Abstract
Hexose transporters play a pivotal role in the absorption of food-derived monosaccharides in the gastrointestinal tract. Although a basic knowledge of the hexose transporters has already been gained, their detailed distribution and comparative intensities of expression throughout the gastrointestinal tract have not been fully elucidated. In this study, we quantitatively evaluated the expression of SGLT1, GLUT1, GLUT2, and GLUT5 by in situ hybridization and real-time PCR techniques using a total of 28 segments from the gastrointestinal tract of 9-week-old mice. GLUT2 and GLUT5 mRNA expressions were detected predominantly from the proximal to middle parts of the small intestine, showing identical expression profiles, while SGLT1 mRNA was expressed not only in the small intestine but also in the large intestine. Notably, GLUT1 mRNA was expressed at a considerable level in both the stomach and large intestine but was negligible in the small intestine. Immunohistochemistry demonstrated the polarized localization of hexose transporters in the large intestine: SGLT1 on the luminal surface and GLUT1 on the basal side of epithelial cells. The present study provided more elaborate information concerning the localization of hexose transporters in the small intestine. Furthermore, this study revealed the significant expression of glucose transporters in the large intestine, suggesting the existence of the physiological uptake of glucose in that location in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small intestine in mammals plays a central role in the digestion and absorption of nutrients from diets, e.g., carbohydrates, proteins and fats. Glucose derived from carbohydrates serves as the most important metabolic substrate (Wood and Trayhurn 2003). Long-chain carbohydrates, such as starch in the lumen of the small intestine, are digested by α-amylase into short-chain oligo- and/or disaccharides (Caspary 1992) and then further cleaved into monosaccharides by digestive enzymes, such as maltase and sucrase–isomaltase (Alpers et al. 1986; Hoffman and Chang 1991) that are present in the brush border membrane of epithelial cells. The first line of absorption for monosaccharides in the intestinal epithelium depends on the Na+/glucose co-transporter (SGLT) and the facilitated-diffusion glucose transporters (GLUT). Both SGLT1 and GLUT5 are expressed on the apical membrane of epithelial cells in the small intestine. SGLT1 mainly mediates the transport of glucose, and to a lesser extent galactose (Dong et al. 1997; Ferraris 2001; Kojima et al. 1999), whereas GLUT5 mediates the uptake of fructose, but not glucose transport (Burant and Saxena 1994; Burant et al. 1992; Douard and Ferraris 2008). In the second step, the absorbed glucose and fructose passively move out of enterocytes with the aid of the basolaterally expressed passive transporter GLUT2 (Ferraris and Diamond 1997). In addition, it is recently suggested that apically expressed GLUT2 plays a role in glucose absorption especially when a diet containing high-glycemic index sugars was ingested (Gouyon et al. 2003; Kellett and Brot-Laroche 2005).
Although a basic knowledge of the arrangement and role of intestinal hexose transporters is available, their detailed distribution and comparative intensities of expression throughout the gastrointestinal tract have not been elucidated yet. No comprehensive studies have tried to analyze the expression profiles of relevant hexose transporters using the same samples. Furthermore, the segmentation of the gastrointestinal tract for analyses was fairly rough (e.g., quadrisection of small intestine) and the segmental expression was evaluated mainly by Northern blot analysis (Kojima et al. 1999). Northern blot analysis has a lower quantitative ability compared with real-time PCR (Bieche et al. 1999; Vandenbroucke et al. 2001; Wang and Brown 1999) and cannot provide any information about the cellular localization of signals in contrast to in situ hybridization techniques.
In this study, we evaluated the expression of relevant GLUT members (GLUT1-7) and two SGLT subtypes (SGLT1 and SGLT2) by an in situ hybridization technique in the gastrointestinal tract of mice. SGLT1, GLUT1, GLUT2, and GLUT5 with significant expressions were further quantitatively analyzed by a real-time PCR method using a total of 28 segments from the stomach to the anus. For some transporters, we conducted an immunohistochemical analysis to investigate the subcellular localization at a protein level. The present study revealed the significant expression of SGLT1 and GLUT1 in the large intestine, suggesting the existence of a mechanism for glucose uptake there, as reported in the chicken, rat and lactating bovine (Bindslev et al. 1997; De La Horra et al. 2001; Gonzalez Bosc et al. 1998; Zhao et al. 1998). Moreover, unique expression patterns of hexose transporters were found in the follicle-associated epithelium (FAE) of Peyer’s patches and lymphoid follicles.
