.8=good, 0.8–0.7=fair, 0.7–0.6=poor and 0.6–0.5=fail
Discussion
In 1998 Jones published a reappraisal of infrared thermal imaging of the skin. It was well known that abnormalities such as malignancies, inflammation and infection cause localised increases in temperature, which are shown as hot spots or as asymmetrical patterns in an infrared thermograph. The transfer of military technology to medical use has prompted this reappraisal of infrared thermography in medicine. If thermographs are captured under controlled conditions, they may be interpreted readily to diagnose certain conditions and to monitor the reaction of a patient’s physiology to thermal and other stresses (Jones 1998).
CRPS1 is associated with complex disturbances of the sympathetic nervous system, which also controls microcirculation of the skin. Circulatory skin changes are in turn reflected by altered superficial thermal emission, which can be reliably imaged by thermography. Such thermographic findings often appear before skin or roentgenographic changes become manifest and may therefore be helpful in early diagnosis (Pochaczevsky 1987).
Thermography has been shown to be an effective way to monitor near-surface blood flow in the limbs and to be sensitive to changes accompanying painful conditions. The usefulness of this technique for early detection of CRPS1 has been demonstrated (Karstetter and Sherman 1991).
Although thermography is still believed to be non-specific, one of the most exciting advantages of infrared videothermography is the readily available display which detects precisely hitherto unrecognised problems that affect a patient’s physiology. At first sight in a number of cases, intensity and the extensiveness of the problem could be judged in comparison with the unaffected hand.
In our setup, the reproducibility of repeated videothermographic measurements proved to be over 95%. The computerised regression of the number of pixels per interval of 0.1°C was easily displayed regardless of whether symmetry existed between both hands or not. To date, the mean areas (mm2) of temperature above basal peak temperature, e.g. in ranges of 0.2°C, have usually been calculated (Koyama et al. 2000).
Under normal conditions the degree of thermal asymmetry between opposite sides of the body is very small. Using computerised telethermography in normal persons, the skin temperature difference between sides of the body was only 0.24 (±0.073)°C. In contrast, in patients with peripheral nerve injury, the temperature of the skin innervated by the damaged nerve deviated by an average of 1.55°C (Baron et al. 2002). Data obtained from 40 matched regions of the body surface of 90 asymptomatic normal individuals showed temperature differences for the leg of 0.27 (±0.021)°C and for the foot 0.38 (±0.033)°C. These values were reproducible in both short- and long-term follow-up measurements over a period of 5 years (Uematsu et al. 1988). In our population of 18 patients, we found a mean temperature difference between both hands, as determined by videothermography, of 1.4 (±1.3)°C (range 0.3–4.7°C).
In a study by Oerlemans et al. (1999a), in healthy volunteers, skin temperature differences between both hands were measured with an infrared tympanic thermometer to provide insight into the relationship between dorsal and palmar temperature differences. Skin temperature of the hand differed with the site where it was measured; differences between sites changed over time. The mean absolute differences in skin temperature between dorsal and palmar aspects of the hands were similar (0.30°C and 0.25°C respectively) and they were comparable with differences found by Uematsu et al. (1988). We found that the temperature differences between involved and univolved hands were significantly higher. This could be due to the disadvantages of contact thermography and infrared tympanic thermometry which are limited by the number of spots which can be monitored following a standardised matrix protocol (Sherman et al. 1996). Both the surface area of a certain skin temperature and the temperature range are not considered using these methods.
The usefulness of thermographs for the evaluation of chronic pain has been investigated earlier. Sherman et al. (1987) described that the stability and symmetry of thermographic patterns over time among both healthy subjects and subjects whose pain remained at the same intensity across several recordings were both high and consistent. They reported thermography to be an excellent tool for monitoring changes in pain related to variations in near-surface blood flow. They found a good relationship between changes in pain intensity and changes in symmetry of heat patterns. Thermography had mixed usefulness in differentiating pain-free from pained subjects reporting knee pain (efficiency 98%), leg pain and back pain (efficiency 56%). In a later study these investigators reported new findings concerning the clinical usefulness of skin temperature patterns for tracking CRPS1 by assessing changes in pain. In chronic CRPS1 patients, thermographs were usually cooler on the most painful side, but the amount of relative coolness was not proportional to pain intensity (Sherman et al. 1994). The authors therefore concluded that videothermography is not an appropriate tool to use alone for single-session diagnosis of CRPS1. In a large population of healthy soldiers it was impossible to predict from any thermographic measurement on the lower limbs which soldiers were most likely to develop lower limb pain as a result of training. On the contrary, soldiers reporting lower limb pain produced abnormal thermographs. It was concluded that, in general, thermographs were of little value for predicting stress-induced lower limb pain (Sherman et al. 1995).
