Abstract
The perichromatin region is an elusive zone of the cell nucleus located at the periphery of the condensed chromatin areas. This region is visible at the electron microscope level under special staining treatments, otherwise it is merged with the border of condensed chromatin. In this 200 nm-thick area, several fundamental cell processes take place, such as replication, DNA repair and transcription. In addition, RNA processing occurs in the perichromatin region, including 5′-capping and 3′-polyadenylation as well as splicing. Recently, it has become clear that also some epigenetics modifications take place there, such as methylation of DNA and RNA on cytosine and adenosine. In summary, this thin interface between chromatin and the interchromatinic space represents the zone where the majority of the functions of DNA in interphase occur, in a place where there is no steric hindrance of condensed chromatin, the products can easily move away toward their target and the enzymes can freely dock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A brief history of the perichromatin region
The observation of organelles, structures and visible parts of the cell or of the tissue constitute an obvious way of relating structure to function. Many different cellular functions have been attributed to definite structures over the years and following the progress of microscopy. The cell nucleus, mitochondria, centrioles have been described first by light then by electron microscopy (EM).
There is, however, an elusive zone (or region) of the cell nucleus which can be roughly defined as immaterial. Strictly speaking, the perichromatin region (PR) is visible under special staining treatments, otherwise it is merged with the border of condensed chromatin.
It must be underlined that the immaterial PR overlaps with a long-standing morphological and functional feature of the cell nucleus: the definition of euchromatin in relation to heterochromatin. Morphologically, as defined in classic Feulgen-stained nuclei, euchromatin is loose, non-condensed and potentially active chromatin. At EM, however, selectively stained DNA in thin section reveals that loose chromatin is mainly confined at the periphery of condensed chromatin areas, on the surface of the dense clumps. Consequently, euchromatin lies within the PR. The latter is the region where euchromatin works but also where functions different from those pertaining to euchromatin (but correlated) are carried out (Figs 1, 2).
a Schematic drawing of condensed chromatin areas (Ch) and the nuclear envelope. The area between chromatin and the purple line corresponds roughly to the PR. Np, nuclear pore. b Rat liver, osmium ammine staining for DNA. The line marking the PR is roughly 100 nm from the limit of stained DNA. c Rat liver, EDTA staining for RNPs. Chromatin is bleached and RNP-containing structures are present at its border in the PR. d Rat liver, terbium staining for RNA. Although less contrasted than in C, the structures present at the chromatin periphery are perichromatin fibres. Bar 200 nm

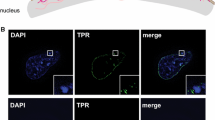
Reprinted from Trentani et al. Eur J Histochem 47:195–200, 2003, with permission. Bar = 30 nm, b Rat thymocyte, anti-5mC labelling. Most of the labelling occurs at the surface of chromatin, while the more distant few grains most probably label methylated RNA. c Hep-2 cell. After a short pulse of BrdU, beginning of replication is indicated by the labelling (green dots) present at the periphery of the chromatin. d Hep-2 cell. Labelling with an anti-DNA/RNA hybrid. The gold grains localized at the sites of transcription where the hybrids are present. Bar 200 nm
a Hep-2 cell. The large gold grain labels BrU incorporated into nascent RNA, while the small ones indicate the m3G cap of RNA.
The first description of the PR comes from the pioneering work of Bernhard (1969) and Monneron and Bernhard (1969). In these two papers, a new staining technique was proposed as suitable for pinpointing nuclear and nucleolar components generally constituted by ribonucleoproteins (RNPs). As stated by Bernhard, the EDTA regressive technique is preferential for nuclear/nucleolar RNPs but not for RNA alone. Moreover, positive material found in the cytoplasm should be tested by other techniques for RNPs. In the nucleus, the regressive staining clearly showed the presence of structures yet unknown at the time, like the perichromatin fibrils (PF) and the coiled bodies (now called Cajal bodies), but, in addition, confirmed the presence of some already described structures such as perichromatin granules (PG) or interchromatin granules (IG) also called nuclear speckles (Bernhard and Granboulan 1963; Swift 1959; Ris 1961; Watson 1962).