Materials and methods
Animals
Six male BALB/c mice at 8 weeks of age were purchased from Japan SLC (Shizuoka, Japan). The mice were housed under non-barrier circumstances in our animal facilities with controlled temperature (23 ± 1°C), relative humidity (40–60%), and lighting (light-on at 09:00–21:00). They were allowed ad libitum access to normal rodent chow (Labo-MR stock; nitrogen-free extracts = 54.7%; Nihon Nosan Kogyo, Tokyo, Japan) and water for an acclimatization period of 7 days.
This experiment was conducted in accordance with the guidelines for studies with laboratory animals of the Kyoto Prefectural University Experimental Animal Committee and the Guidelines for Animal Experimentation, Graduate School of Medicine, Hokkaido University.
Dissection and sample collection
After the acclimatization, all mice were fasted overnight with food withdrawn. Fasting started at 6:00 p.m. on the day before sampling and the dissection was performed at 9:00–10:00 a.m. (15–16 h fasting). Each mouse was anaesthetized with an intraperitoneal injection of 30 μl of sodium pentobarbital (Schering-Plough, Osaka, Japan) and then exsanguinated. After an abdominal incision, the entire digestive tract, from the cardiac end of the stomach to the anus, was removed. Samples for in situ hybridization and immunohistochemical analyses were obtained from three of six mice and samples for real-time PCR analysis were from the other three mice.
For in situ hybridization and immunohistochemical analyses, five and three segments were collected from the small and large intestine, respectively, and termed as D (duodenum), J-1, J-2 (jejunum), I-1, I-2 (ileum), Ce (cecum), Co (colon), and R (rectum), as shown in Fig. 1. In the in situ hybridization analysis, the obtained tissues were washed sufficiently with physiological saline and directly embedded into a freezing medium (OCT compound; Sakura Finetechnical Co. Ltd., Tokyo, Japan). These embedded tissues were quickly frozen in liquid nitrogen and stored at −80°C until use.
Sampling regions for real-time PCR and in situ hybridization. The entire length of the gastrointestinal tract was segmented and approximately 30 mg of specimens for real-time PCR analysis was obtained from 28 hatched regions in the figures: 3 in the stomach, 20 in the small intestine, and 5 in the large intestine. Three segments from the stomach correspond to the forestomach (#1), glandular stomach (#2), and pyloric antrum (#3). For in situ hybridization (ISH), five specimens from the small intestine and three specimens from the large intestine were obtained and termed D, J-1, J-2, I-1, I-2, Ce, Co, and R
For real-time PCR analysis, we obtained a total of 28 tissue segments from the whole length of the gastrointestinal tract (Fig. 1): 3 segments from the stomach, 20 segments from the small intestine, and 5 segments from the large intestine (1 from the cecum and 4 from the colon). Approximately 30 mg of the samples was taken for real-time PCR analysis from each segment. All samples were immersed and fixed in RNAlater (Sigma, Tokyo, Japan) overnight at 4°C. The samples in RNAlater were then stored at −80°C until further analysis.
