Abstract
Purpose
To investigate the objective function of the inner retinal layer in each stage of primary open angle glaucoma (POAG) using the photopic negative response (PhNR) measured by RETeval full-field electroretinography (ERG), and to identify which PhNR parameter is the most useful.
Methods
Ninety eyes of 90 patients with POAG (30 with mild POAG (mean deviation (MD) ≥ -6 dB) and 60 with moderate-to-advanced POAG (MD < -6 dB)) and 76 eyes of 76 control cases were examined. We investigated six PhNR parameters and their relationships with the results of the Humphrey 30–2 visual field test and the thickness of the circumpapillary retinal nerve fiber layer (cpRNFL) obtained from optical coherence tomography. The following PhNR parameters were assessed: base-to-trough (BT), peak-to-trough (PT), 72msPhNR, the W-ratio, P-ratio, implicit time (IT), and a-wave and b-wave amplitudes on ERG.
Results
All PhNR parameters other than IT significantly differed between the all POAG (all stages) and control groups and between the moderate-to-advanced POAG and control groups. BT and 72msPhNR in the mild POAG group, significantly differed from those in the control group. Regarding the relationships between PhNR parameters and the visual field and between these parameters and cpRNFL thickness, correlations were observed between all PhNR parameters, except PT and IT, and both the visual field and cpRNFL thickness in the all and moderate-to-advanced POAG groups. 72msPhNR correlated with cpRNFL thickness in the mild POAG group. The area under the receiver operating characteristic curve was greater for BT than for the other PhNR parameters in both the mild and moderate-to-advanced POAG groups. The discriminant linear function for examining the presence or absence of POAG and the threshold for diagnosis were quantitatively obtained as follows. Regarding BT: discriminant = 0.505 × BT + 2.017; threshold = positive for POAG, negative for no POAG; correct answer rate = 80.7%. Concerning 72msPhNR: discriminant = 0.533 × 72msPhNR + 1.553; threshold = positive for POAG and negative for no POAG; correct answer rate = 77.1%.
Conclusion
RETeval-measured PhNR parameters were useful for an objective evaluation of visual function in moderate-to-advanced POAG. BT appeared to be the most diagnostically useful parameter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
The guidelines of the American Academy of Ophthalmology define primary open angle glaucoma (POAG) as “a chronic, progressive optic neuropathy in adults in which there is a characteristic acquired atrophy of the optic nerve and the loss of retinal ganglion cells and their axons” and state that POAG is associated with the open anterior chamber angle on gonioscopy [1]. In 2020, the most recent year for which information is available, an estimated 53 million individuals worldwide had POAG, with a prevalence of 3.0% in the population aged 40 to 80 years [1].
Glaucoma is the second leading cause of blindness after cataracts worldwide [1]. In Japan, glaucoma is the most common cause of blindness with a prevalence of 5% in individuals older than 40 years old [2, 3].
The standard functional assessment of retinal ganglion cells (RGCs) in glaucoma is a visual field test; however, it is subjective and dependent on a patient’s condition. In addition, it is necessary for the patient to understand the visual field test properly in order to make an accurate evaluation. Since this limitation may increase the difficulty of performing an examination of the elderly, an objective evaluation of RGC function is often necessary. The main methods for the objective evaluation of RGC function are an assessment of the photopic negative response (PhNR) and a reversal pattern electroretinogram (PERG) [4]. However, neither of these tests has been fully evaluated for the early detection or follow-up of glaucoma. Therefore, we herein investigated various PhNR parameters to identify which is the most useful for an objective functional assessment of glaucoma.
Since PhNR measurements using conventional ERG devices are cumbersome and mildly invasive due to the requirement of contact lens electrodes, we used a RETeval ERG system (LKC Technologies Inc., Gaithersburg, MD). The RETeval ERG system uses skin electrodes, which are lightweight and convenient to carry, making it easier to use and less invasive than conventional ERG devices. Furthermore, the RETeval device records the PhNR according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standard full-field ERG protocol [5,6,7,8,9]. Previous studies recorded the PhNR of POAG using the RETeval system [5, 8, 9], and we herein investigated the relationships between each PhNR parameter and the visual field and morphological evaluation in each stage of POAG.
