Abstract
Aim
To investigate the exposure-risk relationship for occupational chromium (VI) exposure and lung cancer in order to establish exposure limits.
Methods
We systematically searched for studies reporting on occupational Cr(VI) exposure and cancers of the respiratory tract. To be included, studies needed to provide data for more than one level of occupational Cr(VI) exposure, adequately consider the confounder smoking and be of adequate methodological quality. Because direct genotoxicity was considered the predominant mechanism of carcinogenesis of Cr(VI), linear models were applied in order to fit risk data. Relative risks were calculated based on these linear regression models and then used to estimate excess absolute risks.
Results
Five studies of two cohorts of chromium production workers in Baltimore, Maryland, and Painesville, Ohio, were included. Based on different estimates for the exposure effect, the absolute excess risk was found to be “acceptable” (less than 4 per 10,000 according to the German Committee on Hazardous Substances, “AGS”) at a Cr(VI) concentration of 0.1 μg/m3, and became “intolerable” (more than 4 per 1,000) beyond a Cr(VI) concentration of 1 μg/m3.
Conclusion
Occupational exposure limits for Cr(VI) based on excess absolute risks can be derived from published data identified by a systematic literature review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium (VI) compounds [Cr(VI) compounds] have been classified as human carcinogens by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) and by authorities of most industrialized nations. This classification is based mainly on the results of epidemiological studies linking Cr(VI) to lung cancer. In addition, experimental studies in animals and both in vivo and in vitro mutagenicity and genotoxicity assays support this conclusion, with direct genotoxicity being the dominant carcinogenic principle. Moreover, welding fumes that contain Cr(VI) compounds are considered possibly carcinogenic to humans (IARC 1990). According to a directive of the European Union (First directive on classification, packaging and labelling of dangerous substances; Council Directive 67/548/EEC), zinc chromate and Cr(VI) oxide are classified as human carcinogens (Category 1). All soluble Cr(VI) compounds and the chromates of potassium, sodium, calcium, and strontium are classified as carcinogenic to animals (Category 2). However, no exposure limits have been defined by the EU directive.
In Germany, occupational cancer caused by chromium and its compounds can be recognized and compensated as an occupational disease (No. 1103 of the German list of occupational diseases). However, while there are officially recommended conversion values (exposure equivalents for carcinogenic substances, “EKA” values) that relate Cr(VI) air concentrations to blood and urinary concentrations (Deutsche Forschungsgemeinschaft 2012), workplace limits for occupational exposure to Cr(VI) compounds are lacking. The German Committee on Hazardous Substances (German “Ausschuss für Gefahrstoffe”, AGS) proposed a graduated risk concept for exposure limits, with recommendation for acceptable and tolerable excess risks based on cumulative occupational exposures (assuming a working lifetime of 40 years). According to this recommendation, an excess risk of 4 or less per 10,000 is considered “acceptable” and an excess risk between 4 per 10,000 and 4 per 1,000 is considered “tolerable”. An excess risk exceeding 4 per 1,000 is “not tolerable”. The acceptable risk limit is scheduled to be changed to 4 per 100,000 by 2018 (AGS 2008).
We aimed to determine the exposure-risk relationship for Cr(VI) and cancer of the respiratory tract based on the results of existing epidemiological studies in order to establish “tolerable” and “acceptable” limits for occupational exposure; we followed the “Guide for the quantification of cancer risk figures after exposure to carcinogenic hazardous substances for establishing limit values at the workplace” (AGS 2008). The exposure-risk relationship (risk assessment) we describe may also provide a basis for the REACH procedure (Registration, Evaluation, Authorization, and Restriction of Chemical substances), the European Community regulation on chemicals.
Methods
Systematic literature search
A search strategy was developed based on search terms describing Population-Exposure-Outcome (PEO) criteria. To focus the search to a population of workers, the search terms recommended by Mattioli et al. (2010) were used. The outcome (O) terms refer to “cancer” and its synonyms in order to gather as many relevant studies as possible. We searched PubMed (1947 to December 31, 2010) and applied the following search terms:
(work[tw] OR works*[tw] OR work’*[tw] OR worka*[tw] OR worke*[tw] OR workg*[tw] OR worki*[tw] OR workl*[tw] OR workp*[tw] OR occup* OR employ*) AND (chrome* OR chromate* OR chromium*) AND (cancer OR neoplasm* OR tumour OR tumor OR carcinog*) AND (cohort* OR case-control OR case-referent OR case-cohort OR evaluation OR longitudinal* OR follow* OR mortality OR inciden*).
Only the limit “abstract available” was applied. Acceptable cancer types were restricted to lung and other respiratory cancers. In accordance with the AGS (2008) recommendation, studies reporting lung cancer mortalities were considered to be almost equivalent to lung cancer incidences due to the high lethality of lung cancers.
A second search of Medline was performed in order to identify populations with considerable occupational risk of exposure to chromium. The industrial sectors and occupational features searched closely follow those listed by the US EPA (US Environmental Protection Agency) (1998) and the ATSDR (Agency for Toxic Substances and Disease Registry) (2000) as having a risk of chromium exposure. Moreover, the search was expanded by searching the ISI Web of Science database, with the search terms adapted accordingly.