In situ hybridization
Two non-overlapping 45-mer antisense oligonucleotide probes specific for each gene were synthesized. The targeted nucleotide residues of probes for GLUT1–GLUT7 were described in our previous studies (Nagai et al. 2010; Takebe et al. 2009). Antisense probes for SGLTs were newly designed to be complementary to the following sequences: 585–629 and 1086–1130 of mouse SGLT1 (slc5a1, NM_019810), and 781–825 and 1341–1385 of SGLT2 (slc5a2, NM_133254). These oligonucleotides were labeled with 33P-dATP using terminal deoxynucleotidyl transferase (TOYOBO, Osaka, Japan). Ten-micrometer-thick fresh frozen sections were prepared and mounted on glass slides precoated with 3-aminopropyltriethoxysilane. They were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 15 min and then acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine–HCl (pH 8.0) for 10 min. Hybridization was performed at 42°C for 10 h by adding 10,000 cpm/μl of 33P-labeled oligonucleotide probes. Slides were rinsed at room temperature for 30 min in 2× SSC (1× SSC: 150 mM sodium chloride, 15 mM sodium citrate) containing 0.1% sarkosyl, twice at 55°C for 40 min in 0.1× SSC containing 0.1% sarkosyl, dehydrated through a graded series of ethanol, and air-dried. The slides were either exposed to BioMax MR films (Kodak, Rochester, NY) for a week or dipped into autoradiographic emulsion (NTB-2; Kodak) and exposed at 4°C for 4–8 weeks. Some of the hybridized sections for transporters with significant expressions (SGLT1, GLUT1, GLUT2, and GLUT5) were counterstained with hematoxylin after development.
Our in situ hybridization analysis was performed using two individual probes for each mRNA. The in situ hybridization technique using the two non-overlapping antisense probes exhibited identical labeling in all the tissues examined. The specificity of the hybridization was also confirmed by the disappearance of signals upon the addition of an excess of an unlabeled antisense probe.
RNA extraction and cDNA synthesis
Total RNA was extracted from each tissue stored in RNAlater and purified with QuickGene RNA tissue kit S II (FUJIFILM, Tokyo, Japan) and a RNase-free DNase set (TAKARA, Shiga, Japan) according to the manufacturer’s instructions. cDNA was then synthesized from 150 ng of the total RNA with ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan) according to the manufacturer’s instructions.
Real-time PCR
Real-time PCR was performed using a LightCycler 480 (Roche Applied Science, Tokyo, Japan). The expressions of four genes—SGLT1, GLUT1, GLUT2, and GLUT5—were evaluated in each segment of the gastrointestinal tract. For the housekeeping gene, the expression of β-actin gene was assessed in this study since our preliminary study using external control RNA indicated that the expression of other frequently used housekeeping genes, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hypoxantin phosphoribosyl transferase (HPRT) and 18S ribosomal RNA (18S rRNA), considerably differed among the tissues (data not shown). Specific primers and probe sets for each gene were designed according to the GenBank accession number with online software provided by Roche Applied Science (https://www.roche-applied-science.com/). The sequences of the primers and probes used in this study are listed in Table 1. PCR for all genes was performed under the following conditions: an initial denaturation at 95°C for 5 min, followed by 50 cycles of denaturation at 95°C for 10 s and combined annealing–extension at 60°C for 30 s. All reactions were conducted with LightCycler 480 Probes Master and Universal ProbeLibrary Set (Roche) according to the manufacturer’s instructions.
Immunohistochemical analysis
For immunohistochemistry, another group of three male mice were heavily anesthetized and then perfused through the left ventricle of the heart with a physiological saline and subsequently with 4% paraformaldehyde plus 0.2% picric acid in 0.1 M phosphate buffer, pH 7.4. The intestinal tissues were dissected out and immersed in the same fixative for an additional 6 h. The fixed tissues were dipped in 30% sucrose solution overnight at 4°C, embedded in OCT compound, and quickly frozen in liquid nitrogen. Frozen sections of 10 μm in thickness were mounted on poly-l-lysine-coated glass slides and stained by the immunofluorescence method. They were pretreated with 0.3% Triton X-100-containing PBS (pH 7.2) to enhance penetration of the antibodies. Frozen sections were incubated overnight with either a goat anti-SGLT1 antibody (sc-20582; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:200 or a rabbit anti-mouse GLUT1 antibody (donated by Prof. Watanabe, Hokkaido University, Sapporo, Japan) at a concentration of 1 μg/ml, followed by incubation with Cy3-labeled donkey anti-goat or rabbit IgG (1:200 in dilution; Jackson ImmunoResearch, West Grove, PA). After rinsing in PBS, the same sections were incubated shortly with a nucleic acid dye, SOYTOX (Invitrogen, Camarillo, CA), and observed under a confocal laser scanning microscope (Fluoview; Olympus, Tokyo, Japan). The specificity of immunoreactions on sections was confirmed according to a conventional procedure, including absorption tests. The immunoreactivities were completely eliminated using the primary antibodies preabsorbed with corresponding antigens (human GLUT1 and SGLT1 from Santa Cruz Biotechnology).
