Abstract
Adipose tissue contains a stroma that can be easily isolated. Thus, human adipose tissue presents an source of multipotent stromal cells. In order to determine the implication of hematopoietic markers in adipocyte biology, we have defined part of the phenotype of the human adipose tissue-derived stromal cells, and compared this to fully differentiated adipocytes. Flow cytometry demonstrates that the protein expression phenotype of both cell types are similar and includes the expression of CD10, CD13, CD34, CD36, CD55, CD59 and CD65. No significant difference between subcutaneous and omental adipose tissue could be demonstrated concerning the expression of these markers. However, the expression of CD34, CD36 and CD65 is cell-dependent. While the expression of CD36 and CD65 doubled between stromal cells and mature adipocytes, the expression of CD34 decreased, despite this protein being present on the mature adipocyte. As CD34 is described as a stem cell marker and it being unlikely to be expressed on differentiated cells, this result was confirmed by immunostaining and western blot. The clear function of this protein on the adipocyte membrane remains to be determined. The characterization of new proteins on mature adipocytes could have broad implications for the comprehension of the biology of this tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adipose cell plays a major role in the appearance of obesity. Thus, a great deal of research has been directed towards the study of this particular cell, using primary cultures of preadipocytes or human adipocytes, derived in general from lipoaspirations. The isolated cell precursors are able in an adapted medium, to differentiate into mature adipocytes, thus providing a model of choice for the study of the mechanisms of differentiation, as well as for the study of adipocyte metabolic regulation (Bornstein et al. 2000; Bouloumie et al. 2001; Tiraby et al. 2003). In addition, characteristics of isolated mature adipocytes in vitro are, without doubt, more physiologically relevant than murine immortalized cell lines (Fain et al. 2003, 2004; Motoshima et al. 2002). However, the short-survival time of adipocytes in culture does not make it possible to study long-term phenomena.
A number of studies have also been carried out in order to compare subcutaneous and deep (omental) adipose tissue in humans. These studies show in particular that omental fat has a higher lipid turnover than subcutaneous fat (Marin et al. 1996; Martin et al. 1991). Moreover, omental adipocytes express more glucocorticoid receptors (Rebuffe-Scrive et al. 1990). Among certain very obese individuals, these adipocytes express lower rates of lipoprotein lipase (LPL) than subcutaneous adipocytes (Fried et al. 1993). Lastly, it has been shown that the rates of expression of leptin mRNA were higher in subcutaneous adipose tissue than in omental adipose tissue (Montague et al. 1997; van Harmelen et al. 2002), while contradictory results were observed with adiponectin (Lihn et al. 2004; Motoshima et al. 2002). There do not seem, however, to be major differences (except for leptin) with regard to gene expression rates (Montague et al. 1998) nor even to the capacity for differentiation of cells from subcutaneous and omental origins (Shahparaki et al. 2002; van Harmelen et al. 2002).
As more and more studies considered adipose cells (precursors or mature adipocytes) as immune cells, we wanted to determine the evolution of the expression of around 20 characteristic surface markers for hematopoietic cells, both on precursor cells (stromal vascular fraction, SVF) and isolated mature adipocytes, from subcutaneous or omental adipose tissue, derived from normal or slightly overweight women. We have shown that the cell precursors express very strongly CD34, CD13, and CD59, while mature adipocytes express CD34 on their surface, usually described as being specific to stem cells. CD36 and CD59 are also highly expressed on the surface membrane of mature adipocytes. Whatever the origin of the adipose tissue, the expression of the various surface markers tested does not seem to differ.
Materials and methods
Subjects
Subcutaneous and omental tissue specimens of white human fat were obtained from normal weight or slightly overweight human subjects (exclusively females, body mass index <26 kg/m2) undergoing abdominal surgery (aged between 40 years and 65 years, mean 55 years) or liposuction (aged between 35 years and 50 years, mean 42 years). Indications for surgery were colon cancer, pancreatic cancer, gastric cancer or inguinal hernia. A total of 13 samples (seven subcutaneous and six omental) were obtained from 13 patients. The study was approved by the Ile de la Réunion ethics committee for the protection of persons undergoing biomedical research.