The titles and abstracts of the studies identified by the electronic database searches were screened independently by two reviewers. Studies that clearly did not fulfil the inclusion criteria were excluded from further consideration at this point. To determine the relevance of the job-specific studies, a subset of 50 randomly selected studies was evaluated by screening titles and abstracts. The same procedure was applied to the search results from ISI Web of Science in order to evaluate if any added benefit was to be gained from searching ISI Web of Science. It had been agreed a priori that if at least one of these randomly selected studies contained relevant data, then all the other studies identified by the respective search would be considered.
Selection of included studies
The full-texts of the remaining articles were then screened by two reviewers; for inclusion the studies needed to report risk estimates for more than one level of cumulative occupational Cr(VI) exposure (according to AGS 2008). An additional inclusion requirement was that the studies needed to have adjusted for or otherwise adequately considered potential confounding by smoking, the most critical confounding factor in studies on respiratory cancers.
Studies eligible for inclusion based on the above criteria were then examined independently by two reviewers that assessed their methodological quality; we used a combination of the criteria described by SIGN (Scottish Intercollegiate Guidelines Network 2008) and CASP (Critical Appraisal Skills Programme of the British NHS Appraisal Tools 2008). The studies were assessed on a three-level scale (++, +, −) according to SIGN (Table 1). Studies were classified to be of low quality (SIGN −) when methodological weaknesses were believed to considerably influence the core results.
Deriving the exposure-risk relationships
High-quality studies (SIGN ++ or +) were then used to determine exposure-risk relationships. Risk estimates and associated Cr(VI) exposures were extracted from the publications. If mean values for the exposure categories were given, these values were used. Otherwise, means were approximated by calculating averages of the minimum and maximum values of the exposure ranges. If CrO3 concentrations were reported, these were converted to Cr(VI) concentrations and expressed in mg/m3-years.
Because Cr(VI) is known to be genotoxic (De Flora et al. 1990), a linear model
was applied to the data, where β is the estimate of exposure effect and SMR0 is the calculated standardized mortality ratio (SMR) for the cohort in the absence of any occupational Cr(VI) exposure. The least square approach was used to fit the linear model (lm, R Development Core Team 2011) to the crude extracted data with Cr(VI)-years as the explanatory (independent) variable and SMR as the response (dependent) variable. The linear regression model was fitted with each data point weighted by person-years to represent the sample sizes contributing to each exposure category. As a sensitivity analysis, the unweighted estimates of exposure effects β were calculated, but these did not substantially differ from the weighted β values. As the SMR0 (y-intercept) tended to diverge from 1, in our main analysis, we calculated relative risks (RR) as follows:
To combine the included studies in a meta-analysis, we calculated an unweighted mean β value from the individual estimates.
Computation of excess absolute risks
In accordance with the AGS (2008) recommendation, the lifetime excess (absolute) risk associated with a defined cumulative occupational exposure to Cr(VI), P excess(x) was estimated by subtracting the background lung cancer risk found in the general population. As the epidemiological studies of Cr(VI) exposure report the cancer risk only for male workers, a male reference population was used. In the case of a linear dose–response relationship, the excess lifetime risk P excess(x) is calculated by multiplying the lifetime risk in the reference population (LRnonexposed) by the excess relative risk due to a given cumulative Cr(VI) exposure:
In our first approach, the cumulative lifetime risks of dying from lung cancer between ages 0 and 74 were obtained from the Globocan project for the European region (Globocan 2008). We varied the Cr(VI) workplace air concentrations between 0.0025 and 50 μg/m3. When we give excess risks for specific Cr(VI) air concentrations, a lifetime working time of 40 years is assumed. Therefore, the excess risk for a given workplace Cr(VI) air concentration k [μg/m3] may be interpreted as the excess risk for the cumulative Cr(VI) air concentration of 40 k [μg/m3- years].
However, problems have been reported with this method of calculating excess risk; for example, there may be a tendency for this formula to overestimate excess risk when it is calculated for age ranges extending beyond 70 years (Goldbohm et al. 2006). Therefore, as a second approach, we compared excess lifetime risks for 1 μg/m3 Cr(VI) workplace air concentration for Germany (Globocan 2008) applying the conditional method up to age 74 and the life-table method up to age 74, 80, or 89. Moreover, we calculated excess lifetime risks for 1 μg/m3 Cr(VI) workplace air concentration based on a 7 % lifetime risk among males of developing (incident) lung cancer in Germany (Robert Koch Institut 2012).
Results
Results of the systematic literature search
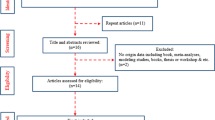
The PubMed search yielded 386 hits of which 237 were excluded on the basis of titles and abstracts. The full-texts of the remaining 131 articles were examined to determine whether the inclusion criteria were met. In particular, the reporting of an exposure-risk relationship and the consideration of smoking were examined. Only 6 studies were found to fulfil our criteria for inclusion (Fig. 1).
Systematic literature search: PRISMA flow diagram (Liberati et al. 2009), basic search in Medline (PubMed)
The search of Medline (via OVID interface) regarding occupational fields with a risk of exposure to Cr(VI) yielded altogether 446 publications. To determine the relevance of these job-specific studies, a subset of 50 randomly selected studies was evaluated by screening titles and abstracts. As no study fulfilled both of the criteria that smoking was considered and that an exposure-risk relationship was reported, the remaining 396 studies were not evaluated.