Statistical analysis
The relative expression level of each mRNA was calculated by the comparative Ct method as described by Nishimura et al. (2003). Briefly, the relative expression of each mRNA was calculated by subtracting the Ct value of the target mRNA from the Ct value of the β-actin mRNA. Differences among the tissues—stomach, small intestine and large intestine—were analyzed with one-way ANOVA followed by the Sheffe’s post hoc test. Statcel (Seiunsha, Inc., Tokyo), which is an add-on for Microsoft Excel (Microsoft, Seattle, USA), was used for the statistical analysis. The difference among means was considered significant at P < 0.05.
Data are presented as fold-differences compared to the expression level of the 1st segment of the small intestine except for GLUT1. For GLUT1 expression, the 1st segment of the small intestine showed a considerably low expression; thus, data were normalized to the expression level in the cardiac end of the stomach. All data are expressed as mean ± standard error (SE).
Results
In situ hybridization analysis
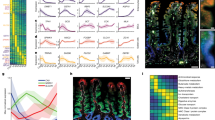
In situ hybridization analyses were performed to survey the expression of seven members of GLUT (GLUT1-7) and two SGLT subtypes (SGLT1 and SGLT2) from the stomach to the anus. X-ray film images of hybridized sections demonstrated significantly intense expressions of SGLT1, GLUT1, GLUT2, and GLUT5 (Fig. 2). Signals for other members of GLUT and SGLT examined were faint or negative on X-ray film images. Signals of SGLT1 mRNA were intense from the duodenum (D) to the end of the ileum (I-2), but still intense in the proximal colon (Co) and rectum (R). The expression of GLUT2, which is a counterpart of SGLT1, was restricted to the small intestine, with a negligible expression at the terminal ileum (I-2). A similar expression pattern was recognized for GLUT5. In contrast, GLUT1 was expressed only in the glandular stomach and in the large intestine from the cecum to rectum (Figs. 2, 3).
X-ray film images showing mRNA expressions for SGLT1, GLUT1, GLUT2, and GLUT5 in the gastrointestinal tract of a mouse. Serial sections from the duodenum (D) to the rectum (R) were arranged on each glass slide and hybridized. The expression of SGLT1 mRNA is intense throughout the small intestine (D to I-2) and still significant in the large intestine. Signals of GLUT1 mRNA are detectable only in the large intestine. The expressions of GLUT2 and GLUT5 are equally restricted to the small intestine. Bar 5 mm
Real-time PCR analysis
Real-time PCR analysis showed almost identical expression patterns with in situ hybridization analysis for all transporters throughout the gastrointestinal tract. The expression level of SGLT1 evaluated by the real-time PCR method was considerably high from the duodenum to the end of the small intestine (Fig. 4). The 2nd segment of the small intestine showed the highest expression, while the expression tended to decrease in the distal regions of the small intestine. The large intestine—except for the cecum (the 1st segment of large intestine)—showed a weak but significant expression of SGLT1 mRNA.
Gene expressions of the hexose transporters (SGLT1, GLUT1, GLUT2, and GLUT5) along the gastrointestinal tract analyzed by real-time PCR. The expression of each gene was normalized to that of β-actin. Data are presented as fold-differences compared with the expression level of the 1st segment of the small intestine except for GLUT1. Data of GLUT1 are presented as fold-differences compared to the expression in the forestomach (#1 of stomach). Bars represent the mean ± SE. P values of statistical analyses are presented on the statistical tables
GLUT2 mRNA was expressed exclusively in the small intestine (Fig. 4). The GLUT2 mRNA expression also peaked at the 2nd segment and was mainly detected from the proximal to middle parts of the small intestine (until the 13th segment), while it decreased in intensity toward the terminal ileum.