Tissue preparation and cell isolation
Liposuction tissue or visceral tissue (after having been cut into small pieces) samples were incubated for 10 min at room temperature in Blood Lysis Buffer (BLB). Samples were then digested for 45 min at 37°C on a rotating shaker in Ringer-Lactate buffer containing 1.5 mg/ml collagenase (NB4, SERVA, Germany, PZ activity 0.170 U/mg). The dispersed tissue was filtered through an 80 μm nylon mesh (for stromal vascular fraction) or directly centrifuged for 1 min at 200 g (for mature adipocytes). The floating adipocytes (mature adipocytes) were collected, resuspended in Krebs-Ringer and rinsed twice, then resuspended in PBS. For stromal vascular fraction, after filtration, the tissue was re-centrifuged for 10 min at 600 g. Sedimented cells were resuspended in BLB and incubated for 10 min at room temperature. After 10 min at 600 g, the cells were washed twice with PBS and resuspended in the same buffer (stromal vascular fraction, SVF).
Flow cytometry analysis
Mature adipocytes and SVF cells (approximately 3×104 cells) were incubated with 10 μl of individual IOTest monoclonal primary antibodies (Immunotech Beckman Coulter, Marseille, France, Table 1), coupled to either phycoerythrin [PE] or fluorescein isothiocyanate [FITC] (Table 2), in 50 μl PBS for 30 min in the dark and at room temperature. Cells were then washed twice with 1 ml PBS (cells were centrifugated and resuspended with PBS for SVF, or recuperation of floating cells for mature adipocytes) and diluted in 150 μl PBS and analyzed with a FACScan flow cytometer (Becton Dickinson). We resuspended adipocytes in a low volume (150 μl) in order to facilitate the aspiration of floating adipocytes for analyzing in the FACScan. Cells incubated with anti-CD20 and anti-CD38 were used as negative controls for PE and FITC, respectively. CD20 and CD38 were used for negative controls as they are typical human B-lymphocyte surface molecule (Shubinsky et al. 1997; Tedder et al. 1988). Moreover, we failed to effectively find their expression in SFV or in mature adipocytes (immunocytochemistry and flow cytometry) from more than 20 patients (personal results). Results were analyzed with WinMDI version 2.8 software.
Immunohistochemistry and immunocytochemistry
Fat tissue
Formalin-fixed white fat tissue, from subcutaneous or omental origins were sectioned (7 μm), deparaffinized and unmasked by using heat treatment in sodium citrate buffer (10 mM) at 95°C for 15 min. Sections were preincubated with 5% BSA and exposed to a 1:50 dilution of monoclonal mouse anti-human CD34 antibody (Kindly provided by Dr P. Gasque, University of Wales College of Medicine, Cardiff, UK). Sections were then incubated with the secondary antibody (1/200 coupled to FITC), washed and mounted with Fluoprep (bioMerieux, Lyon, France) mounting medium.
Mature adipocytes
Mature adipocytes were incubated with 10 μl of individual primary antibodies (Immunotech Beckman Coulter, Marseille, France, Table 1), coupled to either phycoerythrin [PE] or fluorescein isothiocyanate [FITC] (Table 2), in 50 μl PBS for 30 min in the dark at room temperature. Cells were then washed twice with 1 ml PBS (recuperation of floating cells) and diluted in 150 μl PBS. An amount of 5 μl of the agitated suspension were collected, put on a plastic culture flask, and analyzed with a TE200-U inverted microscope (Nikon, Champigny-sur-Marne, France). Mature adipocytes are visualized at the top of the PBS drop.
SVF cells
Cells isolated from the SVF were plated on a glass slide in a tissue culture flask at a concentration of 30, 000 cells/cm2 in 199 medium supplemented with 10% fetal bovine serum (FBS) (PAN Biotech, France), 100 U/ml penicillin, 100 μg/ml streptomycin, 25 ng/ml amphotericin B and 100 ng/ml transferin. The primary cells were cultured for 2 days on glass coverslips, fixed with 0,4% paraformaldehyde (Sigma, Saint Quentin Fallavier, France) and washed three times in PBS. Cells were pre-incubated with 5% BSA and then exposed to a 1:50 dilution of monoclonal mouse anti-human CD34 antibody. After washing, cells were incubated with the secondary antibody (1/100 coupled to FITC), washed and mounted with Fluoprep mounting medium (bioMerieux, Lyon, France).
