Abstract
Purpose
To investigate the distribution of hyperreflective foci (HRF) in eyes with dry age-related macular degeneration (AMD).
Methods
We retrospectively reviewed optical coherence tomography (OCT) images of 58 dry AMD eyes presenting HRF. The distribution of HRF according to the early treatment diabetic retinopathy study area was analyzed according to the presence of subretinal drusenoid deposits (SDDs).
Results
We classified 32 eyes and 26 eyes into the dry AMD with SDD group (SDD group) and dry AMD without SDD group (non-SDD group), respectively. The non-SDD group had higher prevalence and density of HRF at the fovea (65.4% and 1.71 ± 1.48) than the SDD group (37.5% and 0.48 ± 0.63, P = 0.035 and P < 0.001, respectively). However, the prevalence and density of HRF in the outer circle area of the SDD group (81.3% and 0.11 ± 0.09) were greater than those of the non-SDD group (53.8% and 0.05 ± 0.06, p = 0.025 and p = 0.004, respectively). The SDD group showed higher prevalence and mean densities of HRF in the superior and temporal area than in the non-SDD group (all, p < 0.05).
Conclusion
HRF distributions in dry AMD varied according to the presence of SDDs. This might support that the degenerative features may be different between dry AMD eyes with and without SDDs.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a disease characterized by drusen and associated degenerative changes in retinal pigment epithelium (RPE), photoreceptors, Bruch’s membrane, and choriocapillaris [1]. The pathogenesis of AMD is not entirely understood; however, previous studies suggested that RPE integrity is essential for photoreceptor function and RPE degeneration have an essential role during development and progression of the disease in most AMD cases [1].
Among the various optical coherence tomography (OCT) biomarkers for AMD, hyperreflective foci (HRF) have been suggested to be an important risk factor for the progression of early AMD to advanced AMD [2, 3]. HRF are typically detected in the neurosensory retina in areas with RPE atrophy and outer retinal atrophy associated with drusen, which has been suggested as an indicator of overall disease activity [4]. Several studies suggested that the HRF on OCT and hyperpigmentation on color fundus photography had highly specific spatial correlation [5, 6].
With the development of ophthalmic imaging techniques, subtypes of drusen had been classified into several categories and different clinical features according to subtype have been suggested [7]. Subretinal drusenoid deposits (SDDs), also known as reticular pseudodrusen, have different features when compared with soft drusen [7,8,9]. Eyes with SDDs have different degenerative features during disease progression manifested by outer retina degeneration and RPE in the absence of drusen [10]. In addition, these eyes also exhibit greater risk of development of type 3 macular neovascularization (MNV) and multifocal geographic atrophy (GA) [11,12,13]. These different clinical features support the idea that soft drusen and SDDs might have different underlying pathogenesis [14, 15].
Based on recent studies supporting the role of varied degenerative features during AMD progression according to subtype in early AMD, we proposed that the features of RPE migration into the retina may be different according to drusen type [10, 11, 13, 14]. In this study, we investigated HRF distribution in eyes with early AMD with or without SDDs and its associated characteristics.
Methods
This study was performed following the Declaration of Helsinki after the approval of the Institutional Review Board of Korea University Medical Center. We retrospectively reviewed the medical records of consecutive patients who were diagnosed with early or intermediate AMD between January 2018 and June 2021 at Korea University Medical Center. To investigate the features of HRF, we included early or intermediate AMD eyes with HRF in the macular area and excluded patients with bilateral late AMD including neovascular AMD or GA [16]. Eyes with a history of vitreoretinal surgery, laser treatment, retinal vascular disease including retinal vein occlusion, diabetic retinopathy or retinal arterial occlusion, and/or high myopia with an axial length greater than 26.0 mm were excluded.