GLUT5 mRNA expression analyzed by the real-time PCR analysis showed a similar pattern with GLUT2. The significant expression of GLUT5 mRNA was restricted to the small intestine (Fig. 4). In the small intestine, the proximal 1st to 5th segments showed a considerably higher expression of GLUT5 mRNA than the distal segments. Although the in situ hybridization analysis failed to detect any visible signals for GLUT5 mRNA in the large intestine (Fig. 2), the real-time PCR detected a faint expression of GLUT5 mRNA in the colon.
The gene expression pattern of GLUT1 was distinct from those of other transporters which possessed predominant expression sites in the small intestine. GLUT1 mRNA was considerably intense in all segments of the stomach and large intestine, but extremely low or negligible in most segments of the small intestine, except for the 1st and 2nd segments with their significant expressions (Fig. 4).
Light microscopic observation of mRNA and protein expressions
Light microscopic observation of hybridized sections revealed an intense expression of SGLT1 mRNA in the villous epithelium in all regions of the small intestine (Fig. 5). The signals in crypts fell under detectable levels throughout the small intestine. The SGLT1 mRNA of the large intestine was expressed predominantly in crypt regions of the cecum and rectum, while the intense expression was restricted to the surface layer in colonic mucosa (Fig. 5e). Immunohistochemical analysis using the anti-SGLT1 antibody confirmed the existence of SGLT1 protein on the luminal surface of enterocytes in the large intestine (Fig. 5f), as is the case for the small intestine.
Light microscopic observation of tissue sections hybridized for SGLT1 mRNA and immunostained for SGLT1 protein. Silver grains showing the mRNA expression aggregate in the villous epithelium throughout the small intestine (a–d). A significant expression of SGLT1 mRNA is seen in the surface layer of mucosa in the colon (e). Immunofluorescence staining of the colon (f) displays the localization of SGLT1 (red) at the brush border of surface epithelium. Nuclei are stained green with a nucleic acid dye. Bars 100 μm (a–c), 50 μm (d, e), 20 μm (f)
GLUT2 mRNA was expressed in the villous epithelium of the small intestine, except for the terminal ileum (I-2, data not shown in Fig. 6), which completely lacked any signals for GLUT2. The intense signals appeared at the base of the villi, increased in intensity at the middle part of the villi, and decreased toward the villous tips (Fig. 6). The signals were absent in the crypts of all segments observed. The expression pattern of GLUT5 mRNA was almost identical to that of GLUT2 (data not shown). Unlike GLUT2, the terminal ileum maintained a weak but significant expression of GLUT5 mRNA.
Light microscopic observation of tissue sections hybridized for GLUT2 mRNA in the small intestine. Four figures in the right column are dark-field images of the same sections. Silver grains showing mRNA expression of GLUT2 aggregate at the villous epithelium. In the J-1, J-2 and I-1 regions, the signals appear to decrease in intensity around the villus tips. Bar 100 μm
Signals of GLUT1 mRNA in the large intestine accumulated along the surface epithelium and in the upper region of crypts, but not in the deeper crypt (Fig. 7a, b). In the stomach, the signals for GLUT1 mRNA were found in gastric pits including the surface epithelium (data not shown). Immunohistochemical analysis provided further information that the GLUT1 protein was localized in the basolateral membrane of surface epithelial cells in the large intestine (Fig. 7c, d). However, we failed to detect any specific immunoreaction for GLUT1 in the gastric mucosa.
Gene and protein expressions of GLUT1 in the large intestine. Signals for GLUT1 mRNA gather at the surface epithelium and the upper region of crypts in the cecum (a) and colon (b). The GLUT1 immunoreactivity is seen mainly in the basolateral membrane of the surface epithelium in the cecum (c) and colon (d). Bars 50 μm (a, b), 20 μm (c, d)
FAE, the dome-shaped covering epithelium of Peyer’s patches in the small intestine and of lymphoid follicles in the large intestine, showed unique expression patterns different from those of the ordinal epithelium. The FAE of Peyer’s patches did not express SGLT1 and GLUT2—whose expressions were intense in the villous epithelium—but displayed a moderately intense expression of GLUT5 (Fig. 8c). The FAE of lymphoid follicles in the colon was associated with an intense expression of only GLUT1 mRNA (Fig. 8d).