Western-blot
In order to avoid non-adipocyte cell contamination, floating mature adipocytes were rinsed three times in PBS (centrifugation 200 g) before protein extraction. Whole protein extracts (Laemmli buffer, 50 μg per lane) were subjected to SDS-polyacrylamide gel electrophoresis (7.5% acrylamide) and transferred to a nitrocellulose membrane (Bio-Rad, France). The membrane was then blocked with 1% (w/v) FBS in TBS/Tween 20 buffer (250 mM Tris buffer, pH 7.5; 1.37 M NaCl; 0.1% (v/v) Tween 20) for 1 h at room temperature and incubated with mouse anti-human CD34 antibody (Immunotech Beckman Coulter, Marseille, France, Table 1) for 2 h at room temperature. Protein detection was performed by incubating the nitrocellulose membrane for 1 h with an appropriate Horseradish peroxidase anti-mouse IgG antibody (Amersham Bioscience, Orsay, France) and immunoreactivity revealed by exposing the membrane to the ECL detection reagent (Amersham Bioscience, Orsay, France) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using Microsoft Excel software. Percentage differences between SVF cells and mature adipocytes were tested for significance (P<0.001 and P<0.01) by the unpaired Student’s t test.
Results
Many membrane proteins are defined as being characteristic of cells derived from the stromal vascular fraction. In order to characterize the expression of these proteins (Table 1 and 2) on the surface of cells derived from human (female) subcutaneous and omental adipose tissue, a number of different techniques were employed.
Initially, in order to identify protein expression at the surface of cells derived from subcutaneous tissue, a sensitive flow cytometric technique was used. Cells of the vascular stroma and mature adipocytes were incubated with various antibodies conjugated to FITC or PE, and were then analyzed. Figures 1 and 2 show the marked expression of the following proteins: CD59 (complement protectin), CD55 (Decay Accelerating Factor), CD65 (Poly-N-acetyllactosamine), CD10 (common acute lymphocytic leukemia Ag, CALLA), CD36 (fatty acid translocase), CD13 (aminopeptidase N) and CD34 (sialomucin). Whereas, CD10, CD59, CD13 and CD55 are expressed in a relatively homogeneous way at the same time on cell precursors and mature adipocytes, the expression of CD65, CD36 and CD34 varies. Thus (Table 2, Figs. 1, 2, 4), CD36, implicated in lipid metabolism and the transport of fatty acids, is very strongly expressed on the surface of mature adipocytes (intensity of fluorescence 100 times superior, Fig. 1), whereas, the rate of expression on the membrane of the SVF cells is very low. Moreover, there is a two-fold difference in the number of labeled cells between the two cellular types (84% versus 42%, Fig. 2). With regard to CD34, it has been reported in the literature that the protein is highly expressed at the surface of precursor cells (Berenson et al. 1991; Katz et al. 1985). However, contrary to established thought, CD34 is also present at the surface of mature cells (Figs. 1, 2, 4). Our results were similar when identical experiments were carried out on omental adipose tissue (data not shown). Indeed, no difference in terms of the percentage of labeled cells could be determined between subcutaneous adipose tissue and omental tissue, whatever the cellular type (SVF cells or mature adipocytes).