Searching the database ISI Web of Science yielded 286 hits. After studies already assessed were removed from consideration, 50 articles were randomly selected from the remaining 144 articles. No relevant study was found, and we therefore did not evaluate the remaining 94 articles.
Quality assessment
Six articles were found to provide potentially relevant data for establishing an exposure-risk relationship (Crump et al. 2003; Gibb et al. 2000; Gerin et al. 1993; Park et al. 2004; Park and Stayner 2006; Luippold et al. 2003). These studies were based on the data from three retrospective cohorts. We combined the information concerning each cohort and considered data from one cohort published in separate papers as one data set for the further phases of this evaluation. Only studies on two of the cohorts were of acceptable methodological quality, so that they were included in the derivation of the exposure–response relationship (see Table 2); these were studies on the Baltimore cohort (Gibb et al. 2000; Park et al. 2004; Park and Stayner 2006) and the Painesville cohort (Crump et al. 2003; Luippold et al. 2003). Another study (Gerin et al. 1993) did not sufficiently consider smoking and also lacked a detailed description of exposure measurement methods. It was therefore given a low-quality assessment score (“−”) and not considered for the derivation of exposure-risk relationships.
Description of included studies
First described by Gibb et al. in 2000, the retrospective cohort study of a Baltimore, Maryland, chrome production plant observed a large sample of workers over a long period of time. A strength of this study was the availability of ambient Cr(VI) measurements. Ambient levels of Cr(VI) were monitored throughout the entire study period. Although the details of the monitoring varied somewhat over time, the routine “passive” (not incidence-based) monitoring of airborne Cr(VI) levels was unique to this study. Another advantageous characteristic of this study was that information was available for smoking status from the employee records. Smoking status (yes/no) at the beginning of employment was known for over 90 % of the study subjects and was included in the statistical analysis. Smoking was found to be strongly associated with lung cancer mortality. Cumulative Cr(VI) exposure was significantly associated with lung cancer mortality, even after adjustment for smoking. However, this study also included workers who were employed for a short period of time (less than 90 days). While the authors justify the inclusion of these short-term workers for their contribution to the low cumulative dose range, little is known about the remaining work lives of these employees. These employees may have been exposed to carcinogenic substances at other plants or through other work. Based on data from the Baltimore cohort, an excess lifetime risk of lung cancer resulting from Cr(VI) exposure was analysed by means of Poisson regression models (Park et al. 2004). From a linear relationship, a RR of 2.44 (95 % CI 1.54–3.83) for 1 mg CrO3 m−3-year was estimated. The Baltimore cohort data also served as the basis for model calculations by Park and Stayner (2006) who examined the existence of thresholds. The models were compared by the likelihood method; however, a two-stage model did not improve the prediction. Due to the quality of the exposure assessment, the evaluation of smoking, and the large study size this study received an overall quality score of “+”.
The cohort of chrome production workers evaluated by Crump et al. (2003) and Luippold et al. (2003) was employed in the same Painesville, Ohio, plant already examined by Manusco (1997a, b), but had begun working at a later time (i.e. January 1940). This more recent investigation improved on the Manusco study by considering smoking status. However, data regarding smoking status were available only for 41 % of the workers; the prevalence of smoking in the cohort was high (78 %). The distribution of smoking status across all of the cumulative exposure categories was reported to be comparable, suggesting that the standardized mortality ratios (SMR) for the different exposure categories were not confounded. The exposure measurements for this study were taken from 21 industrial hygiene surveys of ambient Cr(VI) levels and extrapolated to estimate the levels over the entire exposure period (1940–1972). These exposure measurements were not reported to be part of a routine monitoring plan; therefore, they may have been incident-based. Exposure information was then linked to the employees using job-exposure matrices (JEM) to calculate each individual’s cumulative occupational Cr(VI) exposure. The workers included in the study had to have worked for at least 1 year, and workers who were transferred to another chrome plant where no exposure information was available were excluded from the study. The Painesville cohort also received a quality assessment score of “+” due to the suitable exposure assessment, the exclusion of persons transferred to other chrome plants and because confounding from smoking was addressed (although smoking status was only known for a minority of the study population).
Exposure-risk relationship
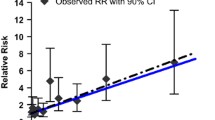
Both Gibb et al. (2000) and Park et al. (2004) analysed data obtained from the Baltimore cohort. However, these studies used different exposure categories to calculate the SMRs (see Fig. 2). Whereas the evaluation by Gibb et al. (2000) was based on four categories where the number of observed deaths was approximately evenly distributed, Park et al. (2004) divided the data into five categories, placing 60 % (N = 72) of the observed deaths into the lowest exposure category and further sub-categorizing the higher exposures. By fitting a linear model to the data provided by Gibb et al. (2000), an estimate of exposure effect (slope) β of 4.52 was obtained when weighting by person-years (Fig. 3); the unweighted β was 4.15. Estimates of the exposure effect β obtained by fitting the linear model to the Park et al. (2004) data were lower (2.82 when weighting by person-years, 2.86 without any weights), but appeared to be better suited to the linear model (see Fig. 4). In contrast to the model’s fit to the Gibb data, the correlation coefficient of the linear model’s fit to the Park data was significant. The relative risk derived from the Park data increased from 1 to 3.82 (2.82 + 1) when Cr(VI) exposure increased from 0 to 1 mg/m3-year.