Skin temperature measurements at all fingertips under resting conditions and continuously monitored during controlled modulation of sympathetic activity have been described recently in CRPS1 patients, in patients with painful limbs of other origin and in healthy individuals (Wasner et al. 2002). The results showed only minor skin temperature asymmetries between both limbs under resting conditions in most CRPS1 patients. During controlled thermoregulation, however, temperature differences between both sides increased dynamically in CRPS1 patients. The sensitivity was 32% in resting conditions and increased up to 76% during controlled alteration of sympathetic activity. Although measurements were performed at fingertips only, the authors concluded that skin temperature differences in the distal limbs are capable of reliably distinguishing CRPS1 from other extremity pain syndromes. A comparison between temperature asymmetry and intensity of pain or immobility was not made in the study (Wasner et al. 2002).
In another study, 185 patients who were considered to have CRPS1 were subjected to thermal stress, after which temperature patterns were evaluated and probability of CRPS1 was compared with clinical diagnostic criteria (Gulevich et al. 1997). Based on clinical criteria and an estimated 50% prior probability, the positive predictive value was 90% and the negative predictive value was 94%. Using the asymmetry factor derived from regression curves of temperature-related pixels of left- and right-sided hands, we also found a positive predictive value of 92% (ROC value 0.923).
However, in chronic pain patients who were classified diagnostically based on computerised thermographic examination, temperature asymmetry accurately discriminated between CRPS1 and non-CRPS1 patients at baseline only. Responses to cold challenge did not discriminate between these two groups (Bruehl et al. 1996). This could be influenced by the mostly observed ‘cold’ CRPS1 at later stages of the disease. Considering these observations, thermographic measurements should be performed at normal conditions (baseline), and after thermal stresses and cold challenge. These results should discriminate between ‘warm’ and ‘cold’ CRPS1.
The question remains whether computerised videothermography is a worthwhile tool to monitor patients who are treated by experimentally applied pharmaceuticals. In our study there is a correlation between momentary pain, duration of CRPS1 and temperature asymmetry. From our data it is clear that calculations based on area under the temperature histograms do not adequatly represent progress of the disease. Parameters such as VAS, MPQ, AROM, oedema volume and temperature difference only indicate presence of the disease when following the criteria as described by Bruehl et al. (1999). Therefore, further study is needed in order to correlate the asymmetry factor and other parameters indicating disease.
References
Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G (2002) Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet 359:1655–1660
Birklein F, Kunzel W, Sieweke N (2001a) Despite clinical similarities there are significant differences between acute limb trauma and complex regional pain syndrome I (CRPS I). Pain 93:165–171
Birklein F, Schmelz M, Schifter S, Weber M (2001b) The important role of neuropeptides in complex regional pain syndrome. Neurology 57:2179–2184
Bruehl S, Lubenow TR, Nath H, Ivankovich O (1996) Validation of thermography in the diagnosis of reflex sympathetic dystrophy. Clin J Pain 12:316–325
Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M (1999) External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain 81:147–154
Bruehl S, Harden RN, Galer BS, Saltz S, Bakonja M, Stanton-Hicks M (2002) Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain 95:119–124
Carlsson AM (1983) Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 16:87–101
Fereidoni M, Ahmadiani A, Semnanian S, Javan M (2000) An accurate and simple method for measurement of paw oedema. J Pharmacol Toxicol Methods 43:11–14
Gulevich SJ, Conwell TD, Lane J, Lockwood B, Schwettmann RS, Rosenberg N, Goldman LB (1997) Stress infrared telethermography is useful in the diagnosis of complex regional pain syndrome, type I (formerly reflex sympathetic dystrophy). Clin J Pain 13:50–59