Flow cytometric analysis of stromal vascular fraction (left side) and mature adipocytes (right side) from subcutaneous human adipose tissue. Cells from a representative individual donor were stained with monoclonal antibodies directed against: (a) CD59; (0b) CD10 or CD36; coupled to fluorescein isothiocyanate (FITC) or against: (c) CD13 or (d) CD34 and coupled to phycoerythrin (PE). Monoclonal antibodies directed against CD38 or CD20 served as a control for FITC or PE, respectively (grey area). M1 zone corresponds to positive cells (102<M1)
Graphical representation of mean percentage of positive cells stained for various proteins after flow cytometric analysis. M1 zone (Fig. 1) define positive cells (>102). Stromal vascular fraction cells or mature adipocytes were stained with monoclonal antibodies directed against CD10, CD36, CD59 or CD65 and coupled to fluorescein isothiocyanate (FITC) or against CD13, CD34 or CD55 and coupled to phycoerythrin (PE). Data are the mean ± SD of n=7 donors. Monoclonal antibodies directed against CD38 or CD20 served as a negative control for FITC or PE. respectively. **P<0.001% versus SVF cells. *P<0.01% versus SVF cells
It should also be noted that there the number of CD65 labeled cells are almost double among the mature adipocytes compared to the SVF cells (51% versus 29%, Fig. 2). There is also a two-fold difference in the intensity of fluorescence between the two cell types when using this labeling method (data not shown).
The expression of CD3, CD4, CD14, CD15, CD19, and CD31 on mature adipocytes was also investigated. The results obtained are presented in Fig. 3 and do not show any labeling by the various antibodies. These results are representative of the purified cells of all of the subjects tested and are valid for mature adipocytes of subcutaneous or omental origins. The results of identical labeling experiments carried out on cells of the SVF are presented in Table 2. It is interesting to note that in this study, we obtained approximately 20% CD31+ cells in the SVF (Table 2).
Flow cytometric analysis of mature adipocytes from subcutaneous human adipose tissue. Cells from a representative individual donor were stained with monoclonal antibodies directed against: (a) CD38 (negative control); (c) CD3; (d) CD15; (f) CD19 and (h) CD31, coupled to fluorescein isothiocyanate (FITC) or against (b) CD20 (negative control); (e) CD4 and (d) CD14, coupled to phycoerythrin (PE)
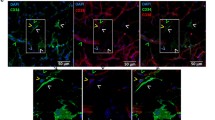
In order to confirm the expression of CD34 on the surface of fully differentiated adipose cells, we used immunohistochemical and immunocytochemical techniques on cells derived from adipose, subcutaneous and omental tissue, as well as directly on tissue sections. The results of these labeling experiments are presented in Fig. 4. It should be noted that the cells resulting from the SVF, in culture (J2), are strongly labeled by the anti-CD34 antibody, which is in agreement with the findings of other groups (Aust et al. 2004; Gronthos et al. 2001; Young et al. 1999). However, this labeling is visible both at the level of mature adipocytes as well as in intact adipose tissue, and is independent of the origin (subcutaneous or omental) (Fig. 4) (data not shown). As adipose tissue (like almost all tissue) is usually widely contaminated with endothelial cells (CD34+), our more convincing result is provided by the labeling of pure mature adipocyte cells (Fig. 4e). Immunolabeling of CD36 is shown as positive control for mature adipocytes. The labeling intensity of CD34 and CD36 (Fig. 4) is in accordance with results obtain by flow cytometry (Fig. 1). THP-1 cells (human immortalized monocyte cell line, mature cells) were used as a negative control for CD34 immunostaining and effectively not shown any staining (Fig. 4i). Day 0 SVF cells were used as positive control (Fig. 4k) in agreement with the results of previous studies (Aust et al. 2004; Gronthos et al. 2001; Young et al. 1999), which demonstrated a strong CD34 staining of these cells.