Patients underwent comprehensive ophthalmic examination including wide-field fundus photography using Optos (Dunfermline, United Kingdom), optical coherence tomography (OCT), and near-infrared and blue reflectance images using the SPECTRALIS HRA system (Heidelberg Engineering, Heidelberg, Germany) [16]. The patients were diagnosed with early or intermediate AMD based on previously suggested criteria on fundus photograph and OCT [17]. SDDs were defined if reticular lesions within the macular area identified on fundus photos and near-infrared or blue reflectance images and the corresponding area showed 5 or more hyper-reflective triangular lesions above the RPE based on OCT [12, 16, 18]. Drusen volume (mm3) was calculated from the thickness map using built-in OCT software. The mean volume of the area between the outer border of the RPE and Bruch’s membrane was obtained after a manual adjustment of segmentation lines. Then, the mean macular drusen volume of each sector of the early treatment of diabetic retinopathy study (ETDRS) grid was used to calculate the total drusen volume in the macular area. The mean ganglion cell-inner plexiform layer (GCIPL) thickness and retinal thickness were obtained from the ETDRS grid. The mean choroidal thickness was defined as the mean of measurements at five points (fovea, 1,500 and 3,000 µm both nasally and temporally from the fovea) using horizontal line scans acquired using a previously described enhanced-depth imaging technique [18].
Based on previous studies, HRF is defined as a discrete, well-circumscribed lesion with a size greater than 15 µm of which reflectivity is similar to or greater than that of the RPE band on OCT and located above the RPE band [6, 19, 20]. Using volume scan images of the OCT, which covers at least a 6- × 6-mm area with a 120-µm interscan distance, two retinal specialists (D. K. and K. N.) independently identified HRF in the retina manually by reviewing the B-scans at 400% magnification and identified the HRF location in the 6-mm zone of the ETDRS area: 1) fovea, 2) superior, nasal, inferior, and temporal sectors of the inner ring 1–3 mm zone, and 3) superior, nasal, inferior, and temporal sectors of the outer ring 3–6 mm zone. Cases with HRF not separated by hyporeflectivity were counted as one HRF [20]. The mean densities of HRF in the superior, nasal, inferior, and temporal sectors were calculated as total number of HRF / area (mm2). If disagreement between the two investigators was present, a third reviewer (C. Y.) reviewed the case and made the final decision.
Considering biological correlations of two eyes from a single patient, one eligible eye was chosen for the analysis. If both eyes were eligible, the right eye was chosen. The kappa coefficient and intraclass correlation coefficients were used to evaluate the interobserver reliabilities for the categorical and continuous variables, respectively. Statistical Package for the Social Sciences version 20.0 for Windows software program (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. Independent t-tests were used for the analysis of continuous variables and chi-squared tests or Fisher’s exact tests were used for categorical variables. A p-value less than 0.05 was considered statistically significant.
Results
A total of 58 eyes from 58 patients were included and 26 eyes and 32 eyes were classified into the non-SDD and SDD groups based on the presence of SDDs, respectively. Mean age, sex, and history of hypertension or diabetes did not differ between the two groups (all p > 0.05; Table 1). The mean choroidal thickness of the non-SDD group (155.39 ± 54.15 µm) was greater than that of the SDD group (129.10 ± 25.80 µm; p = 0.018). Mean visual acuity, retinal thickness, ganglion cell-inner plexiform layer (GCIPL) thickness, AMD stage, and drusen volume were not different between the two groups (all p > 0.05).
Prevalence of HRF
The distribution of HRF in the non-SDD group showed 65.4% at the fovea; 34.6%, 42.3%, 30.8%, and 26.9% at the respective superior, nasal, inferior, and temporal sectors of the inner circle; and 19.2%, 19.2%, 26.9%, and 15.4% at superior, nasal, inferior, and temporal sectors of the outer circle. The SDD group showed HRF of 37.5% at the fovea; 62.5%, 25.0%, 18.8%, and 56.3% at the superior, nasal, inferior, and temporal sectors of the inner circle, respectively; and 59.4%, 40.6%, 12.5%, and 40.6% at the superior, nasal, inferior, and temporal sector of outer circle (p = 0.035, 0.035, 0.163, 0.287, 0.025, 0.002, 0.080, 0.163 and 0.036, respectively) (Fig. 1). At the inner circle (< 3 mm area) area, the non-SDD group showed HRF of 88.5%, similar to the 84.4% of the SDD group (p = 0.654). However, the SDD group showed a higher prevalence of HRF at the outer circle (3 to 6 mm area) area, with 81.3% relative to 53.8% in the non-SSD group (p = 0.025). The kappa coefficients for the presence of HRF in each area ranged from 0.668 to 0.931. Representative cases are presented in Figs. 2 and 3.