In their seminal paper, Monneron and Bernhard stated that PF are “a small rim of predominantly fibrillar structures which keeps its contrast at the periphery of many, but not all, areas of unstained chromatin”. Also, transcription was found located at the periphery of condensed chromatin (Allfrey 1966; Gall and Callan 1962; Monneron and Bernhard 1969).
The need for an open area
In the literature, different theoretical models explaining the way chromatin can regulate its function (Hancock 2012; Cortini and Filion 2018), or help different factors to intervene in its regulation, are present. Basically, the crowding theory recognizes a high impact to the proximity of different chromatin conformation states, ions, enzymes and various factors in the overall functioning of chromatin. Since the functions carried out by chromatin are essentially three, as we will describe below, the region where all these factors can interact is the key to understand its functioning. Chromatin folding reduces the available space for factors to circulate, and unfolding, on the contrary, can actually lead to a more free circulation of the factors necessary for functioning. As a result, a sort of zona franca in which unfolding, remodelling and circulation of the main actors can occur is mandatory.
In addition, a high concentration of calcium/magnesium ions at the periphery of chromatin has been detected by electron spectroscopic imaging (Masiello et al. in preparation) and these ions could participate in the maintenance of chromatin folding/unfolding processes.
The 200 nm rim which constitutes the PR becomes the hot spot: DNA, nascent RNA, regulatory RNAs, RNA Polymerase II, DNA polymerases, methyltransferases have been detected there, i.e., in the region where genes can “expand” and enzymes work.
Proof of the existence
Early autoradiographic studies have been described above. Moreover, the use of cold precursors both for DNA (Raska et al. 1991) and RNA (Cmarko et al. 1999; Trentani et al. 2003) resulted in labelling the peripheral area of the condensed chromatin. However, a very good proof of the existence of PR came from a drastic change in the cell/tissue preparation technique. Puvion and Bernhard (1975) proved that after cryoultramicrotomy of fixed/frozen sections, the rim corresponding to PR was clearly visible when stained with the EDTA technique The same was found after tissues were high-pressure frozen and cryosubstituted in the absence of any chemical agent (von Schack et al 1991). Finally, the last doubt that PR might be a procedural artifact involving the presence of embedding resin was ruled out by Bouquet-Marquis et al. (2006). The authors observed the presence of PR in ultrathin frozen hydrated sections, i.e., in the absence of any embedding material with the exception of vitrified ice.
Taken all together, these data confirm that PR, although elusive in conventionally stained sections, is present and can be rendered visible.
Replication
One of the first processes studied in the perichromatin region is represented by DNA replication. DNA replication is one of the most important processes that ensures the vitality in prokaryotic and eukaryotic cells. In fact, errors during this fundamental mechanism may lead to deleterious consequence for the organism. For this reason, it is deeply investigated. Regarding the eukaryotic cells, one of the biggest deals was to understand where in the nucleus DNA replication takes place. After several years of research and different demonstrations with multiple methodologies, researchers have associated this important role to the perichromatin region.
Among different experimental observations of DNA synthesis at the periphery of condensed chromatin areas, one of the most relevant was performed by means of EM autoradiography (Fakan and Hankok 1974). In this study, early-replicated DNA was marked by the incorporation of 3 H-thymidine. After few seconds of incorporation, the autoradiographic grains were found in the nucleus but in higher percentage at the border zone between the condensed chromatin and the interchromatin space. This initial remark was lately confirmed with other methodologies, for example, by means of electron microscopy, while studying the localization of Cyclin A, an enzyme strongly related with replication (Sobczak-Thepot et al. 1993). After pulse labelling with 5-bromo 2′- deoxyuridine (BrdU), a cold marker of active DNA replication, HeLa cells were labelled with a gold-conjugate anti-Cyclin A antibody. The result of the work was a massive presence of Cyclin A and BrdU mainly near the periphery of condensed chromatin. Despite the purpose of this experiment being not totally focused in the understanding of DNA replication specific site, it helped to highlight the biological function of the PR.