Immunostaining of stromal vascular fraction cells after 2 days of culture (a, b,×200), tissue sections (c, d, ×200) or mature adipocytes (e, f, ×200; g, h, ×400) from human subcutaneous adipose tissue. THP-1 cells (human monocyte cell line) were used as negative control for CD34 (i, ×200). CD13 is used as positive control for THP-1 cells (j, ×200). SVF cells (0 day of culture) were used as positive control for CD34 (k, ×200). FACS analysis of negative and positive control are shown (i, j and k). a and c samples were exposed to mouse anti-human CD34 antibody and stained with FITC-linked secondary antibody; b and d samples were exposed to FITC-linked secondary antibody alone (control); e mature adipocytes were incubated with mouse anti-human CD34 antibody coupled to PE; f mature adipocytes were incubated with mouse anti-human CD20 antibody coupled to PE (negative control); g mature adipocytes were incubated with mouse anti-human CD36 antibody coupled to FITC; h mature adipocytes were incubated with mouse anti-human CD38 antibody coupled to FITC (negative control); i and j THP-1 cells (negative control) were incubated with mouse anti-human CD34 or CD13 antibody coupled to PE; k day purified SVF cells (positive control) were incubated with mouse anti-human CD34 antibody coupled to PE
Last, to determine the total expression of these proteins within SVF cells and adipocytes resulting from subcutaneous and omental tissue (from the same patient), we carried out a western blot (Fig. 5). Thus, the expression of CD34 on cells resulting from the SVF as well as on fully differentiated adipocytes can be confirmed. We find that the expression of CD34 seems to be greater in cells derived from the SVF than fully differentiated adipocytes, in line with the results from flow cytometric analysis. Prior membrane staining with Ponceau red, did not detect any differences in the quantity of proteins loaded on the gel (data not shown).
Western blot analysis of CD34 protein expressed by omental and subcutaneous human mature adipocytes and SVF cells. 50 μg of total protein was separated by SDS-PAGE. Protein detection was performed by the ECL Plus western blotting detection reagent. Panel A: molecular weight ladder; Panel B: proteins of SVF cells from subcutaneous tissue; Panel C: proteins of mature adipocytes from subcutaneous tissue; Panel D: proteins of SVF cells from omental tissue; Panel E: proteins of mature adipocytes from omental tissue
The origin of the adipose tissue (subcutaneous or omental), had no significant difference upon the expression of CD34 among patients analyzed.
Discussion
The results that we present in this study add to our understanding of the role and function of the adipocyte cell. We are indeed the first group to characterize in a differential way the expression of functionally significant proteins on the surface of SVF cells and mature adipocytes from two locations (subcutaneous and omental adipose tissue). Among the six samples of omental adipose tissue, three came from patients treated for hernia (close to normal situation), and three came from patients treated for cancer (pathologic situation). However, it does not seem that this particular point interfered in our study as amongst all the CD analyzed on SVF cells and mature adipocytes, we could not detect significant difference between the normal and the pathological situation.
With regards to the expression of the proteins investigated in this study, no significant difference could be determined between cells from subcutaneous or omental adipose tissue. However, it is clear that these results do not call into question functional differences already demonstrated in other studies (Lundgren et al. 2004; Marin et al. 1996; Martin et al. 1991; Montague et al. 1997; van Harmelen et al. 2002).
The labeling of mature adipocytes enables us to clearly visualize the physiology of expressed proteins in human tissue in a way that is very close to the situation in vivo. The absence of labeling for CD3, CD19, and CD15 (Fig. 3) demonstrates that the cells used in this study are not contaminated (or very little) by immune cells. Our results are also in agreement with the results of previous studies on undifferentiated cells in culture, which showed the expression of CD10, CD13, CD34, CD55, and CD59 (Aust et al. 2004; Gronthos et al. 2001; Young et al. 1999).
We, on the other hand, have not been able to demonstrate the membrane expression of certain proteins such as CD4 or CD14 (Fig. 3). With regards to CD4, our results do not agree with the study by Hazan et al. (2002) that demonstrated the expression of the CD4 receptor on the surface of adipocytes differentiated in culture as well as the immunolabeling of the protein in subcutaneous adipose tissue. The work by Hazan et al. proposed a link between the expression of CD4 and the entry of the HIV virus into adipocytes. The differences observed can certainly be explained by two deciding factors. Certain experiments were carried out upon differentiated cells in vitro, which does not necessarily reflect the actual situation in vivo. In addition, subsequent confirmation of CD4+ labeling was carried out on adipose tissue sections. It is possible that positive labeling occurred as a result of the presence of another cellular type (lymphocytes for CD4 and endothelial cells for CXCR4 and CCR5) (Gupta et al. 1998). Whatever the reason, our results do not show any CD4+ labeling, in either subcutaneous or omental SVF cells, or adipocytes, as confirmed by the use of two different antibodies (clone 13B8.2 and SFCI12T4D11 (data not shown)).