Cumulative Cr(VI) exposure and standardized mortality rate (SMR) based on the data from Gibb et al. (2000). Linear regression was performed weighted by person-years. SMRs are illustrated with confidence intervals (95 % CI). The size of the symbol is proportional to the number of person-years within the respective categories
Cumulative Cr(VI) exposure and standardized mortality rate (SMR) based on the data from Park et al. (2004). Linear regression was performed weighted by person-years. SMRs are illustrated with confidence intervals (95 % CI). The size of the symbol is proportional to the number of person-years within the respective categories
The Cr(VI) exposure range examined for the Painesville cohort was considerably wider than that of the Baltimore cohort: The highest reported cumulative exposure given for the Painesville cohort (Luippold et al. 2003, Crump et al. 2003) was 29 mg/m3-years. However, fitting a linear model to the SMRs reported by Crump et al. (2003), resulted in a slope of the exposure-risk relationship that was smaller than 1. Furthermore, it is remarkable that the highest SMRs were reached already at relatively low Cr(VI) exposure levels.
Meta-analysis: synthesis of the risk estimates
For aggregating the studies, a mean value was calculated from the individual estimates β derived from Crump et al. (2003) and Park et al. (2004). The results from Gibb et al. (2000) were not considered as the Park et al. (2004) evaluation of the Baltimore cohort provides a more detailed representation of the higher cumulative Cr(VI) exposures levels, potentially reducing the effect of the inclusion of workers with short-term chrome exposure and improving comparability to the exposure categories in the study by Crump et al. (2003). A mean β of 1.75 was obtained ([0.68 + 2.82]/2).
Excess risk according to Cr(VI) exposure: derivation of acceptable and tolerable Cr(VI) concentrations
In order to estimate the excess risk due to Cr(VI) exposure, it was assumed that Cr(VI) exposure lasted from age 20 to age 60 (40 years of cumulative occupational exposure) and that the latency period required from exposure to disease incidence was more than 10 years. In our first approach, excess absolute risks were calculated for the European population using the conditional method. Based on relative risk calculations from the pooled weighted mean of β from the Crump and Park studies (i.e. β = 1.75), a “tolerable” excess risk (limit 4 per 1,000) was associated with a Cr(VI) exposure of about 1 μg/m3 (Table 3).
Lung cancer mortality is slightly lower for the German population compared to the European population. Therefore, the excess absolute risk for 1 μg/m3 workplace air concentration of Cr(VI) reduces from 3.3 per 1,000 for the European population (Table 3) to 2.9 per 1,000 for the German population (Table 4; based on the mortality risk up to age 74 years, conditional method). Additionally, excess risks were calculated by a life-table analysis up to ages 74, 80, or 89 years to allow comparison with Goldbohm et al. (2006) who calculated excess risks at the same ages. When the life-table analysis is applied, the excess absolute risk slightly diminishes from 2.9 per 1,000 to 2.3 per 1,000 for the German population (Table 4; based on mortality risk up to 74 years). However, the excess absolute risk rises to 4.1 per 1,000 when mortality is regarded up to age 89 (life-table method). When the calculation of the excess risk is based on the 7 % German lifetime risk of developing (incident) lung cancer, the excess absolute risk is 4.9 per 1,000 for 1 μg/m3 workplace air concentration of Cr(VI) (conditional method).
The German Committee on Hazardous Substances (AGS 2008) recommends that an excess risk of 4 per 100,000 should be reached between 2013 and 2018. Based on this low excess risk, Cr(VI) concentrations (workplace limits) would fall below 0.01 μg/m3 (Table 3). When the cumulative Cr(VI) exposures are calculated for a work-life until age 60, the corresponding cumulative Cr(VI) concentrations would fall below 0.4 μg/m3-years.
Discussion
An extensive literature search was conducted to identify all studies of an acceptable quality reporting data suitable for calculating the exposure-risk relationship between Cr(VI) exposure and respiratory cancer. Based on risk calculations from the pooled weighted mean of β from the Crump and Park studies (i.e. β = 1.75), a “tolerable” excess risk (limit 4 per 1,000) was associated with a Cr(VI) workplace exposure of about 1 μg/m3.
This estimate of an exposure limit needs to be seen in the context of the limitations of our analysis; these limitations arise from the uncertainties of the exposure measurements performed in the included studies, from deficiencies in the assessment of possible co-exposures at the chromate production plants, and from the lack of complete assessment of confounding factors, especially smoking. All these mean that there is considerable uncertainty in our risk calculations. Another important limitation concerns transferability of our risk estimate to workplaces outside chromate production. In principle, our derived exposure limits are applicable to any occupational Cr(VI) exposure. However, they will of course be most valid where working conditions are similar to the conditions investigated in the included studies, that is, in chromate production. Transferability to other workplaces may be limited by different co-exposures (e.g. welding would often mean co-exposure to nickel, itself associated with lung cancer). Dust particle size may also differ in different workplaces and is clearly another important issue that may limit transferability.