Nascent DNA movement was intensely explored by several microscopic technologies either with light microscopy observations (De Boni 1994; Manders et al. 1996) or other ultrastructural studies (Hozak et al. 1993; Mazzotti et al. 1998). Despite the relevance of all these works, no one particularly recognizes precise structural domains inside the nucleus specifically assignable to replication sites. A decisive demonstration was given by a high-resolution in situ analysis of newly replicated DNA kinetics (Jaunin et al. 2000). In this work, cells were incubated with halogenated nucleotides: BrdU, 5-chloro 2′-deoxyuridine (CldU) and 5-iodo 2′-deoxyuridine (IdU). Moreover, as further validation, sites of replication were associated to the presence of gold-labelled DNA-polymerase α. The distribution of the three thymidine analogues, was observed at the electron microscope in incubated cells. In the case of BrdU, the incubation was performed at different times (e.g., 2 min, 5 min, 30 min). After few minutes of BrdU incorporation the signal was present mostly at the periphery of dense chromatin, with very few labelling in the condensed area; after more time the signal was shifted to the dense area meanwhile the periphery was less immuno-labelled. Other cells were, alternatively, administrated IdU for 20 min before a chase period and subsequent incubation with CldU for 5 min. The CldU labelling was demonstrated to be localized within the perichromatin areas, whereas IdU, incubated for longer time, was present especially inside the condensed chromatin.
At last, the labelling of DNA polymerase was found to colocalize with BrdU signal especially in dispersed chromatin areas and at the border of condensed chromatin. All these results gave a strong importance to the perichromatin region: being newly nascent DNA there, together with the most important enzyme for the synthesis, it was manifested that the sites of replication should correspond to this area.
Further on, other advanced approaches were utilized, particularly to obtain a topography of the nuclear DNA and its distribution in the nucleus (Rouquette et al. 2009; Markaki et al. 2011).
To our knowledge there is no additional information about replication occurring in the perichromatin region. Therefore, this topic could be still considered an open field to delve into.
DNA repair
The PR is now considered the nuclear sub-compartment where DNA replication and possibly DNA repair take place. During DNA replication errors are corrected by DNA polymerase, while, other kind of mistakes require the employment of machineries, such as mismatch repair system, base excision repair (BER) system or nucleotide excision repair (NER) system, whose compartmentalization appears important to be understood.
In an important paper by Solimando et al. (2009), known to be the only focused on the localization of DNA repair process, it was observed that by irradiating cells with UV, cyclobutane-pyrimidine dimers (CPD) accumulate in the PR and majorly on condensed chromatin areas, indicating the latter as the most enriched of lesions. Despite this, it was observed that by labelling those proteins involved in the repair by the NER (i.e., the DNA damage recognition and repair factors XPA and XPC), there was a huge accumulation of the enzymes in the PR, tenfold more than in the condensed area, revealing that, the assembly of this complex putatively occurs in this specific nuclear sub-compartment, giving a novel and unique insight into the DNA repair process and triggering the attention for further studies.
Transcription
The probably most documented event occurring in the PR is transcription. For many years, numerous studies concerning the nucleus architecture in terms of the spatial distribution of the principal nuclear functions were conducted, especially at high-resolution level by ultrastructural cytochemistry (Niedojadlo et al. 2011). Several of these papers lead first to the identification of the site in which transcription takes place, i.e., the PR, and then to its more and more detailed characterization.
The first approaches were mainly based on short pulse of both radioactive or halogenated RNA precursors (Fakan and van Driel 2007). Fakan and Bernhard (1971) showed that the incubation with 3H-uridine (3HU) allows to label nascent transcripts since the modified nucleotide can be incorporated in newly synthetized RNAs. In particular, if the incubation time is sufficiently short, RNA molecules remain prevalently in the sites of transcription which can be directly localized by electron microscopy autoradiography with the support of a specific staining technique allowing to bleach chromatin domains (Bernhard 1969). Therefore, after 2–5 min labelling, all radioactivity was clearly localized between EDTA-stained RNP structures and the surface of chromatin, i.e., in the PR (Fakan and Bernhard 1971; Fakan et al. 1976). Later on, these pioneering results were confirmed by labelling bromouridine (BrU), another RNA precursor, together with the hybrid DNA–RNA molecule which is formed during transcription (Testillano et al. 1994): this approach differently mapped the transcription sites in the PR (Trentani et al. 2003). However, the nuclear topography of transcription was studied by means of more recent techniques of super-resolution fluorescence microscopy, that are three-dimensional structured illumination microscopy (3D-SIM) and spectral precision distance/position determination microscopy (SPDM), describing the PR indeed as a nuclear compartment (Markaki et al. 2011).