Huygen FJ, de Bruijn AG, Klein J, Zijlstra FJ (2001) Neuroimmune alterations in the complex regional pain syndrome. Eur J Pharmacol 429:101–113
Huygen FJ, de Bruijn AG, de Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ (2002) Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm 11:47–51
Jones BF (1998) A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging 17:1019–1027
Karstetter KW, Sherman RA (1991) Use of thermography for initial detection of early reflex sympathetic dystrophy. J Am Podiatr Med Assoc 81:198–205
Koyama N, Hirata K, Hori K, Dan K, Yokota T (2000) Computer-assisted infrared thermographic study of axon reflex induced by intradermal melittin. Pain 84:133–139
Lowe NK, Walker SN, McCallum RC (1999) Confirming the theoretical structure of the McGill Pain Questionnaire in acute clinical pain. Pain 39:275–279
Matsumura H, Jimbo Y, Watanabe (1996) Haemodynamic changes in early phase reflex sympathetic dystrophy. Scand J Plast Reconstr Surg Hand Surg 30:133–138
Oerlemans HM, Graff MJ, Dijkstra-Hekkink JB, de Boo T, Goris RJ, Oostendorp vRA (1999a) Reliability and normal values for measuring the skin temperature of the hand with an infrared tympanic thermometer: a pilot study. J Hand Ther 12:284–290
Oerlemans HM, Oostendorp RA, de Boo T, Goris RJ (1999b) Pain and reduced mobility in complex regional pain syndrome I: outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain 83:77–83
Pochaczevsky R (1987) Thermography in posttraumatic pain. Am J Sports Med 15:243–250
Raja SN, Grabow TS (2002) Complex regional pain syndrome I (reflex sympathetic dystrophy). Anesthesiology 96:1254–1260
Sandroni P, Low PA, Ferrer T, Opgfer-Gehrking TL, Willner CL, Wilson PR (1998) Complex regional pain syndrome I (CRPS I): prospective study and laboratory evaluation. Clin J Pain 14:282–289
Schwartzman RJ, Popescu A (2002) Reflex sympathetic dystrophy. Curr Rheumatol Rep 4:165–169
Sherman RA, Barja RH, Bruno GM (1987) Thermographic correlates of chronic pain: analysis of 125 patients incorporating evaluations by a blind panel. Arch Phys Med Rehabil 68:273–279
Sherman RA, Karstetter KW, Damiano M, Evans CB (1994) Stability of temperature asymmetries in reflex sympathetic dystrophy over time and changes in pain. Clin J Pain 10:71–77
Sherman RA, Woerman A, Karstetter KW, May H (1995) Prediction and portrayal of repetitive stress-induced lower limb pain disorders among soldiers in basic training using videothermography. Clin J Pain 11:236–241
Sherman RA, Woerman AL, Karstetter KW (1996) Comparative effectiveness of videothermography, contact thermography, and infrared beam thermography for scanning relative skin temperature. J Rehabil Res Dev 33:377–386
Uematsu S (1985) Thermographic imaging of cutaneous sensory segment in patients with peripheral nerve injury. Skin-temperature stability between sides of the body. J Neurosurg 62:716–720
Uematsu S, Edwin DH, Jankel WR, Kozikowski J, Trattner M (1988) Quantification of thermal asymmetry. Part 1: Normal values and reproducibility. J Neurosurg 69:552–555
Van de Beek WJ, Schwartzman RJ, van Nes SI, Delhaas EM, van Hilten JJ (2002) Diagnostic criteria used in studies of reflex sympathetic dystrophy. Neurology 58:522–526
Veldman PH, Reynen HM, Arntz IE, Goris RJ (1993) Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 342:1012–1016
Wasner G, Schattschneider J, Baron R (2002) Skin temperature side differences—a diagnostic tool for CRPS? Pain 98:19–26
Zuurmond WW, Langendijk PN, Bezemer PD, Brink HE, de Lange JJ, van Loenen AC (1996) Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiol Scand 40:364–367
Acknowledgements
This study was financially supported by the Algesiological Research Foundation, Academic Hospital Dijkzigt, Erasmus MC, Rotterdam. The technical assistance of Dirk Stronks and Nevin Rhandani was appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huygen, F.J.P.M., Niehof, S., Klein, J. et al. Computer-assisted skin videothermography is a highly sensitive quality tool in the diagnosis and monitoring of complex regional pain syndrome type I. Eur J Appl Physiol 91, 516–524 (2004). https://doi.org/10.1007/s00421-003-1037-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-003-1037-6