Exclusion of biomonitoring data of urinary chromium
In our systematic review, it had been defined a priori that only studies based on measurements of air Cr(VI) concentrations were to be included. Therefore, a study by Birk et al. (2006) was not included in our derivation of an exposure-risk relationship because of the lack of direct measurements of the exposure to Cr(VI) in the air in that study, instead, presumed exposure to Cr(VI) was calculated from biomonitoring data of urinary chromium. Measurements of urinary chromium, however, suffer from the disadvantage that it is not possible to distinguish between Cr(VI) and Cr(III) exposure. Cr(VI) undergoes reduction after entering the human organism and urinary chromium can be detected only as Cr(III), regardless of whether it originally derived from Cr(VI) or Cr(III). This limitation is acknowledged by Birk and colleagues. Other work underlines the drawbacks of using urinary chromium as a surrogate for occupational Cr(VI) exposure. Bukowski et al. (1991) described that, for example, exercise, consumption of beer, and diabetic status had significant effects on urinary chromium levels. A relationship between Cr(VI) exposure and cancer risk therefore cannot be confidently derived from the data presented by Birk et al. (2006).
Possible reasons for different relative risks derived from the Baltimore and Painesville cohorts
There were remarkable differences in relative risk estimates between the three publications examined (see Figs. 3, 4, 5) with the highest values for the Gibb study and the lowest values for the study by Crump et al. (2003). Comparing the relative risks derived from the studies of the Baltimore cohort (Gibb et al. 2000; Park et al. 2004), the different values of relative risks were likely due to the different exposure categories assigned in the papers. In Gibb et al. (2000) (Fig. 3), the observed deaths were relatively evenly distributed among the exposure categories, resulting in increased SMRs at low levels of Cr(VI) exposure for the cohort. By contrast, Park et al. (2004) assigned a majority of the observed deaths to the lowest exposure category. Additionally, they subdivided the exposures into five categories, with a small number of person-years in the highest exposure category. The broad lowest exposure category resulted in a slower rise of the SMRs with increasing Cr(VI) exposure. An effect on the exposure-risk relationship can be expected when the choice of cut-points differs (Steenland and Deddens 2004).
Cumulative Cr(VI) exposure and standardized mortality rate (SMR) based on the data from Crump et al. (2003). Linear regression was performed weighted by person-years. SMRs are illustrated with confidence intervals (95 % CI). The size of the symbol is proportional to the number of person-years within the respective categories
Comparing the analyses on the Baltimore cohort with those on the Painesville cohort, the following study characteristics might explain the different exposure ranges: Firstly, the Baltimore cohort included short-term employees (having worked fewer than 90 days at the plant), thereby increasing the number of subjects with low cumulative exposures. Secondly, the employment period of the Painesville cohort began a decade earlier than that of the Baltimore cohort (1940 compared to 1950). According to Crump et al. (2003), the working conditions changed over these years causing a decrease in airborne Cr(VI) concentration at the workplace. However, whereas exposure levels differed by a factor greater than 10, the Cr(VI) concentration decreased by a factor less than 10 during the 1940s (estimation from Fig. 1 of Crump et al. (2003)).
Although the quality of the studies included was found to be acceptable (study quality “+”), several factors could still have diminished the accuracy of the SMR estimations for the two cohorts:
-
The estimation of the SMRs, and especially of the confidence intervals, for the two cohorts could have been influenced by the difference in sample sizes (person-years), because the Painesville cohort was approximately a quarter of the size of the Baltimore cohort.
-
Another factor that may have influenced the SMR estimates for the Painesville cohort is that smoking status was known for only 41 % of the cohort. Although Crump et al. (2003) determined that the smokers were evenly distributed among the exposure categories, the smoking status was unknown for a majority of the cohort. Therefore, it is possible that the SMR estimates for the Painesville cohort may still have been confounded to some extent by an uneven distribution of smokers in the exposure categories. Residual confounding resulting from smoking could have also influenced the SMR estimates for both cohorts, because even if smokers were equally distributed throughout the exposure categories, neither study had information regarding the intensity and duration of smoking (i.e. pack-years). If the smokers in one exposure category happened to smoke more or longer than the smoking workers in another category, the increased risk for respiratory cancers among the heavier smokers would have inflated the SMR estimate for their exposure category.
-
In addition to residual confounding due to smoking, the inclusion of very short-term employees (working less than 90 days) in the Baltimore cohort could also have led to an increased SMR in the low exposure range for this cohort. It seems that the study authors had no information regarding the remaining work histories for these employees. It is therefore possible that other occupational exposures in these employees may have been relevant confounding factors. If some of these employees went on to work in other chromium production plants or were occupationally exposed to other carcinogens, for example, welding by-products or asbestos, this could have led to a misleading increase in the SMR for the lowest Baltimore exposure category.
Potential nonlinearity of the dose–response relationship
Our relative risk was derived from the exposure-risk relationship that was based on SMR risk measures. Intercepts greater than one were obtained from our linear model calculations indicating that the reported SMRs were probably biased, due to an increased risk in the cohorts compared to their reference populations for reasons other than Cr(VI) exposure. As a sensitivity analysis, we divided the slope β by SMR0 to approach a “multiplicative” interaction with unknown “other exposures” (which are thought to have led to an elevated SMR0) and to obtain a “corrected” SMR (or relative risk) of 1 in the absence of Cr(VI). However, confounding at different Cr(VI) exposures may still differ, and this approach is only a crude estimation which may not reflect the true relative risk. The resulting β’ values were lower than the β values, since they were divided by the intercept which was always greater than 1. With this sensitivity analysis, we calculated somewhat lower excess risks. Other approaches to adjust for bias in SMRs have been proposed (Jones and Swerdlow 1998; Morfeld and McCunney 2010), but application of these complicated methods would from our point of view unnecessarily complicate risk communication.