Focusing on the characterization of transcription in the PR, the first strong evidence that PF were the structures containing the rapidly labelled extra-nucleolar RNA, described before, was obtained in cortisone-stimulated rat hepatocytes, showing an increase of the PF number (Nash et al. 1975). Then, the combination of EM autoradiography, immunolabelling and staining methods revealed that the incorporation of BrU as well as the localization of DNA/RNA hybrids occurred properly into selectively stained PF (Biggiogera and Fakan 1998; Biggiogera and Masiello 2017; Trentani et al. 2003). Taken together, these results indicated that perichromatin fibrils could be the ultrastructural appearance of transcripts, becoming consequently the object of further characterization. In fact, these fibrils were later found to be associated with different transcription components. Hence, RNA polymerase II (Pol II) colocalized frequently with BrU on PF, especially after shorter incubation periods, as well as the transcription factor TFIIH, definitively confirming PF as in situ forms of nascent transcripts (Cmarko et al. 1999; Fakan 1994). Moreover, the presence of the splicing factors snRNPs (U1, U2, U4, U5 and U7) or SC-35, which is a non-snRNP component of the spliceosome, revealed splicing as a cotranscriptional event occurring in the PR (Fakan 1986; Spector et al. 1991; Puvion et al. 1984; Fakan et al. 1984, 1986). The assembly of the cotranscriptional spliceosome was described to be dependent on the presence of 7-methylguanosine cap, indirectly suggesting that 5′-end capping is another event involving PR (Gornemann et al. 2005). Nevertheless, capping and splicing are not the only processes which PF undergo in the PR: in fact, poly(A)-polymerase was located together with BrU on PF, showing PR not only as site of mRNA synthesis but also of pre-mRNA processing (Cmarko et al. 1999) and cleavage (Cardinale et al. 2007). Similarly, EM in situ hybridization experiments identified poly-adenylated RNA in PF (Visa et al. 1993; Huang et al. 1994). However, the presence of heterogeneous RNP (hnRNPs) on PF further ascertained the nature of these nuclear structural constituents: this group of RNA binding proteins are mainly localized on pre-mRNA being involved in the processing of heterogeneous nuclear RNA (hnRNA) into mature mRNA (Chaudhury et al. 2010). Finally, it seems interesting to show a new method, combining the high resolution of transmission electron microscopy (TEM) with the study of the transcription dynamics, which foresees the incorporation with two RNA halogenated precursors (iodouridine, IU, and chlorouridine, ClU) to define a time transcription window, in which a single fibril of newly synthetized RNA can be detected, as a parameter of the transcription efficiency and, consequently, of the PR activity (Spedito et al. 2014).
Perichromatin region resulted to be also the accumulation site of perichromatin granules (PG), reasonably originated by at least some PF before leaving the nucleus (Fakan and Puvion 1980). These particular structures were showed to be involved in intranuclear pre-mRNA storage (Vazquez-Nin et al. 1997), prompting the scientific community to consider the further role of the perichromatin region in mRNA sorting to the cytoplasm, probably mediated by proteins usually involved in intracellular motility (Fakan and van Driel 2007).
RNA epigenetic modifications
As previously described, RNA processing occurs in the PR, including 5′-capping and 3′-polyadenylation as well as splicing. However, this event includes also RNA editing through epigenetic modifications, which have recently attracted more and more attention. In a recent study, 5-methylcytosine (5mC) was first localized in the perichromatin region at ultrastructural level on terbium-positive PF with both a modified ribonucleotide (fluorouridine, FU) labelling nascent RNA and a hnRNP core protein as a marker of nascent transcripts: these results, showing mRNA methylation as a cotranscriptional event, elucidated another activity involving the PR, i.e., RNA epigenetic modifications (Masiello and Biggiogera 2017). About that, N6-methyladenosine (m6A) is the prevalent internal RNA modification in eukaryotes, showed to play a crucial role in the 3′-end processing of pre-mRNA transcripts, in particular in the alternative polyadenylation, and to be a splicing regulator (Kasowitz et al. 2018; Yang et al. 2018). Moreover, METTL3 (methyltransferase-like 3) knock-out experiments demonstrated that the recruitment of this methylating enzyme to pre-mRNA is a cotranscriptional event (Xu et al. 2017) and these results were also confirmed by genome-wide profiling studies (Knuckles and Buhler 2018). Since the cotranscriptional processes involve the PR, m6A-methylation could be hypothesized to occur in this region.