Distribution of hyperreflective foci (HRF) in the macula according to the early treatment diabetic retinopathy study (ETDRS) area between age-related macular degeneration (AMD) eyes with and without subretinal drusenoid deposits (SDDs). (A) The non-SDD group (AMD eyes without SDDs) showed a higher prevalence of HRF at the fovea while the the SDD group (AMD eyes with SDDs) showed a higher prevalence of HRF in the superior and temporal sectors of the inner and outer circle areas. (B) The SDD group showed higher prevalence of HRF at the outer circle area. Gray-colored areas with an asterisk (*) indicate significant difference of prevalence between the two groups, with a p-value < 0.05
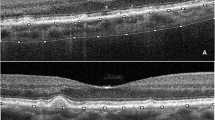
Representative fundus and optical coherence tomography (OCT) scan images of a 79-year-old male patient with dry age-related macular degeneration (AMD) without subretinal drusenoid deposit. (A to B) Soft drusen are located at the fovea. (C) The hyperreflective foci are located at and around the fovea, and the locations are indicated as black dots. (D to G) The hyperreflective foci are indicated with arrows on each OCT scan
Representative fundus and optical coherence tomography (OCT) scan images of a 70-year-old female patient with dry age-related macular degeneration (AMD) with subretinal drusenoid deposits. (A to B) Soft drusen are located at the fovea, and reticular pseudodrusen are found on the macula area (C) The hyperreflective foci are located at the inner and outer circles of the macula superotemporally, and the locations are indicated as black dots. (D to G) The hyperreflective foci are indicated with arrows on each OCT scan image
Number and density of HRF
The mean number of HRF in the SDD group (5.28 ± 2.16) was not different from that of the non-SDD group (4.42 ± 1.30, p = 0.081). The mean number and density of HRF at the fovea of the non-SDD group (1.35 ± 1.16 and 1.71 ± 1.48) were greater than those of the SDD group (0.38 ± 0.49, 0.48 ± 0.63, p < 0.001) (Table 2). The mean number and density of HRF at the inner circle of the SDD group (2.50 ± 1.74 and 0.40 ± 0.28) were not different from those of the non-SDD group (2.00 ± 1.20 and 0.32 ± 0.19, p = 0.219). However, the mean number and density of HRF at the outer circle of the SDD group (2.41 ± 1.92 and 0.11 ± 0.09) were greater than those of the non-SDD group (1.08 ± 1.29 and 0.05 ± 0.06, p = 0.004). The mean numbers and densities of HRF at superior and temporal areas of the inner and outer circles of SDD group were greater than those of non-SDD group (all, p < 0.05). The intraclass correlation coefficients for the mean number of HRF in each area ranged from 0.682 to 0.965.
Discussion
In this study, distributions of HRF differed according to the presence of SDDs. The non-SDD group showed higher prevalence of HRF at the fovea, while the SDD group showed higher prevalence of HRF in outer circle and superior and temporal areas. These different features suggest that the features of RPE degeneration vary in eyes with AMD according to the presence of SDDs.
RPE dysfunction has an important role in AMD disease progression and several lines of evidence suggest that phenotypic characteristics of AMD may come from the RPE dysfunction or degeneration [1, 3, 4, 6, 21]. Among these features, migrating RPE associated with drusen has been shown as HRF in OCT and these features are suggested to be a biomarker for AMD progression [1, 2, 4,5,6, 22]. Previous studies reported presence of HRF over drusen as a sign of RPE atrophy, and that HRF usually occurs in and around the fovea [21,22,23]. In this study, 65.4% and 88.5% of cases in the non-SDD group showed HRF at the fovea and inside the 3 mm area, respectively. This feature is consistent with previous reported studies about HRF in dry AMD that showed that the distribution of drusen and relatively lower presence of HRF outside the 3 mm area may be associated with the topographic distribution of drusen, which are usually centered on the fovea [2, 15]. However, the SDD group showed HRF both inside and outside the 3 mm area in about 80% of cases, and this might be a distinguishing feature compared with those without SDDs.