With regards to CD14 (LPS receptor), our results do not support the findings of Sewter et al. (1999), who demonstrated the expression of the CD14 messenger in human adipocytes. Thus, Sewter et al. explained an increase in TNFα in response to stimulation with LPS by the presence of CD14 on the cell surface. We have only been able to demonstrate approximately 3% of cells expressing CD14, whatever the cellular type or the origin of the tissue investigated. This, as well as the low intensity of fluorescence given by fluorometry, does not enable us to validate the presence of this protein on the surface of the adipocytes. Therefore, the effect of LPS on the adipocyte cannot be directly related to the presence of CD14 on its membrane. However, it is possible that this effect occurs via the secretion of CD14 by the adipose cell (Su et al. 1999), or possibly as a result of another receptor (Frleta et al. 2003; Heine et al. 2003). The expression of CD55 on the mature adipocyte would seem to agree with this hypothesis. It has also been demonstrated that the presence of CD14 is not necessary for activation by LPS (Lynn et al. 1993).
The expression of CD36 on the surface of adipocytes as well as its implication in many cellular processes (scavenger receptor functions, lipid metabolism, fatty acid transport) have already been largely described in rodents as well as in the 3T3-L1 and 3T3-F442A cell lines (Febbraio et al. 1999; Kuniyasu et al. 2002, 2003; Sfeir et al. 1999). Recently, a link has been established between insulin resistance, the expression of CD36 on the surface of macrophages, as well as its function as a scavenger receptor (Liang et al. 2004), whereas, its deficiency had previously been incriminated in the development of insulin resistance (Miyaoka et al. 2001). We show here for the first time the expression of CD36 on the surface of cell precursors (SVF) and on isolated human adipocytes. This expression is dependent upon differentiation and is thus highly pronounced on mature adipocytes (strong labeling intensity, Fig. 1). The expression of CD36 undoubtedly reflects its very significant role in the function of the adipose cell. Looking beyond its implication in the transport of fatty acids, this new data enables us to postulate that the adipose tissue could play a major role in the elimination of LDL oxidized within the human body (Kuniyasu et al. 2002; Stanton et al. 1997; Zhao et al. 2004). Thus, CD36 can be regarded as a very good marker of differentiated adipocyte (Teboul et al. 2001).
The most interesting and surprising result in this study relates to the expression of CD34 on the surface of mature adipocytes derived from subcutaneous or omental adipose tissue. Indeed, this receptor is generally regarded as a marker of cell lines or juvenile cells (Berenson et al. 1991; Katz et al. 1985), while its expression on the surface of differentiated cells remains limited (Delia et al. 1993; Drew et al. 2002; Fina et al. 1990).
The use of three different visualization techniques as well as two distinct antibodies in this study greatly supports the validity of our results. Moreover, the fact that various CD markers of immune cells (CD19: plasmocytes; CD3: T cells; CD15: macrophages) or of endothelial cells (CD31) are not found in the mature adipocyte preparations used in this study demonstrates that these preparations are not contaminated by other cell types generally found within adipose tissue. The absence of these markers could not be due to collagenase digestion as we could find them in the SVF. Moreover, differential expression of CD36 between SVF cells (very low staining) and mature adipocytes (very strong staining) show, without ambiguity, that mature adipocytes preparation are not contaminated with cells from the SVF as no cell of the SVF show such a strong staining.
Despite the results of experiments undertaken within our laboratory as well as what is currently known from the literature, we are unable to explain the function of the CD34 receptor on the surface of adipocytes. However, CD34 has been implicated in cellular adhesion (Healy et al. 1995; Majdic et al. 1994) and more particularly in lymphocyte recruitment by endothelial cells (Baumhueter et al. 1994). Indeed, in endothelial cells, the CD34 receptor, a ligand of L-selectins, is highly expressed (Baumhueter et al. 1994). Thus, it is possible that this function also exists within adipose tissue. Our results agree with a recent study showing that adipocytes and endothelial cells share a common precursor (Planat-Benard et al. 2004). Moreover, mature adipocytes are able to undergo dedifferentiation acquiring an endothelial phenotype (Planat-Benard et al. 2004). Thus, it is not surprising to find identical proteins expressed on the membranes of these two cell types. Last, we have demonstrated the expression of CD65, a ligand for E-selectins (Noguchi et al. 2001), on mature adipocytes. This result consolidates the hypothesis that adipocytes are implicated in leucocyte recruitment via CD34 and CD65.