Multiple roles of this proper nuclear compartment have already been described above. However, other epigenetic/regulative events were demonstrated to have a perichromatin localization. In fact, Morlando et al. (2008) showed that the pri-miRNA processing is a cotranscriptional event. Interestingly, experiments of chromatin immunoprecipitation coupled with DNA/RNA sequencing (ChIP-seq) revealed first the physical association of some RNAi factors, such as Drosha and Dgcr8, with chromatin during transcription and, in particular, their binding to nascent transcripts to control their stability or induce their degradation (Knuckles et al. 2017). Therefore, these results seem to indicate that RNAi takes place in the perichromatin region.
As far as epigenetics is concerned, some related modifications and, consequently, chromatin remodelling could be also reasonably localized in the perichromatin region. For instance, among many other modifications localized in the transcribed decondensed chromatin (Muller et al. 2007), the methylation of lysine 4 at histone 3 seems to characterize the dispersed chromatin fibres nearby chromatin surface (Ruthenburg et al. 2007), suggesting the occurrence of histone modifications and also of chromatin remodelling pathways, mediated by specific protein readers, in the perichromatin region. The PR as site of the modification of histone tails was then demonstrated by localizing Tip60, a histone acetyltransferase, properly in the interface between the interchromatin space and compact chromatin domains (Wee et al. 2014). Moreover, polycomb group (PcG) proteins represent a heterogeneous class of polypeptides involved in epigenetic silencing in higher eukaryotes through DNA methylation or histone modifications: EM immuno-gold labelling showed an increase of some PcG proteins at the border of chromatin surface exerting their gene silencing function in the PR where transcriptionally active loci are also present (Cmarko et al. 2002). However, while transcription and RNA processing at PR are well-defined events, the epigenetic regulation occurring in the perichromatin region has yet to be deeply investigated.
Concluding remarks
The immaterial perichromatin region is a “fluid” site where all the most important biological processes occur. In the future, a putatively new function could be addressed to this region. For instance, we believe that the PR could be the site for the action of energetic cargo of some enzymes resident here, such as polymerases or enzymes implicated in RNA modifications; it could also be the site where phospholipids and inositol phosphates can modify histones and where many other different processes, still unexplored, may take place. Being such a sophisticated area only particular conditions will allow its study, certainly the advent of the new microscopy era, advanced technologies and new methodologies will give new insights. In conclusion, we can say that the perichromatin is a movable feast, a inhomogeneous region made of differently active areas, a place where genes are expressed and everything then begins.
References
Allfrey VG (1966) Control mechanisms in ribonucleic acid synthesis. Cancer Res 26(9):2026–2040
Bernhard W (1969) A new staining procedure for electron microscopical cytology. J Ultrastruct Res 27(3):250–265
Bernhard W, Granboulan N (1963) The fine structure of the cancer cell nucleus. Exp Cell Res 24:19–53
Biggiogera M, Fakan S (1998) Fine structural specific visualization of RNA on ultrathin sections. J Histochem Cytochem 46:389–395
Biggiogera M, Masiello I (2017) Visualizing RNA at electron microscopy by terbium citrate. Methods Mol Biol 1560:277–283
Bouchet-Marquis C, Dubochet J, Fakan S (2006) Cryoelectron microscopy of vitrified sections: a new challenge for the analysis of functional nuclear architecture. Histochem Cell Biol 125(1–2):43–51
Cardinale S, Cisterna B, Bonetti P, Aringhieri C, Biggiogera M, Barabino SM (2007) Subnuclear localization and dynamics of the Pre-mRNA 3′ end processing factor mammalian cleavage factor I 68-kDa subunit. Mol Biol Cell 18(4):1282–1292