Methods
Patients
One-hundred and sixty-six patients attending or hospitalized in the Department of Ophthalmology, University of Miyazaki Hospital (Miyazaki, Japan) between September 2015 and June 2022 were enrolled, and assigned to a POAG group of 90 eyes in 90 patients or a control group of 76 eyes in 76 patients. The POAG group included two patients with normal tension glaucoma. The present study was approved by the Research Ethics Committee of the Faculty of Medicine, University of Miyazaki (study number: O-0670) and adhered to the tenets of the Declaration of Helsinki. All patients provided informed consent and public announcements of this study were performed. Details of the study were made available on the university website. The demographic characteristics of participants are shown in Table 1.
All patients in the control group met the following four criteria: intraocular pressure (IOP) < 21 mmHg; no optic nerve abnormalities (e.g., enlarged optic disc cupping, disc hemorrhage, focal rim notching, and RNFL defects); no other obvious abnormal findings in the optic nerve or retina; no medical history of diabetes. IOP was measured using a non-contact tonometer in the control group. The ocular axial length was measured with an IOL Master 700 (Carl Zeiss Meditec).
Patients in the POAG group were diagnosed according to the Japan Glaucoma Society Guidelines for Glaucoma (5th Edition) [10]. We identified characteristic glaucomatous optic neuropathy and the loss of RGCs and associated visual field abnormalities. All patients in the POAG group met the following three criteria: abnormal visual fields in the Humphrey 30–2 Swedish Interactive Threshold Algorithm Fast test (Humphrey-Zeiss Systems, Dublin, CA), specifically a glaucomatous visual field disorder based on the Anderson-Pattela classification; no optic nerve disease or retinal disease other than glaucoma; no medical history of diabetes. IOP was measured using a Goldmann applanation tonometer in the POAG group.
We subdivided the POAG group into two groups: mild and moderate-to-advanced POAG. Patients with a mean deviation (MD) ≥ -6 dB were considered to have mild POAG (n = 30 eyes; MD average: -3.17 ± 1.28 dB; MD range: -0.86 to -5.26 dB), while those with MD < -6 dB were considered to have moderate-to-advanced POAG (n = 60 eyes; MD average: -17.42 ± 6.51 dB; MD range: -6.36 to -31.01 dB).
ERG recording
We measured ERG and the PhNR using a RETeval ERG system. RETeval is an ERG measuring device with skin electrode specifications. Since it is handheld and small, it may be installed anywhere. ERG is measured according to the ISCEV standard full-field ERG protocol using RETeval. The ISCEV extended protocol uses a short stimulus (< 5 ms) of red light (630–660 nm; 1.0–2.5 phot cd s/m2) on a blue-saturated background (450–485 nm; 10 phot cd/m2) after pupil dilation and 10 min of photopic adaptation [11]. We dilated the pupils of participants maximally using tropicamide and phenylephrine. After wiping the skin with ethanol, RETeval sensor-strip skin electrodes were attached 2 mm from the lower eyelid margin. Stimuli consisting of 1.0 cds/m2 red flashes (621 nm) on a 10 cd/m2 blue background (470 nm) were presented with a full-field LED stimulator. The stimulus light was emitted at a frequency of 3.4 Hz, and one PhNR measurement was performed 100 times. The average of the three PhNR measurements was used in analyses.
We investigated 6 PhNR parameters and 2 ERG parameters as follows: (1) base-to-trough (BT, the difference between the pre-stimulus baseline and the PhNR depression); (2) peak-to-trough (PT, the difference between the peak of the b-wave and the PhNR trough); (3) 72msPhNR (the PhNR amplitude at 72 ms); (4) the W-ratio; (5) the P-ratio; (6) the implicit time (IT) of the minimum PhNR; (7) the a-wave amplitude of ERG; and (8) the b-wave amplitude of ERG [12]. The W-ratio and P-ratio were calculated using the following formulas, which were used in previous studies [13, 14]:
Optical coherence tomography and visual field measurement
We measured optical coherence tomography (OCT) using swept-source (SS) OCT (DRI-OCT Triton; Topcon Corporation, Tokyo). SS-OCT was performed after maximal pupillary dilation. We used an optic disc 3D scan protocol with a 6 × 6 mm optic disc circumference and a macula 3D scan protocol with a 7 × 7 mm square of the macula. The averages of the circumpapillary (cp) RNFL and macular ganglion cell inner plexiform layer (mGCIPL) were investigated.
All glaucoma patients underwent a visual field examination with a Humphrey Visual Field Analyzer (HFAIII model 860; Carl Zeiss Meditec, Dublin, CA). MD was measured as the average sensitivity of the entire visual field and the pattern standard deviation (PSD) as a parameter of a visual field shape irregularity showing variations in sensitivity at each test point from the age-corrected normal pattern. The ERG recording, OCT measurements, and visual field test were all conducted within approximately 1 month.