In situ hybridization analysis showing the mRNA expression of hexose transporters in the follicle-associated epithelium (FAE). Dark-field images of three serial sections containing a Peyer’s patch (PP) of the J-2 region show a moderately intense expression of GLUT5 in the FAE (arrow in c), but a lack of signals for SGLT1 and GLUT2 (a, b). FAE in a lymphoid follicle of the colon are associated with an intense expression of GLUT1 (d). Arrows in all figures indicate FAE which cover lymphoid follicles. V intestinal villi, bars 100 μm
Discussion
Hexose transporters are responsible for the absorption of food-derived monosaccharides in the gastrointestinal tract, where the small intestine is believed to be the main site for the absorption (Monteiro et al. 1999; Toloza and Diamond 1992). This study using two analyzing techniques thoroughly evaluated the distribution and cellular expression of GLUT1, 2, 5 and SGLT1 in the murine gastrointestinal tract at a mRNA level and demonstrate the significant expression of glucose transporters in the large intestine.
Reportedly, GLUT2, GLUT5, and SGLT1 were intensely expressed in the small intestine, while the expressions of other hexose transporters evaluated were negligible in the small intestine at least at mRNA levels. Here, GLUT2 and GLUT5 mRNAs were predominantly expressed in the proximal half of the small intestine; their expression peaked at the second segment of the small intestine (around 4 cm from the pylorus) and decreased in intensity toward the terminal ileum. The expression pattern of SGLT1 was similar to some extent with those of GLUT2 and GLUT5. However, the distal regions of the small intestine maintained a considerably intense mRNA expression of SGLT1 compared with GLUT2 and GLUT5, being in agreement with findings from the rat intestine (Shimada et al. 2009). In addition, our preliminary evaluation by real-time PCR indicated that mRNA expression pattern of GLUT7, a newly found intestinal hexose transporter (Cheeseman 2008), was similar to those of GLUT2 and GLUT5, although the expression level was relatively low as in situ hybridization failed to detect the signals (data not shown). It is safe to conclude that the duodenum is the major site responsible for monosaccharide absorption and the absorptive ability is maintained until the end of the small intestine.
Light microscopic observation of hybridized sections further revealed that GLUT2, GLUT5, and SGLT1 mRNAs were expressed abundantly from the crypt–villus junction to the middle part of the intestinal villi but remarkably decreased in intensity at the villous tips. Crypts of the small intestine completely lacked signals for all hexose transporters analyzed in this study. The mRNA expression patterns along the crypt–villus axis are similar with that of SGLT1 reported in the rat small intestine (Dong et al. 1997). The expression gradient along the intestinal villi indicates that the transcription of three hexose transporters begins together with the differentiation of enterocytes, continues in the middle part of the villi, but rapidly decreases around the summit of the villi. The synthesized proteins may continue to hold until the villous tips even with the reduced mRNA expressions (Balen et al. 2008; Yoshida et al. 1995).
This study demonstrates the significant expression of SGLT1 and GLUT1 in the large intestine by the use of different analytical methods for mRNA expression. The present immunohistochemical analysis further demonstrated the polarized localization of glucose transporters in the large intestine: SGLT1 on the luminal surface and GLUT1 on the basal side of epithelial cells. We concurrently confirmed the mRNA expressions of both SGLT1 and GLUT1 in the large intestine of humans and pigs, suggesting that the existence of the two transporters in the large intestine is a common phenomenon among animals (article in preparation). Indeed, existence of glucose transport in the colon has been reported in the chicken, rat and lactating bovine (Bindslev et al. 1997; De La Horra et al. 2001; Gonzalez Bosc et al. 1998; Zhao et al. 1998). Previous studies in rodents, however, did not recognize any significant expressions of glucose transporters in the large intestine under normal conditions (Kojima et al. 1999; Yoshida et al. 1995; Younes et al. 1996), although an expression of GLUT1 has been reported in the human colon with carcinoma (Younes et al. 1996). The failure to detect them in normal conditions may be partially due to the lower methodological sensitivity as compared with our study using real-time PCR and in situ hybridization methods. Although some previous studies might actually have detected weak expressions of SGLT1 and GLUT1 (Lambert et al. 2002; Toloza and Diamond 1992), the “unexpected” distribution of glucose transporters was disregarded and not analyzed further. The functional significance of the glucose transporters in the large intestine remains to be elucidated. We recently noted that a low but detectable level of glucose exists in cecal contents of both 16-h-fasted and non-fasted mice (approximately 0.8 and 2.1 mM, respectively) as reported in rats and other animals (Ferraris et al. 1990). Thus, it is possible that luminal glucose in the large intestine is absorbed via the apically expressed SGLT1, supplying an energy source for enterocytes. Furthermore, we found tenfold higher expressions of SGLT1 mRNA in the large intestine of germ-free mice than conventional mice (our unpublished data). It is likely that the large intestine of the germ-free mice may readily absorb remnant glucose via SGLT1 as an energy source. This may be explained by the fact that the large intestine in these mice cannot produce short-chain fatty acids which are another important energy substrate for enterocytes, due to the lack of intestinal bacteria.