Chaudhury A, Chander P, Howe PH (2010) Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1’s multifunctional regulatory roles. RNA 16:1449–1462
Cmarko D, Verschure PJ, Martin TE, Dahmus ME, Krause S, Fu XD, Fakan S (1999) Ultrastructural analysis of transcription and splicing in the cell nucleus after bromo-UTP microinjection. Mol Bio Cell 10:211–223
Cmarko D, Verschure PJ, Otte AP, van Driel R, Fakan S (2002) Polycomb group gene silencing proteins are concentrated in the perichromatin compartment of the mammalian nucleus. J Cell Sci 116:335–343
Cortini R, Filion GJ (2018) Theoretical principles of transcription factor traffic on folded chromatin. Nat Commun 9(1):1740
De Boni U (1994) The interphase nucleus as a dynamic structure. Int Rev Cytol 150:149–171
Fakan S (1994) Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol 4:86–90
Fakan S, Bernhard W (1971) Localisation of rapidly and slowly labelled nuclear RNA as visualized by high resolution autoradiography. Exp Cell Res 67:129–141
Fakan S, Hankok R (1974) Localization of newly-synthesized DNA in a mammalian cell as visualized by high resolution autoradiography. Exp Cell Res 81:95–102
Fakan S, Puvion E (1980) The ultrastructural visualization of nucleolar and extranucleolar RNA synthesis and distribution. Int Rev Cytol 65:255–299
Fakan S, van Driel R (2007) The perichromatin region: a functional compartment in the nucleus that determines large-scale chromatin folding. Semin Cell Dev Biol 18:676–681
Fakan S, Puvion E, Sphor G (1976) Localization and characterization of newly synthesized nuclear RNA in isolate rat hepatocytes. Exp Cell Res 99:155–164
Fakan S, Leser G, Martin TE (1986) Immunoelectron microscope visualization of nuclear ribonucleoprotein antigens within spread transcription complexes. J Cell Biol 103:1153–1157
Gall JG, Callan HG (1962) H3 uridine incorporation in lampbrush chromosomes. Proc Natl Acad Sci 48:562–570
Görnemann J, Kotovic KM, Hujer K, Neugebauer KM (2005) Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol Cell 19:53–63
Hancock R (2012) Structure of metaphase chromosomes: a role for effects of macromolecular crowding. PLoS One 7(4):e36045
Hozak P, Hassan AB, Jackson DA, Cook PR (1993) Visualization of replication factories attached to a nucleoskeleton. Cell 73:361–373
Huang S, Deerinck TJ, Ellisman MH, Spector DL (1994) In vivo analysis of the stability and transport of nuclear poly(A) + RNA. J Cell Biol 126:877–899
Jaunin F, Visser AE, Cmarko D, Aten JA, Fakan S (2000) Fine structural in situ analysis of nascent DNA movement following DNA replication. Exp Cell Res 260:313–323
Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, Wang PJ (2018) Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLOS Genet 14:e1007412
Knuckles P, Bühler M (2018) Adenosine methylation as a molecular imprint defining the fate of RNA. FEBS Lett. https://doi.org/10.1002/1873-3468.13107. (Epub ahead of print)
Knuckles P, Carl SH, Musheev M, Niehrs C, Wenger A, Bühler M (2017) RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat Struct Mol Biol 24:561–569
Manders EMM, Stap J, Strackee J, van Driel R, Aten JA (1996) Dynamic behavior of DNA replication do- mains. Exp Cell Res 226:328 – 335
Markaki Y, Gunkel M, Schermelleh L, Beichmanis S, Neumann J, Heidemann M, Leonahardt H, Eick D, Cremer C, Cremer T (2011) Functional nuclear organization of transcription and DNA replication: a topographical marriage between chromatin domains and the interchromatin compartment. Cold Spring Harb Symp Quant Bio 75:475–492
Masiello I, Biggiogera M (2017) Ultrastructural localization of 5-methylcytosine on DNA and RNA. Cell Mol Life Sci 74:3057–3064
Mazzotti G, Gobbi P, Manzoli L, Falconi M (1998) Nuclear morphology during the S phase. Microsc Res Tech 40:418–431
Monneron A, Bernhard W (1969) Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res 27(3):266–288
Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ (2008) Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol 15:902–909
Müller WG, Rieder D, Karpova TS, John S, Trajanoski Z, McNally JG (2007) Organization of chromatin and histone modifications at a transcription site. J Cell Biol 177:957–967