Statistical analysis
The normality of data distribution was investigated with a normal quantile–quantile plot (Q-Q plot). Values are expressed as the mean ± standard deviation (SD). In statistical analyses, we used the Kruskal–Wallis test with Dunn’s multiple test and Spearman’s rank correlation coefficient. P-values < 0.05 indicated a significant difference. Statistical analyses were performed with GraphPad Prism 9 (GraphPad Software, San Diego, CA) and IBM SPSS statistics (version 27.0; IBM, Armonk, NY).
Data quality checks
We used data quality checks to perform a discriminant analysis. A discriminant analysis may be used to provide thresholds for lesion diagnosis using diagnostic indices. In the diagnosis of glaucoma using the PhNR, previous studies compared the diagnostic ability of various PhNR parameters using a receiver operating characteristic curve (ROC) analysis; however, this analysis has not clearly identified diagnostic thresholds suggestive of POAG for the individual parameters. In the present study, validity was verified by giving this diagnostic threshold and showing its effect. A discriminant analysis is a method that calculates the possibility of the discrimination of categorical data or ordinal data, or a discriminant criterion, and has the characteristic of being able to clarify results even with a small number of data points. However, the quality of statistical data is critical for unlocking this powerful capability. The following two criteria must be met.
-
1.
Each group shows a normal distribution for the diagnostic parameters.
-
2.
All groups have the same covariance matrix (homogeneous variance).
Regarding criterion 2, each group uses a different covariance matrix (a scalar matrix if the number of independent variables is one, as in this analysis) to calculate the Mahalanobis distance and find equidistant points. By using a discriminant line (surface), it may be removed from the precondition. This method gives correct results when the normal distribution holds. In this analysis, the precondition of 2 was removed by this method.
Confirmation of a normal distribution
The normalized Quantile–Quantile (Q-Q) plot confirmed whether each diagnostic parameter followed a normal distribution. Supplemental tables 1a and 1b show descriptive statistics for each RETeval parameter in each group. Supplemental figures 1a and 1b are Q-Q plots for BT and 72msPhNR, which were later judged to have a high diagnostic effect. The X-axis is the observed value and the Y-axis is the expected normal frequency. Since the central part does not markedly deviate from the 45° line for any parameter in any groups, it was apparent that a discriminant analysis was applicable to search for the discriminant function and optimize the threshold value.
Results
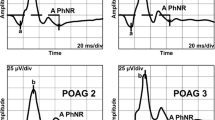
We recorded the PhNR in the control and POAG groups using RETeval. Representative waveforms of the PhNR in the control and POAG groups are shown in Fig. 1a and b. The PhNR waveform was smaller in the POAG group than in the control group.
Comparison of individual PhNR parameters between POAG and control groups
Supplemental table 2a shows the mean values for PhNR and ERG parameters. The means of BT and 72msPhNR significantly differed between the mild POAG and control groups (p = 0.0041 and p = 0.0229, respectively). Significant differences were observed in BT (p < 0.0001), PT (p < 0.0001), 72msPhNR (p < 0.0001), the W-ratio (p < 0.0001), and the P-ratio (p < 0.0001) between the moderate-to-advanced POAG and control groups. Significant differences were also noted in mean BT (p < 0.0001), PT (p = 0.0004), 72msPhNR (p < 0.0001), the W-ratio (p < 0.0001), and the P-ratio (p < 0.0001) between the all POAG (all stages) and control groups. Neither the a-wave nor b-wave on ERG significantly differed between the mild, moderate-to-advanced, or all POAG groups and the control group.
Supplemental table 2b shows the area under the curve (AUC) values for each parameter between the control and POAG groups. Figure 2a and b show comparisons of ROC curves for each PhNR parameter. According to AUC, BT was the most useful parameter for distinguishing patients with mild or moderate-to-advanced POAG from control patients.
a ROC curves for each PhNR parameter between control and mild POAG groups. BT: purple; PT: green; the W-ratio: yellow; 72msPhNR: pink; the P-ratio: black; IT: orange. b ROC curves for each PhNR parameter between control and moderate-to-advanced POAG groups. BT: purple; PT: green; the W-ratio: yellow; 72msPhNR: pink; the P-ratio: black; IT: orange
The discriminant linear function for examining the presence or absence of POAG and the threshold for diagnosis were calculated as follows: regarding BT: discriminant = 0.505 × BT + 2.017; threshold = positive for POAG, negative for no POAG; correct answer rate = 80.7%. Concerning 72msPhNR: discriminant = 0.533 × 72msPhNR + 1.553; threshold = positive for POAG and negative for no POAG; correct answer rate = 77.1%.