The expression of GLUT1 mRNA in the gastric mucosa was demonstrated by both in situ hybridization and real-time PCR methods but did not accompany the expression of SGLT1 mRNA. The surface mucous cells constituting the gastric pits contained a diffuse immunoreactivity for GLUT1 in the basal cytoplasm. It is generally accepted that GLUT1 is a unidirectional transporter and essentially responsible for the uptake of glucose for fundamental cellular activities (Takata et al. 1997). Therefore, the expression of GLUT1 in the stomach may be involved in the uptake of glucose from the bloodstream as an energy source of gastric pit cells. However, it is worth noting that GLUT1 in the gastric pit cells did not show a membrane-associated localization, as does GLUT1 in the blood-tissue barriers (Takata et al. 1997).
The expression patterns of glucose transporters in the FAE of Peyer’s patches and lymphoid follicles differed from those of the ordinal intestinal epithelium. The lack of SGLT1 and GLUT2 in FAE may be assumed, because the FAE does not contribute to the absorption of nutrients. However, FAE displayed an elevated expression of GLUT1 and GLUT5, especially the former, in lymphoid follicles of the large intestine. It is currently unclear whether these hexose transporters relate to a particular role of FAE such as the uptake of antigens or they are simply retained during the differentiation into FAE (Clark et al. 2000). Further study is required to reveal the functional significance of the hexose transporters in FAE.
In conclusion, this study provided more specific information about the localization of SGLT1, GLUT2, and GLUT5, which regulate monosaccharide absorption throughout the gastrointestinal tract. Notable findings included the expression of SGLT1 and GLUT1 in the large intestine and the unique expression profile of hexose transporters in the FAE of lymphoid tissues. The expression in the large intestine implies the ability for monosaccharide uptake and/or transport via the large intestine in some conditions as reported in the chicken, rat and lactating bovine (Bindslev et al. 1997; De La Horra et al. 2001; Gonzalez Bosc et al. 1998; Zhao et al. 1998). The expression profile in the FAE must be related to the specialization of this epithelium.