Nash RE, Puvion E, Bernhard W (1975) Perichromatin fibrils as components of rapidly labeled extranucleolar RNA. J Ultrastr Res 53:395–405
Niedojadlo J, Perret-Vivancos C, Kalland KH, Cmarko D, Cremer T, Van Driel R, Fakan S (2011) Transcribed DNA is preferentially located in the perichromatin region of mammalian cell nuclei. Exp Cell Res 317:433–444
Puvion E, Bernhard W (1975) Ribonucleoprotein components in liver cell nuclei as visualized by cryoultramicrotomy. J Cell Biol 67(1):200–214
Puvion E, Viron A, Assens C, Leduc EH, Jeanteur P (1984) Immunocytochemical identification of nuclear structures containing snRNPs in isolated rat liver cells. J Ultrast Res 87:180–189
Raska I, Michel LS, Jarnik M, Dundr M, Fakan S, Gasser S, Gassmann M, Hübscher U, Izaurralde E, Martinez E et al (1991) Ultrastructural cryoimmunocytochemistry is a convenient tool for the study of DNA replication in cultured cells. J Electron Microsc Tech 18:91–105
Ris H (1961) Ultrastructure and molecular organization of genetic systems. Can J Genet Cytol 3:95–120
Rouquette J, Genoud C, Vazquez-Nin GH, Kraus B, Cremer T, Fakan S (2009) Revealing the high-resolution three-dimensional network of chromatin and interchromatin space: a novel electron-microscopic approach to reconstructing nuclear architecture. Chromosome Res 17:801–810
Ruthenburg AJ, Allis CD, Wysocka J (2007) Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell 25:15–30
Sobczak-Thepot J, Harper F, Florentin Y, Zindy F, Brechot C, Puvion E (1993) Localization of cyclin A at the sites of cellular DNA replication. Exp Cell Res 206:43–48
Solimando L, Luijsterburg MS, Vecchio L, Vermeulen W, van Driel R, Fakan S (2009) Spatial organization of nucleotide excision repair proteins after UV-induced DNA damage in the human cell nucleus. J Cell Sci 122:83–91
Spector DL, Fu XD, Maniatis T (1991) Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J 10:3467–3481
Spedito A, Cisterna B, Malatesta M, Biggiogera M (2014) Use of halogenated precursors to define a transcription time window after treatment with hypometabolizing molecules. Histochem Cell Biol 141:243–249
Swift H (1959) Studies on nuclear fine structure. Brookhaven Symp Biol 12:134–152
Testillano PS, Gorab E, Risueno MC (1994) A new approach to map transcription sites at the ultrastructural level. J Histochem Cytochem 42:1–10
Trentani A, Testillano PS, Risueño MC, Biggiogera M (2003) Visualization of transcription sites at the electron microscope. Eur J Histochem 47:195–200
Vázquez Nin GH, Echeverría OM, Ortiz R, Ubaldo E, Fakan S (1997) Effects of hypophyseal hormones on transcription and RNA export to the cytoplasm. Exp Cell Res 236:519–526
Visa N, Puvion-Dutilleul F, Harper F, Bachellerie JP, Puvion E (1993) Intranuclear distribution of poly(A) RNA determined by electron microscope in situ hybridization. Exp Cell Res 208:19–34
von Schack ML, Fakan S, Villiger W (1991) Some applications of cryosubstitution in ultrastructural studies of the cell nucleus. Biol Cell 72(1–2):113–119
Watson M (1962) Observations on a granule associated with chromatin in the nuclei of cells of rat and mouse. J Cell Biol 13:162–167
Wee CL, Teo S, Oey NE, Wright GD, VanDongen HMA, VanDongen AMJ (2014) Nuclear Arc interacts with the histone acetyltransferase Tip60 to modify H4K12 acetylation. eNeuro. https://doi.org/10.1523/ENEURO.0019-14.2014
Xu K, Yang Y, Feng GH, Sun BF, Chen JQ, Li YF, Li W (2017) Metl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res 27:1100–1114
Yang Y, Hsu PJ, Chen YS, Yang YG (2018) Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res 28:616–624
Acknowledgements
This research was supported by the Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022)—Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia (to M.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Masiello, I., Siciliani, S. & Biggiogera, M. Perichromatin region: a moveable feast. Histochem Cell Biol 150, 227–233 (2018). https://doi.org/10.1007/s00418-018-1703-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1703-8