Schuman et al. reported that HRF in AMD eyes were signs of outer retinal atrophy characterized by photoreceptor thinning and RPE atrophy associated with drusen, which are precursors of GA [23]. However, recent studies suggested that outer retinal atrophy can be developed in an area with SDDs during their progression and regression [10, 12]. This type of retinal atrophy was suggested to be a new late form of AMD in addition to classic GA and neovascular AMD [10, 12]. In addition to the outer retinal atrophy associated with SDDs, concurrent loss and degeneration of the RPE are key features in eyes with SDDs [12, 24]. Previous studies about the histologic features of the RPE and its associated in vivo imaging data showed that eyes with SDD are characterized by retinal changes from the RPE to the outer nuclear layer accompanied by the RPE damage [25, 26]. The aberrant displacement of the RPE toward the outer retina was supposed to be a sign of RPE abnormality or dysfunction and might suggest an advanced stage of degeneration [25, 26]. The different distribution of HRF in AMD eyes with SDDs might be associated with the different features of degeneration in eyes with SDDs [10, 12]. The SDDs usually develop at the perifoveal area, initially sparing the fovea, and propagate diffusely to the adjacent area of the macula, which is different from that of soft drusen [15, 27]. As the degenerative process associated with SDDs is supposed to start at the perifoveal area following the sequence of SDD development, the degeneration of RPE and HRF might also follow topographic features [13, 28]. In addition to those topographic features, the diffuse and multifocal chorioretinal degenerative features in eyes with SDDs, which have been suggested to be associated with underlying overall choroidal ischemia, might also contribute to diffuse HRF distribution in the macula [18, 29].
The SDD group showed a higher prevalence of HRF in the superior and temporal sector of the macula than the non-SDD group in this study. The HRF has been considered to be a precursor for late AMD and the topographic features of these HRF might be associated with the location of late AMD [2, 4,5,6]. In GA, previous studies reported that the location of GA has no predilection; however, these studies did not consider the presence of SDDs [30, 31]. Recently, GA in eyes with SDDs has also been reported to have different characteristics when compared with those without SDDs [8, 13]. These eyes show a higher prevalence of perifoveal and multifocal GA [28, 31, 32]. However, the location of initial GA or nascent GA in eyes with SDDs have not been precisely reported.
Recently, Reiter et al. reported that the superior and temporal macular areas were associated with faster GA progression and photoreceptor degeneration in eyes with SDDs [28]. The authors suggested that these features might be associated with the distribution of SDDs, which are usually initially seen in the superior macula. Thus, the outer retina and RPE degeneration may start at the superior macula and follow the area with SDD propagation. This might contribute to the finding of the current study, which shows a higher prevalence of HRF in the superior and temporal macula in AMD eyes with SDDs.
In addition to GA, type 3 MNV is a neovascular form of late AMD that is strongly associated with SDDs [33, 34]. Type 3 MNV is likely associated with the migrated RPE in the inner retina and focally changed vascular endothelial growth factor concentration caused by the RPE [35]. These type 3 MNV have topographic characteristics with predilection for the superior and temporal macula and these features are supposed to be associated with similar topographic features of SDDs and type 3 MNV [36]. Overall, the topographic features of retinal degeneration and neovascularization in eyes with SDDs, which are predominantly located in the superior and temporal area, might also be associated with the topographic features of HRFs in eyes with SDDs in this study.
This study has several limitations. This study has a retrospective design and includes a small number of cases from one tertiary medical center. Lack of follow up data may limit the clinical importance of the study. Second, we did not investigate lesions outside the 6 × 6 mm ETDRS macular area. Thus, investigation of the limited area might not represent overall retinal changes. Third, even though three investigators assessed the HRF, the identification of HRF may depend on the investigator’s judgement and the lack of an objective method for assessment might have adversely affected data interpretation. Fourth, because the interscan distance of the OCT scans was 120 μm, there might be missing lesions between the scans.
In conclusion, dry AMD eyes showed different features of HRF according to the presence of SDDs. Diffuse and superotemporal distribution of HRF in eyes with SDDs might suggest that RPE degenerative features are different between dry AMD eyes with and without SDDs.
Code availability
Not applicable.