In accordance with the AGS (2008) recommendation, we assumed a linear exposure-risk relationship between cumulative Cr(VI) exposure and the relative risk estimates. However, the exposure-risk relationship may be nonlinear. The data of the Painesville cohort (Crump et al. 2003) indicate a linear rise for the lower cumulative Cr(VI) concentrations examined, but at higher cumulative concentrations (that were not evaluated in the Baltimore cohort) the risk appears to level off. Under certain assumptions a plateau at high exposures may be biologically plausible. Steenland and Deddens (2004) postulated that saturation of a metabolic pathway at high concentrations may have such an effect. Another reason for the occurrence of a plateau may be the depletion of susceptible individuals at high exposures (Steenland and Deddens 2004). Moreover, Steenland and Deddens (2004) discuss that also mismeasurements and bias can result in a plateau. Healthy worker and/or survivor effects may account for this bias, with only more “resilient” persons remaining employed long enough to reach the higher cumulative exposition levels. Workers experiencing, for example, an ulceration of the nasal septum, chronic bronchitis or contact dermatitis due to their occupational Cr(VI) exposures may have ceased working before reaching higher cumulative exposure levels. Consequently, as the plateau at high exposures in the Painesville cohort might be explained by selection bias, there is no sufficient reason to not use the linear model.
Consequences of relative risk derivation from extracted categorized SMRs
All of our calculations of risk were performed based on linear regressions of the SMR estimates for various Cr(VI) exposure levels published for two retrospective cohorts. Unfortunately, only categorized exposure data were available from the published references. As mentioned above, we observed that the slope (β) of the linear regression was affected by the categorization of the exposure data selected by the original study authors (see Fig. 2). Moreover, the goodness of the model fit and the corresponding ability of the model to accurately predict risk may have been influenced by the choice of categorization. Concerning the uncertainty associated with calculating the slope of the exposure-risk relationships (β), an aggregation of the exposure-risk relationships from the different studies is problematic. We calculated the mean of the βs without any weighting. Regrettably, an unbiased and accurate aggregation of the data would only have been possible with the individual subject data. Nevertheless, the unweighted mean of the estimated βs serves as an estimate within the range of the βs obtained from the published studies.
Calculation of excess lifetime risk: comparison of the conditional and life-table methods
The excess risk was slightly higher when it was calculated by the conditional method compared to the life-table method. An overestimation of excess risk based on the conditional method is also discussed by Goldbohm et al. (2006). This overestimation results from the fact that the conditional calculations do not adjust for population dynamics. It can be expected that the size of a cohort decreases with increasing age. Therefore, the life-table method should in principle provide a better representation of true excess risks. However, the life-table method is more complicated, and it is more difficult to reproduce the results of the life-table analysis. In the light of relatively small differences between the results of the conditional and life-table analysis, we regard the conditional method as preferable.
Excess risk derived in this report and excess risk calculated in other studies
Based on an exposure level of 1 μg/m3 Cr(VI), as a “best estimate”, an excess risk between 2.3 and 4.9 per 1,000 was calculated (applying different reference populations, different age ranges, and different calculation methods with a β value of 1.75). The excess lifetime risk (up to age 74) calculated in this report lies within the range of excess risks given by Goldbohm et al. (2006). In contrast to our report, where the excess risks were calculated using a mean of the Crump et al. (2003) and the Park et al.’s. (2004) results for the Painesville and Baltimore cohorts, respectively, Goldbohm et al. (2006) provided individual excess risks for both cohorts (using the data from Gibb et al. (2000) for the Baltimore cohort).
The SCOEL (2004) estimation of excess risk was based on a meta-analysis by Steenland et al. (1996). These authors calculated a relative risk of 2.78 for Cr(VI) exposure summarizing 10 studies (see Tab. VIII in Steenland et al. 1996). None of these studies was eligible for inclusion in our review: eight of the studies did not fulfil the criterion that bias from smoking had to be adequately considered. The other two studies (Alderson et al. 1981; Takahashi and Okubo 1990) did not provide any quantitative Cr(VI) exposure information. In order to calculate an estimate for exposure (β), a cumulative Cr(VI) exposure of 7.5 mg/m3-years was assumed for all 10 studies. This assumption appears to account for the relatively small excess risk calculated by SCOEL.
According to the AGS (2008) recommendation, the lifetime risk should be reduced to 4 per 100,000 by 2018. Based on our calculations, such a lifetime risk would be associated with Cr(VI) concentrations below 0.01 μg/m3 resulting in a cumulative (40 years) occupational Cr(VI) exposure below 0.4 μg/m3-years. According to the WHO (2000) guidelines, a limit of 0.00025 μg/m3 is proposed which is even lower than the concentrations derived in this report. However, the lower value corresponds to the higher excess (lifetime) risks described by the WHO.
Excess risks were calculated based on either the background cancer risk by the conditional method or the life-table method, both using a reference population with minimal Cr(VI) exposure. However, this population includes non-smokers as well as smokers. As the lung cancer risk of non-smokers is lower than that of smokers, the excess risk based on a non-smoking population (or one smoking less) would be lower compared to the risk calculated here.