Relationships between MD, PSD, and each PhNR parameter
Supplemental table 3 shows average MD and PSD values in each stage of POAG. Supplemental tables 4a (MD) and 4b (PSD) show the relationships between the visual field index and each PhNR parameter.
In the mild POAG group, none of the PhNR parameters correlated with MD. In the moderate-to-advanced POAG group, MD correlated with BT (p < 0.0001), 72msPhNR (p = 0.0023), the W-ratio (p = 0.0002), and the P-ratio (p = 0.0028), but not with PT or IT. In the all POAG group (all stages), MD correlated with BT (p < 0.0001), 72msPhNR (p < 0.0001), the W-ratio (p < 0.0001), and the P-ratio (p < 0.00001), but not with PT or IT.
In terms of PSD, PSD did not correlate with any PhNR parameter in the mild POAG group. In the moderate-to-advanced POAG group, only PT correlated with PSD (p = 0.0202). In the all POAG group, PSD correlated with BT (p = 0.0007), 72msPhNR (p = 0.0008), the W-ratio (p < 0.0001), and the P-ratio (p = 0.0030), but not with PT or IT.
Relationships between OCT parameters and each PhNR parameter
The following results were obtained for OCT parameters. Supplemental table 5 shows average cpRNFL and GCIPL in each stage of POAG. Supplemental tables 6a (cpRNFL) and 6b (GCIPL) showed the relationships between OCT parameters and each PhNR parameter. In the mild POAG group, cpRNFL did not correlate with any PhNR parameter. In the moderate-to-advanced POAG group, cpRNFL correlated with BT (p = 0.0120), 72msPhNR (p = 0.0015), the W-ratio (p = 0.0454), and the P-ratio (p = 0.0036), but not with PT or IT. In the all POAG group, cpRNFL correlated with BT (p < 0.0001), 72msPhNR (p < 0.0001), the W-ratio (p < 0.0001), and the P-ratio (p < 0.00001), but not with PT or IT.
GCIPL did not correlate with any PhNR parameter in the mild or moderate-to-advanced POAG group. In the all POAG group, GCIPL correlated with BT (p = 0.0252), 72msPhNR (p = 0.0031), the W-ratio (p = 0.0343), and the P-ratio (p = 0.0316), but not with PT or IT.
Relationship between axial length and each PhNR parameter
We investigated the relationship between the axial length of the eye and individual PhNR parameters in the control group. The relationship between axial length and PhNR parameters was not examined in the POAG groups in order to exclude the effects of RGC loss. The axial length of the eye did not correlate with BT (n = 60, r = -0.141, p = 0.142), PT (n = 60, r = 0.039, p = 0.384), 72msPhNR (n = 60, r = -0.067, p = 0.305), the W-ratio (n = 60, r = 0.013, p = 0.460), or the P-ratio (n = 60, r = 0.096, p = 0.232) in the control group.
Discussion
The present study investigated whether an electrophysiological evaluation of RGC function in glaucomatous eyes may be performed by measuring the PhNR using RETeval, a non-invasive and convenient ERG measurement device. To establish whether a functional evaluation of RGCs in glaucoma was possible using the PhNR, we initially examined whether PhNR parameters significantly differed between the control and POAG groups using RETeval. The results obtained revealed significant differences in PhNR parameters between the groups. Previous studies investigated the relationship between the PhNR and severity of glaucoma [14,15,16,17,18,19], and at least two measured the PhNR in glaucoma to assess the feasibility of measuring the PhNR of full-field ERG using skin electrodes instead of conjunctival electrodes for open angle glaucoma [9, 15]. To the best of our knowledge, this is the first study using RETeval to establish thresholds for individual PhNR parameters that are diagnostic for the presence or absence of POAG. Therefore, the present results will make an important contribution to the future diagnosis of POAG.