References
Alpers DH, Helms D, Seetharam S, May VL, Strauss AW (1986) In vitro translation of intestinal sucrase–isomaltase and glucoamylase. Biochem Biophys Res Commun 134:37–43
Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I (2008) Revised immunolocalization of the Na+–d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295:C475–C489
Bieche I, Laurendeau I, Tozlu S, Olivi M, Vidaud D, Lidereau R, Vidaud M (1999) Quantitation of MYC gene expression in sporadic breast tumors with a real-time reverse transcription-PCR assay. Cancer Res 59:2759–2765
Bindslev N, Hirayama BA, Wright EM (1997) Na/d-glucose cotransport and SGLT1 expression in hen colon correlates with dietary Na+. Comp Biochem Physiol A Physiol 118:219–227
Burant CF, Saxena M (1994) Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. Am J Physiol 267:G71–G79
Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO (1992) Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267:14523–14526
Caspary WF (1992) Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr 55:299S–308S
Cheeseman C (2008) GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrionol Metab 295:E238–E241
Clark MA, Hirst BH, Jepson MA (2000) Lectin-mediated mucosal delivery of drugs and microparticles. Adv Drug Deliv Rev 43:207–223
De La Horra MC, Cano M, Peral MJ, Calonge ML, Ilundáin AA (2001) Hormonal regulation of chicken intestinal NHE and SGLT-1 activities. Am J Physiol Regul Integr Comp Physiol 280:R655–R660
Dong R, Srai SK, Debnam E, Smith M (1997) Transcriptional and translational control over sodium-glucose-linked transporter (SGLT1) gene expression in adult rat small intestine. FEBS Lett 406:79–82
Douard V, Ferraris RP (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295:E227–E237
Ferraris RP (2001) Dietary and developmental regulation of intestinal sugar transport. Biochem J 360:265–276
Ferraris RP, Diamond J (1997) Regulation of intestinal sugar transport. Physiol Rev 77:257–302
Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM (1990) Luminal glucose concentrations in the gut under normal conditions. Am J Physiol 259:G822–G837
Gonzalez Bosc LV, Vidal Na, Prieto R, Tur JA (1998) Effect of atrial natriuretic peptide on alpha-methyl-D-glucoside intestinal active uptake in rats. Peptides 19:1249–1253
Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E (2003) Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol 552:823–832
Hoffman LR, Chang EB (1991) Determinants of regional sucrase–isomaltase expression in adult rat small intestine. J Biol Chem 266:21815–21820
Kellett GL, Brot-Laroche E (2005) Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 54:3056–3062
Kojima T, Nishimura M, Yajima T, Kuwata T, Suzuki Y, Goda T, Takase S, Harada E (1999) Developmental changes in the regional Na+/glucose transporter mRNA along the small intestine of suckling rats. Comp Biochem Physiol B Biochem Mol Biol 122:89–95
Lambert DW, Wood IS, Ellis A, Shirazi-Beechey SP (2002) Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br J Cancer 86:1262–1269
Monteiro IM, Jiang L, Ferraris RP (1999) Dietary modulation of intestinal fructose transport and GLUT5 mRNA expression in hypothyroid rat pups. J Pediatr Gastroenterol Nutr 29:563–570
Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T (2010) Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta 31:126–133
Nishimura M, Ueda N, Naito S (2003) Effects of dimethyl sulfoxide on the gene induction of cytochrome P450 isoforms, UGT-dependent glucuronosyl transferase isoforms, and ABCB1 in primary culture of human hepatocytes. Biol Pharm Bull 26:1052–1056
Shimada M, Mochizuki K, Goda T (2009) Feeding rats dietary resistant starch shifts the peak of SGLT1 gene expression and histone H3 acetylation on the gene from the upper jejunum toward the ileum. J Agric Food Chem 57:8049–8055
Takata K, Hirano H, Kasahara M (1997) Transport of glucose across the blood-tissue barriers. Int Rev Cytol 172:1–53
Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Yajima T, Iwanaga T (2009) Cellular expression of a monocarboxylate transporter (MCT1) in the mammary gland and sebaceous gland of mice. Histochem Cell Biol 131:401–409
Toloza EM, Diamond J (1992) Ontogenetic development of nutrient transporters in rat intestine. Am J Physiol 263:G593–G604
Vandenbroucke II, Vandesompele J, Paepe AD, Messiaen L (2001) Quantification of splice variants using real-time PCR. Nucleic Acids Res 29:E68
Wang T, Brown MJ (1999) mRNA quantification by real time TaqMan polymerase chain reaction: validation and comparison with RNase protection. Anal Biochem 269:198–201
Wood IS, Trayhurn P (2003) Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 89:3–9
Yoshida A, Takata K, Kasahara T, Aoyagi T, Saito S, Hirano H (1995) Immunohistochemical localization of Na(+)-dependent glucose transporter in the rat digestive tract. Histochem J 27:420–426
Younes M, Lechago LV, Lechago J (1996) Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res 2:1151–1154
Zhao FQ, Okine EK, Cheeseman CI, Shirazi-Beechey SP, Kennelly JJ (1998) Glucose transporter gene expression in lactating bovine gastrointestinal tract. J Anim Sci 76:2921–2929
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yoshikawa, T., Inoue, R., Matsumoto, M. et al. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol 135, 183–194 (2011). https://doi.org/10.1007/s00418-011-0779-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0779-1