We would like to illustrate this point with a hypothetical example: Based on data from a multicenter case–control study (Crispo et al. 2004), the cumulative risk of lung cancer among men by age 75 (“lifetime risk”) is 14.3 % in male smokers and 0.6 % in male non-smokers. Assuming a population made up exclusively of male smokers, 10.0 per 1,000 excess lung cancer cases could be expected at a workplace Cr(VI) concentration of 1 μg/m3 based on a relative risk of 1.07 as found in our systematic review (0.07 × 143 per 1,000). In contrast, in a population made up of male non-smokers only 0.4 per 1,000 excess lung cancer cases could be expected at a workplace Cr(VI) concentration of 1 μg/m3. This example illustrates the “context dependency” of absolute excess risks: To ensure the same safety level at the workplace in terms of absolute excess risks, considerably higher workplace limits would be allowed in otherwise “healthier” populations (i.e. with lower non-occupational cancer risks). This is ethically problematic.
The absolute excess risk estimations based on the life-table method depend on the proportion of smokers in a given population. Moreover, the excess risk estimations depend on the life expectancy of the underlying population and the age limit included in the life-table analysis.
Workplace limits for Cr(VI) compounds
A number of workplace exposure limits for Cr(VI) have been recommended by different organizations. We calculated the excess risk of respiratory cancer mortality associated with these levels of long-term occupational exposure, based on our exposure-risk relationship calculations, and compared these threshold-based excess risks. According to our analysis, the NIOSH workplace limit (1 μg/m3) is (borderline) tolerable, whereas the ACGIH generic limits for water-soluble Cr(VI) (50 μg/m3) as well as the ACGIH generic limits (10 μg/m3) for water-insoluble Cr(VI) are not tolerable according to the AGS recommendations. According to our systematic review, the current OSHA (US Occupational Safety and Health Administration) PEL (permissible exposure limit) of 5 μg/m3 CrO3, equivalent to about 2.5 μg/m3 Cr(VI), would result in an excess lifetime lung cancer risk of 8.4 per 1,000 (Table 3). According to the quantitative risk assessment conducted by Park et al. (2004), the excess lifetime risk of lung cancer mortality based on the OSHA PEL would even be 14 to 16 per 1,000 (Park et al. 2004, their Table V). Taking the AGS (2008) recommendation into consideration, the OSHA PEL therefore would not guarantee adequate health protection at the workplace.
Specification of Cr(VI) exposure (soluble and insoluble Cr(VI) compounds)
All the above calculations of relative risk and excess risk refer to Cr(VI) compounds in general, irrespective of their solubility in water. However, some of the Cr(VI) compounds are water-soluble (chromic acid, chromium trioxide, chromates and dichromates of sodium, potassium, ammonium, lithium, caesium, and rubidium) and others are rather insoluble (chromates of zinc, calcium, lead, barium, strontium, and sintered chromium trioxides). Welding fumes belong to the group of insoluble Cr(VI) compounds. ACGIH (2004) recommends the differentiation of soluble and insoluble Cr(VI) for the risk estimation because the carcinogenic potential of insoluble Cr(VI) compounds was higher than that of soluble ones (IARC 1990). However, the analysis of the Painesville and Baltimore cohorts allows no differentiation between soluble and insoluble Cr(VI) compounds. Therefore, a differentiated evaluation of risk due to soluble and insoluble Cr(VI) compounds remains a task for future research.
Conclusion
Occupational exposure limits for Cr(VI) based on excess absolute risks can be derived from published data identified by a systematic literature review. The methodologically acceptable studies found through our literature search had different strengths and weaknesses. Therefore, the tolerable limit of about 1 μg/m3 workplace Cr(VI) concentration is based on the synthesis of data from both study cohorts (Baltimore and Painesville cohorts). It should be emphasized that extending our calculations of excess risk to more advanced ages would result in lower exposure limits. This may not be unreasonable, considering increasing life expectancy and the long latency period for respiratory cancers. Moreover, excess absolute risks should be calculated for lifetime cancer incidence, not for lifetime cancer mortality. Finally, we would like to point to the “context dependency” of excess absolute risks: With decreasing background risks in a population (due to, for example, decreasing smoking prevalence), the excess absolute risks for a given Cr(VI) air concentration would also decrease.