The study of the expression of adipocyte surface proteins highlights the potential existence of various processes controlling and modeling the cellular interactions within the adipose tissue (omental or subcutaneous). However, the interactions between immunizing cells, endothelial cells, and adipocytes remains to be more clearly determined.
Lastly, the results obtained from mature adipocytes extracted directly from adipose tissue make it possible to highlight specific characteristics of the human adipocyte that are not revealed in primary cultures or cell lines. Thus, the use of this particular in vitro model should help us in elucidating certain functions of adipocytes in vivo.
References
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6:7–14
Baumhueter S, Dybdal N, Kyle C, Lasky LA (1994) Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood 84:2554–2565
Berenson RJ, Bensinger WI, Hill RS, Andrews RG, Garcia-Lopez J, Kalamasz DF, Still BJ, Spitzer G, Buckner CD, Bernstein ID et al (1991) Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood 77:1717–1722
Bornstein SR, Abu-Asab M, Glasow A, Path G, Hauner H, Tsokos M, Chrousos GP, Scherbaum WA (2000) Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes 49:532–538
Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M (2001) Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 50:2080–2086
Delia D, Lampugnani MG, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti MA, Greaves MF (1993) CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood 81:1001–1008
Drew E, Merkens H, Chelliah S, Doyonnas R, McNagny KM (2002) CD34 is a specific marker of mature murine mast cells. Exp Hematol 30:1211
Fain JN, Kanu A, Bahouth SW, Cowan GS, Lloyd Hiler M (2003) Inhibition of leptin release by atrial natriuretic peptide (ANP) in human adipocytes. Biochem Pharmacol 65:1883–1888
Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW (2004) Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145:2273–2282
Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL (1999) A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274:19055–19062
Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75:2417–2426
Fried SK, Russell CD, Grauso NL, Brolin RE (1993) Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 92:2191–2198
Frleta D, Noelle RJ, Wade WF (2003) CD40-mediated up-regulation of Toll-like receptor 4-MD2 complex on the surface of murine dendritic cells. J Leukoc Biol 74:1064–1073
Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM (2001) Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 189:54–63
Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM (1998) Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem 273:4282–4287
van Harmelen V, Dicker A, Ryden M, Hauner H, Lonnqvist F, Naslund E, Arner P (2002) Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes 51:2029–2036
Hazan U, Romero IA, Cancello R, Valente S, Perrin V, Mariot V, Dumonceaux J, Gerhardt CC, Strosberg AD, Couraud PO, Pietri-Rouxel F (2002) Human adipose cells express CD4, CXCR4, and CCR5 [corrected] receptors: a new target cell type for the immunodeficiency virus-1? Faseb J 16:1254–1256
Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T (1995) The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci USA 92:12240–12244
Heine H, El-Samalouti VT, Notzel C, Pfeiffer A, Lentschat A, Kusumoto S, Schmitz G, Hamann L, Ulmer AJ (2003) CD55/decay accelerating factor is part of the lipopolysaccharide-induced receptor complex. Eur J Immunol 33:1399–1408
Katz FE, Tindle R, Sutherland DR, Greaves MF (1985) Identification of a membrane glycoprotein associated with haemopoietic progenitor cells. Leuk Res 9:191–198
Kuniyasu A, Hayashi S, Nakayama H (2002) Adipocytes recognize and degrade oxidized low density lipoprotein through CD36. Biochem Biophys Res Commun 295:319–323
Kuniyasu A, Ohgami N, Hayashi S, Miyazaki A, Horiuchi S, Nakayama H (2003) CD36-mediated endocytic uptake of advanced glycation end products (AGE) in mouse 3T3-L1 and human subcutaneous adipocytes. FEBS Lett 537:85–90
Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR (2004) Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 113:764–773
Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B (2004) Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol 219:9–15
Lundgren M, Buren J, Ruge T, Myrnas T, Eriksson JW (2004) Glucocorticoids down-regulate glucose uptake capacity and insulin-signaling proteins in omental but not subcutaneous human adipocytes. J Clin Endocrinol Metab 89:2989–2997
Lynn WA, Liu Y, Golenbock DT (1993) Neither CD14 nor serum is absolutely necessary for activation of mononuclear phagocytes by bacterial lipopolysaccharide. Infect Immun 61:4452–4461
Majdic O, Stockl J, Pickl WF, Bohuslav J, Strobl H, Scheinecker C, Stockinger H, Knapp W (1994) Signaling and induction of enhanced cytoadhesiveness via the hematopoietic progenitor cell surface molecule CD34. Blood 83:1226–1234
Marin P, Lonn L, Andersson B, Oden B, Olbe L, Bengtsson BA, Bjorntorp P (1996) Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81:1018–1022
Martin ML, Jensen MD (1991) Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88:609–613
Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y (2001) CD36 deficiency associated with insulin resistance. Lancet 357:686–687
Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S (1997) Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46:342–347
Montague CT, Prins JB, Sanders L, Zhang J, Sewter CP, Digby J, Byrne CD, O’Rahilly S (1998) Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 47:1384–1391
Motoshima H, Wu X, Sinha MK, Hardy VE, Rosato EL, Barbot DJ, Rosato FE, Goldstein BJ (2002) Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab 87:5662–5667
Noguchi M, Sato N, Sugimori H, Mori K, Oshimi K (2001) A minor E-selectin ligand, CD65, is critical for extravascular infiltration of acute myeloid leukemia cells. Leuk Res 25:847–853
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109:656–663
Rebuffe-Scrive M, Bronnegard M, Nilsson A, Eldh J, Gustafsson JA, Bjorntorp P (1990) Steroid hormone receptors in human adipose tissues. J Clin Endocrinol Metab 71:1215–1219
Sewter CP, Digby JE, Blows F, Prins J, O’Rahilly S (1999) Regulation of tumour necrosis factor-alpha release from human adipose tissue in vitro. J Endocrinol 163:33–38
Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N (1999) CD36 antisense expression in 3T3-F442A preadipocytes. Mol Cell Biochem 192:3–8
Shahparaki A, Grunder L, Sorisky A (2002) Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism 51:1211–1215
Shubinsky G, Schlesinger M (1997) The CD38 lymphocyte differentiation marker: new insight into its ectoenzymatic activity and its role as a signal transducer. Immunity 7:315–324
Stanton LA, van de Venter M, Litthauer D, Oelofsen W (1997) Effect of lipoproteins on the differentiation of 3T3-L1 and human preadipocytes in cell culture. Comp Biochem Physiol B Biochem Mol Biol 116:65–73
Su GL, Dorko K, Strom SC, Nussler AK, Wang SC (1999) CD14 expression and production by human hepatocytes. J Hepatol 31:435–442
Teboul L, Febbraio M, Gaillard D, Amri EZ, Silverstein R, Grimaldi PA (2001) Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. Biochem J 360:305–312
Tedder TF, Streuli M, Schlossman SF, Saito H (1988) Isolation and structure of a cDNA encoding the B1 (CD20) cell-surface antigen of human B lymphocytes. Proc Natl Acad Sci USA 85:208–212
Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D (2003) Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278:33370–33376
Young HE, Steele TA, Bray RA, Detmer K, Blake LW, Lucas PW, Black AC Jr (1999) Human pluripotent and progenitor cells display cell surface cluster differentiation markers CD10, CD13, CD56, and MHC class-I. Proc Soc Exp Biol Med 221:63–71
Zhao SP, Zhang DQ (2004) Atorvastatin enhances cellular uptake of oxidized LDL in adipocytes from hypercholesterolemic rabbits. Clin Chim Acta 339:189–194
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Festy, F., Hoareau, L., Bes-Houtmann, S. et al. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol 124, 113–121 (2005). https://doi.org/10.1007/s00418-005-0014-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0014-z