In the present study, we investigated six PhNR parameters (BT, PT, 72msPhNR, the W-ratio, P-ratio, and IT) and two ERG parameters (the a-wave amplitude and b-wave amplitude). Kita et al. also investigated PhNR parameters in POAG and found that four PhNR parameters (BT, 72msPhNR, the W-ratio, and P-ratio) significantly differed between controls and patients with POAG [9]. They also demonstrated that 72msPhNR performed the best for correlation strength and generality with MD and OCT, and the AUC value of the best-performing PhNR parameters were BT in mild POAG and the W-ratio in moderate-to-advanced POAG. These findings suggest the potential of the RETeval ERG system for detecting moderate-to-advanced POAG. In the present study, the AUC values of PhNR parameters were higher in the moderate-to-advanced POAG group than in the mild POAG group. In addition, the relationships between PhNR parameters and MD and cpRNFL were stronger in the moderate-to-advanced POAG group than in the mild POAG group, which demonstrated that PhNR parameters were more useful for the detection of moderate-to-advanced POAG than mild POAG. Furthermore, the AUC value was the highest for BT and the second highest for 72msPhNR in both the mild and moderate-to-advanced POAG groups in the present study. The four best-performing RETeval PhNR parameters (BT, 72msPhNR, the W-ratio, and P-ratio) proposed by Kita et al. also had significant AUC values in the present study in both the mild and moderate-to-advanced POAG groups; therefore, the present results are consistent with the findings of Kita et al.
In clinical settings, the detection of early-stage or preperimetric POAG is important, particularly in those without obvious damage in the visual field or RNFL. However, in the present study, RETeval-measured PhNR parameters were more useful for an objective evaluation of visual function in the moderate-to-advanced POAG group than in the mild POAG group. Regarding PERG, which is similar to the PhNR, changes have been reported to occur prior to visual field losses [13]. While similar findings have been reported for the PhNR, it was not reduced in ocular hypertension; therefore, further studies are warranted [13]. Since RGC damage in mild POAG is localized, full-field PhNR is not useful for mild glaucoma because it cannot detect localized RGC damage [19]. To overcome this issue, the focal PhNR may be measured to more sensitively detect local RGC damage [20]. However, in the present study, the focal PhNR was difficult to measure using RETeval. Furthermore, the amplitude of the PhNR was smaller than that of the PhNR using a conventional contact lens electrode, which increases the difficulties associated with detecting early glaucoma. The disadvantage of the PhNR measurement using RETeval is that it cannot capture the small changes that occur in mild POAG. Therefore, other methods, such as focal PhNR and PERG, are considered better for assessing the objective function of the inner retinal layer in mild POAG.
We used the W-ratio and P-ratio as the PhNR/b-wave ratio because previous studies demonstrated that this approach had several advantages. Mortlock et al. found that the ratio of the b-wave to the PhNR amplitude had the lowest level of inter-individual, inter-session, and inter-ocular variability of all measurement techniques [14]. Moreover, Fortune et al. reported that the PhNR/b-wave ratio varied less than absolute amplitude responses in rhesus monkeys [21]. Machida et al. demonstrated that the b-wave/PhNR amplitude ratio more strongly correlated with the increasing severity of glaucomatous field defects and retinal nerve fiber layer thickness than absolute amplitude values [20]. In the present study, the W-ratio and P-ratio in the mild, moderate-to-advanced, and all POAG groups significantly different from those in the control group, and these two PhNR parameters correlated with MD or cpRNFL thickness in the moderate-to-advanced POAG and all POAG groups. However, the AUC value was the highest in BT in the mild or moderate-to-advanced POAG group. According to a review of the PhNR reported by Prencipe et al., BT is the most reliable parameter for patient evaluations [12]. However, since the most useful PhNR parameters slightly differed among studies, further studies are needed.
Relationships have been reported between the PhNR and visual field [9, 17, 18, 22,23,24,25,26]. Viswanathan et al. [17] noted that the PhNR was markedly reduced in the eyes of macaque monkeys with experimental glaucoma when visual field defects were measured by behavioral perimetry. They were also the first group to show that the PhNR amplitude linearly correlated with MD measured by static visual field tests [18]. In addition, the PhNR has been shown to correlate with the visual field in other diseases. Park et al. [22] reported relationships between the PhNR elicited using full-field ERG and the Humphrey visual field mean deviation in idiopathic intracranial hypertension. Moon et al. [23] demonstrated that the PhNR/b-wave amplitude ratio correlated with postoperative MD and the temporal visual field in patients with chiasmal compression. In the present study, several PhNR parameters correlated with the MD values of static visual field tests in the all POAG group. In a previous study using RETeval, Kita et al. reported that four PhNR parameters (BT, the W-ratio, 72msPhNR, and the P-ratio) correlated with MD values in patients with moderate-to-advanced POAG [9]. In the present study, the same four PhNR parameters also correlated with MD values in the moderate-to-advanced POAG group. Moreover, Kita et al. reported that PhNR parameters more strongly correlated with MD than with PSD, which was consistent with the present results.