References
ACGIH (American Conference of Industrial Hygienists) (2004) Chromium and inorganic compounds; Chromium(VI), water-soluble fume
AGS (2008) Announcement 910 June 2008. Announcement on hazardous substances, risk figures and exposure-risk relationships in activities involving carcinogenic hazardous substances. URL http://www.baua.de/en/Topics-from-A-to-Z/Hazardous-Substances/TRGS/pdf/Announcement-910.pdf?__blob=publicationFile&v=2. Accessed 14 Aug 2011
Alderson M, Rattan N, Bidstrup L (1981) Health of workmen in the chromate-producing industry in Britain. Br J Ind Med 38:117–124
ATSDR (Agency for Toxic Substances & Diseases Registry) (2000) URL http://www.atsdr.cdc.gov. Accessed 14 Apr 2011
Birk T, Mundt KA, Dell LD, Luippold RS, Miksche L, Steinmann-Steiner-Haldenstaett W, Mundt DJ (2006) Lung cancer mortality in the German chromate industry, 1958–1998. J Occup Environ Med 48:426–433
Bukowski JA, Goldstein MD, Johnson BB (1991) Biological markers in chromium exposure assessment confounding variables. Arch Environ Health 46:230–236
CASP (Critical Appraisal Skills Programme of the British NHS Appraisal Tools) (2008) URL http://www.phru.nhs.uk/Pages/PHD/CASP.htm. Accessed 14 Apr 2011)
Crispo A, Brennan P, Jöckel KH, Schaffrath-Rosario A, Wichmann HE, Nyberg F, Simonato L, Merletti F, Forastiere F, Boffetta P, Darby S (2004) The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br J Cancer 91:1280–1286
Crump C, Crump K, Hack E, Luippold R, Mundt K, Liebig E, Panko J, Paustenbach D, Proctor D (2003) Dose-response and risk assessment of airborne hexavalent chromium and lung cancer mortality. Risk Anal 23:1147–1163
De Flora S, Bagnasco M, Serra D, Zanacchi P (1990) Genotoxicity of chromium compounds. A review. Mutat Res 238:99–172
Deutsche Forschungsgemeinschaft (DFG) (ed) (2012) MAK- und BAT-Werte-Liste 2012: maximale Arbeitsplatzkonzentrationen und Biologische Arbeitsstofftoleranzwerte, vol 48. Wiley-VCH, Weinheim
Gerin M, Fletcher AC, Gray C, Winkelmann R, Boffetta P, Simonato L (1993) Development and use of a welding process exposure matrix in a historical prospective study of lung cancer risk in European welders. Int J Epidemiol 22(Suppl 2):S22–S28
Gibb HJ, Lees PS, Pinsky PF, Rooney BC (2000) Lung cancer among workers in chromium chemical production. Am J Ind Med 38(2):115–126
Globocan (2008) Cancer incidence, mortality and prevalence worldwide in 2008. URL http://globocan.iarc.fr/. Accessed 19 Mar 2012
Goldbohm RA, Thielemans LJP, Heederik D, Rubingh CM, Dekkers S, Willems MI, Kroese ED (2006) Risk estimation for carcinogens based on epidemiological data: a structured approach, illustrated by an example on chromium. Reg Tox Pharm 44:294–310
International Agency on Research on Cancer (IARC) (1990) IARC monographs on the evaluation of carcinogenic risks to humans, chromium, nickel and welding, 49, Lyon
Jones ME, Swerdlow AJ (1998) Bias in the standardized mortality ratio when using general population rates to estimate expected number of deaths. Am J Epidemiol 148:1012–1017
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS 6:1–28
Luippold RS, Mundt KA, Austin RP, Liebig E, Crump C, Crump K, Proctor D (2003) Lung cancer mortality among chromate production workers. Occup Environm Med 60:451–457
Manusco TF (1997a) Chromium as an industrial carcinogen: part I. Am J Ind Med 31:129–139
Manusco TF (1997b) Chromium as an industrial carcinogen: part II. Chromium in human tissues. Am J Ind Med 31:140–147
Mattioli S, Zanardi F, Baldasseroni A, Schaafsma F, Cooke RMT, Mancini G, Fierro M, Santangelo C, Farioli A, Fucksia S, Curti S, Violante FS, Verbeek J (2010) Search strings for the study of putative occupational determinants of disease. Occup Environ Med 67:436–443
Morfeld P, McCunney RJ (2010) Bayesian bias adjustment of the lung cancer SMR in a cohort of German carbon black production workers. J Occup Med Tox 5:23
SIGN (Scottish Intercollegiate Guidelines Network) (2008) URL http://www.sign.ac.uk/methodology/checklists.html. Accessed 14 Apr 2011
Park RM, Stayner LT (2006) A search for thresholds and other nonlinearities in the relationship between hexavalent chromium and lung cancer. Risk Anal 26:79–88
Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS (2004) Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal 24:1099–1108
Robert Koch Institut (RKI) (2012) Krebs in Deutschland 2007/2008. Gesundheitsberichterstattung des Bundes. RKI 2012. http://edoc.rki.de/documents/rki_fv/re2vZ21t28Ir8Y/PDF/22aJOdYnmXV0I.pdf. Accessed 19 Mar 2012
SCOEL (2004) Recommendation from the scientific committee on occupational exposure limits: risk assessment for hexavalent chromium, SCOEL/SUM/86, December 2004
Steenland K, Deddens JA (2004) A practical guide to dose-response analyses and risk assessment in occupational epidemiology. Epidemiology 15:63–70
Steenland K, Loomis D, Shy S, Simonsen N (1996) Review of occupational lung carcinogens. Am J Ind Med 29:317–324
Takahashi K, Okubo T (1990) A prospective study of chromium plating workers in Japan. Arch Environm Health 45:107–111
R Development Core Team (2011) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. URL http://www.r-project.org/
US EPA (1998) Toxicological Review of hexavalent chromium (CAS No. 18540-29-9) in support of summary information on the Integrated Risk Information System (IRIS). US Environmental Protection Agency. URL http://www.epa.gov/iris/toxreviews/0144tr.pdf. Accessed 9 Oct 2012
WHO (2000) Air quality guidelines, 2nd edn. WHO Regional Office for Europe, Copenhagen
Acknowledgments
The authors thank the German Federal Institute for Occupational Safety and Health for funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seidler, A., Jähnichen, S., Hegewald, J. et al. Systematic review and quantification of respiratory cancer risk for occupational exposure to hexavalent chromium. Int Arch Occup Environ Health 86, 943–955 (2013). https://doi.org/10.1007/s00420-012-0822-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-012-0822-0