References
Bird A (2021) Role of retinal pigment epithelium in age-related macular disease: a systematic review. Br J Ophthalmol 105:1469–1474. https://doi.org/10.1136/bjophthalmol-2020-317447
Christenbury JG, Folgar FA, O’Connell RV, Chiu SJ, Farsiu S, Toth CA, Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study G (2013) Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 120:1038–1045. https://doi.org/10.1016/j.ophtha.2012.10.018
Nassisi M, Lei J, Abdelfattah NS, Karamat A, Balasubramanian S, Fan W, Uji A, Marion KM, Baker K, Huang X, Morgenthien E, Sadda SR (2019) OCT risk factors for development of late age-related macular degeneration in the fellow eyes of patients enrolled in the HARBOR study. Ophthalmology 126:1667–1674. https://doi.org/10.1016/j.ophtha.2019.05.016
Cao D, Leong B, Messinger JD, Kar D, Ach T, Yannuzzi LA, Freund KB, Curcio CA (2021) Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Invest Ophthalmol Vis Sci 62:34. https://doi.org/10.1167/iovs.62.10.34
Folgar FA, Chow JH, Farsiu S, Wong WT, Schuman SG, O’Connell RV, Winter KP, Chew EY, Hwang TS, Srivastava SK, Harrington MW, Clemons TE, Toth CA (2012) Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci 53:4626–4633. https://doi.org/10.1167/iovs.12-9813
Ho J, Witkin AJ, Liu J, Chen Y, Fujimoto JG, Schuman JS, Duker JS (2011) Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 118:687–693. https://doi.org/10.1016/j.ophtha.2010.08.010
Spaide RF (2018) Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 38:708–716. https://doi.org/10.1097/IAE.0000000000001689
Chen L, Messinger JD, Zhang Y, Spaide RF, Freund KB, Curcio CA (2020) SUBRETINAL DRUSENOID DEPOSIT IN AGE-RELATED MACULAR DEGENERATION: histologic insights into initiation, progression to atrophy, and imaging. Retina 40:618–631. https://doi.org/10.1097/iae.0000000000002657
Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF (2013) Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina 33:265–276. https://doi.org/10.1097/IAE.0b013e31827e25e0
Spaide RF (2013) Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 33:1800–1808. https://doi.org/10.1097/IAE.0b013e31829c3765
Kim JH, Kim JR, Kang SW, Kim SJ, Ha HS (2013) Thinner choroid and greater drusen extent in retinal angiomatous proliferation than in typical exudative age-related macular degeneration. Am J Ophthalmol 155(743–749):749.e741–742. https://doi.org/10.1016/j.ajo.2012.11.001
Spaide RF, Ooto S, Curcio CA (2018) Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol 63:782–815. https://doi.org/10.1016/j.survophthal.2018.05.005
Xu L, Blonska AM, Pumariega NM, Bearelly S, Sohrab MA, Hageman GS, Smith RT (2013) Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina 33:1850–1862. https://doi.org/10.1097/IAE.0b013e31828991b2
Curcio CA (2018) Soft drusen in age-related macular degeneration: biology and targeting via the oil spill strategies. Invest Ophthalmol Vis Sci 59: Amd160-amd181. https://doi.org/10.1167/iovs.18-24882
Curcio CA (2018) Antecedents of soft drusen, the specific deposits of age-related macular degeneration, in the biology of human macula. Invest Ophthalmol Vis Sci 59: Amd182-amd194. https://doi.org/10.1167/iovs.18-24883
Ueda-Arakawa N, Ooto S, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N (2013) Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina 33:490–497. https://doi.org/10.1097/IAE.0b013e318276e0ae
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR (2013) Clinical classification of age-related macular degeneration. Ophthalmology 120:844–851. https://doi.org/10.1016/j.ophtha.2012.10.036
Yun C, Ahn J, Kim M, Hwang SY, Kim SW, Oh J (2016) Ocular perfusion pressure and choroidal thickness in early age-related macular degeneration patients with reticular pseudodrusen. Invest Ophthalmol Vis Sci 57:6604–6609. https://doi.org/10.1167/iovs.16-19989
Lei J, Balasubramanian S, Abdelfattah NS, Nittala MG, Sadda SR (2017) Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 255:1551–1558. https://doi.org/10.1007/s00417-017-3693-y
Echols BS, Clark ME, Swain TA, Chen L, Kar D, Zhang Y, Sloan KR, McGwin G Jr, Singireddy R, Mays C, Kilpatrick D, Crosson JN, Owsley C, Curcio CA (2020) Hyperreflective foci and specks are associated with delayed rod-mediated dark adaptation in nonneovascular age-related macular degeneration. Ophthalmol Retina 4:1059–1068. https://doi.org/10.1016/j.oret.2020.05.001