A correlation between the PhNR amplitude and RNFL thickness has also been reported [15, 16, 19, 22,23,24,25,26,27,28,29,30]. In glaucoma, Cvenkel et al. [30] showed that the PhNR/b-wave ratio had the strongest relationship with a peripapillary retina and macular RNFL thickness, and peripapillary RNFL thickness in eyes with mild glaucoma showed the strongest correlation with the PhNR amplitude and PERG P50 amplitude. Kim et al. [25] found that RNFL thickness correlated with the amplitudes and implicit times of the PhNR. Moreover, Machida et al. [19] reported that the PhNR amplitude and PhNR/b-wave amplitude ratio correlated with the RNFL thickness, the rim area of the optic disc, and the cup/disc area ratio. Regarding diseases other than glaucoma, Park et al. [22] performed OCT for patients with idiopathic intracranial hypertension, and noted relationships between the full-field PhNR amplitude and GCC volume, the latter of which was measured within a 3-mm cylinder centered on the fovea. In a linear regression analysis, Abed et al. revealed that the PhNR amplitude and RNFL thickness positively correlated in childhood optic gliomas [29]. Wu et al. used skin electrodes to record the PhNR in glaucoma patients, [15] and demonstrated that the PhNR/B ratio correlated with RNFL thickness, decreasing by 0.02 units on average for every 10-μm reduction in RNFLT. Kita et al. reported correlations between the four best-performing PhNR parameters (BT, the W-ratio,72msPhNR, and the P-ratio) and the average of cpRNFL in all study patients [9]. In the present study, the same four PhNR parameters correlated with cpRNFL and GCIPL in the all POAG group (all stages). In addition, these four PhNR parameters correlated with cpRNFL in the moderate-to-advanced POAG group. The present results suggest that the PhNR plays a similar role to OCT as a test to supplement the floor effect of visual field test results in advanced POAG. Regarding GCIPL, GCL thickness may be a more sensitive parameter than RNFL, particularly in mild POAG. However, in the present study, GCIPL did not correlate with any PhNR parameter in the mild POAG group. Since the method used in the present study was full-field PhNR, it may have been difficult to detect a localized deterioration in GCIPL in the mild POAG group.
Regarding the relationship between the PhNR and axial length, Kato et al. showed that the W-ratio correlated with axial length [31]. We also investigated the relationship between PhNR parameters and axial length in the control group and found no correlations. However, since limited information is currently available on the relationship between the PhNR and axial length, future studies are needed to accumulate sufficient data. In the present study, age significantly differed between the control and mild POAG groups (Table 1). Joshi et al. reported that the correlations between age and PhNR parameters were weak and were not significant after the application of Holm’s correction for multiple comparisons [32]. Therefore, we herein decided to use the data shown in Table 1 without corrections.
Glaucoma occurs due to RGC damage, whereas the functions of bipolar cells and photoreceptors are not markedly affected. The visual evoked potential (VEP) and PERG are objective RGC function tests; however, VEP does not directly reflect the function of RGCs because it uses electroencephalograms [33]. When we measure PERG, additional steps, such as refractive correction and the preparation of a pattern stimulator, are required. There are three advantages to using the PhNR to evaluate RGC functions [33]. The PhNR may be measured using a conventional ERG system. Furthermore, a-waves and b-waves may be simultaneously recorded, and the functions of the outer and middle layers of the retina may also be evaluated because the PhNR is one of the components of ERG. In addition, the PhNR does not require additional steps (refractive correction, meticulous fixation monitoring, and ocular media transparency). In brief, the PhNR measurement is convenient.
In the present study, we measured the PhNR using RETeval. RETeval also has three advantages. It is a small and portable system that use skin electrodes, which are easy to attach to subjects, and we are able monitor whether patients open their eyes when measuring ERG. Due to these advantages, the PhNR may be examined in childhood glaucoma and glaucoma patients in the early postoperative period using RETeval.
Conclusion
PhNR parameters in eyes with POAG significantly differed from those in control subjects, and each PhNR parameter correlated with the MD value of visual field tests and RNFL thickness. Furthermore, the correlations between PhNR parameters and the visual field and those between PhNR parameters and RNFL thickness were stronger in moderate-to-advanced than mild glaucoma. These results suggest that the measurement of the PhNR in full-field ERG using the RETeval system improves the electrophysiological evaluation of the inner retinal layer function of glaucoma, particularly moderate-to-advanced glaucoma. We quantitatively obtained the discriminant linear function for examining the presence or absence of POAG and the threshold for diagnosis in the present study. Further studies are needed to investigate the relationship between the degree of disease progression and the PhNR in glaucoma, and we aim to examine the progression of visual function deterioration in those who cannot take visual field tests, such as children and the elderly with dementia.
References
Gedde SJ, Vinod K, Wright MM et al (2021) Primary open-angle glaucoma preferred practice pattern®. Ophthalmology 128:71–150
Iwase A, Suzuki Y, Araie M et al (2004) The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 111(9):1641–1648
Morizane Y, Morimoto N, Fujiwara A et al (2019) Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol 63(1):26–33
Senger C, Moreto R, Watanabe SES et al (2020) Electrophysiology in Glaucoma. J Glaucoma 29(2):147–153
Tang J, Hui F, Coote M et al (2018) Baseline detrending for the photopic negative response. Transl Vis Sci Technol 7(5):9
Wu Z, Hadoux X, Hui F et al (2016) Photopic negative response obtained using a handheld electroretinogram device: determining the optimal measure and repeatability. Transl Vis Sci Technol 5(4):8
LKC Technologies, Inc. (2023) RETeval™ Device User Manual. https://lkc.com/wp-content/uploads/2023/02/96-023-EN-RETeval-Device-User-Manual-2023-02-08_A4.pdf
Tang J, Hui F, Hadoux X et al (2018) A comparison of the reteval sensor strip and DTL electrode for recording the photopic negative response. Transl Vis Sci Technol 7(6):27
Kita Y, Holló G, Saito T et al (2020) RETeval portable electroretinogram parameters in different severity stages of glaucoma. J Glaucoma 29(7):572–580
The Japan Glaucoma Society (2022) The Japan glaucoma society guidelines for glaucoma (5th Edition). Nippon Ganka Gakkai Zasshi 126:85–177
Frishman L, Sustar M, Kremers J et al (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol 136(3):207–211
Prencipe M, Perossini T, Brancoli G et al (2020) The photopic negative response (PhNR): measurement approaches and utility in glaucoma. Int Ophthalmol 40:3565–3576
Preiser D, Lagrèze WA, Bach M et al (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54(2):1182–1191
Mortlock KE, Binns AM, Aldebasi YH et al (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121(2):123–134
Wu Z, Hadoux X, Fan Gaskin JC et al (2016) Measuring the photopic negative response: viability of skin electrodes and variability across disease severities in glaucoma. Transl Vis Sci Technol 5(2):13
Macihda S (2012) Clinical applications of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. https://doi.org/10.1155/2012/397178
Viswanathan S, Frishman LJ, Robson JG et al (1999) The Photopic negative response of the macaque electroretinogram; reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Viswanathan S, Frishman LJ, Robson JG et al (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Machida S, Gotoh Y, Toba Y et al (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207
Machida S, Toba Y, Ohtaki A et al (2008) Photopic negative response of focal electroretinograms in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(12):5636–5644
Fortune B, Bui BV, Cull G et al (2004) Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates. Exp Eye Res 78(1):83–93
Park JC, Moss HE, McAnany JJ (2018) Electroretinography in idiopathic cranial hypertension: comparison of the pattern ERG and the photopic negative response. Doc Ophthalmol 136(1):45–55
Moon CH, Hwang SC, Kim BT et al (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52(11):8527–8533
Shen X, Huang L, Fan N et al (2013) Relationship among photopic negative response, retinal nerve fiber layer thickness, and visual field between normal and POAG eyes. ISRN Ophthalmol. https://doi.org/10.1155/2013/182021
Kim HD, Park JY, Ohn YH (2010) Clinical application of photopic negative response (PhNR) for the treatment of glaucoma and diabetic retinopathy. Korean J Ophthalmol 24(2):89–95
Huang L, Shen X, Fan N et al (2012) Clinical application of photopic negative response of the flash electroretinogram in primary open-angle glaucoma. Eye Sci 27(3):113–118
Miyata K, Nakamura M, Kondo M et al (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48(2):820–824
Wang J, Cheng H, Hu YS et al (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53(3):1315–1323
Abed E, Piccardi M, Rizzo D et al (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56(4):2469–2474
Cvenkel B, Sustar M, Perovšek D (2017) Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc Ophthalmol 135:17–28
Kato K, Sugawara A, Nagashima R et al (2020) Factors affecting photopic negative response recorded with ret eval system: study of young healthy subjects. Trans Vis Sci Technol 9(9):19
Joshi NR, Ly E, Viswanathan S (2017) Intensity response function of the photopic negative response (PhNR): effect of age and test-retest reliability. Doc Ophthalmol 135(1):1–16
Machida S (2011) PhNR and Neuro-ophthalmology- clinical application of phnr to optic nerve and inner retinal diseases. Neuro-ophthalmol Jpn 28(2):141–152
Acknowledgements
We would like to express our gratitude to Specially Appointed Professor Kenji Araki (Faculty of Engineering, University of Miyazaki) for his advice on statistical analyses in this research.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP 20K18385.