Leuschen JN, Schuman SG, Winter KP, McCall MN, Wong WT, Chew EY, Hwang T, Srivastava S, Sarin N, Clemons T, Harrington M, Toth CA (2013) Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology 120:140–150. https://doi.org/10.1016/j.ophtha.2012.07.004
Klein ML, Ferris FL 3rd, Armstrong J, Hwang TS, Chew EY, Bressler SB, Chandra SR, Group AR (2008) Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology 115:1026–1031. https://doi.org/10.1016/j.ophtha.2007.08.030
Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA (2009) Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 116:488-496 e482. https://doi.org/10.1016/j.ophtha.2008.10.006
Xu X, Liu X, Wang X, Clark ME, McGwin G Jr, Owsley C, Curcio CA, Zhang Y (2017) Retinal pigment epithelium degeneration associated with subretinal drusenoid deposits in age-related macular degeneration. Am J Ophthalmol 175:87–98. https://doi.org/10.1016/j.ajo.2016.11.021
Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29:95–112. https://doi.org/10.1016/j.preteyeres.2009.11.003
Greferath U, Guymer RH, Vessey KA, Brassington K, Fletcher EL (2016) Correlation of histologic features with in vivo imaging of reticular pseudodrusen. Ophthalmology 123:1320–1331. https://doi.org/10.1016/j.ophtha.2016.02.009
Zhang Y, Sadda SR, Sarraf D, Swain TA, Clark ME, Sloan KR, Warriner WE, Owsley C, Curcio CA (2022) Spatial dissociation of subretinal drusenoid deposits and impaired scotopic and mesopic sensitivity in AMD. Invest Ophthalmol Vis Sci 63:32. https://doi.org/10.1167/iovs.63.2.32
Reiter GS, Told R, Schranz M, Baumann L, Mylonas G, Sacu S, Pollreisz A, Schmidt-Erfurth U (2020) Subretinal drusenoid deposits and photoreceptor loss detecting global and local progression of geographic atrophy by SD-OCT imaging. Invest Ophthalmol Vis Sci 61:11. https://doi.org/10.1167/iovs.61.6.11
Nam KT, Chung HW, Jang S, Kim SW, Oh J, Yun C (2020) Features of the macular and peripapillary choroid and choriocapillaris in eyes with nonexudative age-related macular degeneration. Retina 40:2270–2276. https://doi.org/10.1097/iae.0000000000002758
Mauschitz MM, Fonseca S, Chang P, Göbel AP, Fleckenstein M, Jaffe GJ, Holz FG, Schmitz-Valckenberg S (2012) Topography of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 53:4932–4939. https://doi.org/10.1167/iovs.12-9711
Schmitz-Valckenberg S, Sahel JA, Danis R, Fleckenstein M, Jaffe GJ, Wolf S, Pruente C, Holz FG (2016) Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology 123:361–368. https://doi.org/10.1016/j.ophtha.2015.09.036
Fleckenstein M, Mitchell P, Freund KB, Sadda S, Holz FG, Brittain C, Henry EC, Ferrara D (2018) The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 125:369–390. https://doi.org/10.1016/j.ophtha.2017.08.038
Chang YS, Kim JH, Yoo SJ, Lew YJ, Kim J (2016) Fellow-eye neovascularization in unilateral retinal angiomatous proliferation in a Korean population. Acta Ophthalmol 94:e49-53. https://doi.org/10.1111/aos.12748
Sawa M, Ueno C, Gomi F, Nishida K (2014) Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina 34:761–767. https://doi.org/10.1097/01.iae.0000434566.57189.37
Spaide RF (2019) New proposal for the pathophysiology of type 3 neovascularization as based on multimodal imaging findings. Retina 39:1451–1464. https://doi.org/10.1097/iae.0000000000002412
Kim JH, Chang YS, Kim JW, Kim CG, Lee DW (2020) CHARACTERISTICS OF TYPE 3 NEOVASCULARIZATION LESIONS: focus on the incidence of multifocal lesions and the distribution of lesion location. Retina 40:1124–1131. https://doi.org/10.1097/iae.0000000000002489
Funding
This work was supported by a Korea University Ansan Hospital Grant number (K2212011).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dongwan Kang, Young Joo Lee, Ki Tae Nam, Mihyun Choi and Cheolmin Yun. The first draft of the manuscript was written by Dongwan Kang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review board of the Korea University Medical Center. This study does not contain contents about animals.
Consent to participate
This type of study does not require informed consent.
Consent for publication
This type of study does not require informed consent.
Conflicts of interest/Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, D., Lee, Y.J., Nam, K.T. et al. Hyperreflective foci distribution in eyes with dry age-related macular degeneration with subretinal drusenoid deposits. Graefes Arch Clin Exp Ophthalmol 261, 2821–2828 (2023). https://doi.org/10.1007/s00417-023-06127-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06127-9