Funding
This study was funded by JSPS KAKENHI in the form of a Grant-in-Aid for Young Scientists (B) (Grant number JP 20K18385). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of the Faculty of Medicine, University of Miyazaki and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Research Ethics Committee of the Faculty of Medicine, University of Miyazaki (study number: O-0670).
Informed consent
All patients provided informed consent and public announcements of this study were performed. Details of the study were made available on the university website.
Conflict of interest
Hidaka T and Chuman H certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ikeda Y has received financial support from Alcon Japan Ltd., Senju Pharmaceutical Co., Ltd., Santen Pharmaceutical Co., Ltd., HOYA Corporation, Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., and Ellex, Inc. Ikeda Y has received personal financial interest from Vixion Corporation. Ikeda Y has served as a consultant for Novartis Pharma K.K., HOYA Corporation, and Vixion Corporation. Ikeda Y has a patent to ID Pharma Co., Ltd. Ikeda Y has received royalties from Bayer Yakuhin, Ltd., Kowa Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Vixion Corporation, Chugai Pharmaceutical Co., Ltd., NIDEK Co., Ltd., HOYA Corporation, Santen Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., and Novartis Pharma K.K.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
417_2023_6193_MOESM1_ESM.docx
Supplementary file1. Supplemental Figure 1 a: Q-Q plots for BT. The X-axis is the observed value and the Y-axis is the expected normal frequency. Supplemental Figure 1b: Q-Q plots for 72msPhNR. The X-axis is the observed value and the Y-axis is the expected normal frequency (DOCX 176 KB)
417_2023_6193_MOESM2_ESM.docx
Supplementary file2. Supplemental Table 1a: Descriptive statistics for each RETeval parameter in the control group. Supplemental Table 1b: Descriptive statistics for each RETeval parameter in the all POAG group (DOCX 18 KB)
417_2023_6193_MOESM3_ESM.docx
Supplementary file3. Supplemental Table 2a: PhNR and ERG parameters measured using RETeval in control and mild, moderate-to-advanced, and all POAG groups. Supplemental Table 2b: AUC for PhNR parameters measured using RETeval for the control versus mild POAG group and the control versus moderate-to-advanced POAG group (DOCX 16 KB)
417_2023_6193_MOESM4_ESM.docx
Supplementary file4. Supplemental Table 3: MD and PSD of the Humphrey visual field test in mild, moderate-to-advanced, and all POAG groups. Supplemental Table 4a: Relationships between the MD value of the visual field and PhNR parameters in mild, moderate-to-advanced, and all POAG groups (DOCX 15 KB)
417_2023_6193_MOESM5_ESM.docx
Supplementary file5. Supplemental Table 4b: Relationships between the PSD value of the visual field and PhNR parameters in mild, moderate-to-advanced, and all POAG groups (DOCX 17 KB)
417_2023_6193_MOESM6_ESM.docx
Supplementary file6. Supplemental Table 5: Average cpRNFL and GCIPL of OCT in mild, moderate-to-advanced, and all POAG groups (DOCX 15 KB)
417_2023_6193_MOESM7_ESM.docx
Supplementary file7. Supplemental Table 6a: Relationships between cpRNFL and PhNR parameters in mild, moderate-to-advanced, and all POAG groups. Supplemental Table 6b: Relationships between GCIPL and PhNR parameters in mild, moderate-to-advanced, and all POAG groups (DOCX 17 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hidaka, T., Chuman, H. & Ikeda, Y. Evaluation of inner retinal function at different stages of primary open angle glaucoma using the photopic negative response (PhNR) measured by RETeval electroretinography. Graefes Arch Clin Exp Ophthalmol 262, 161–169 (2024). https://doi.org/10.1007/s00417-023-06193-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06193-z