Abstract
Purpose
To evaluate the outcomes of micropulse transscleral laser therapy (MP-TLT) in patients with uncontrolled glaucoma and prior glaucoma aqueous tube shunt.
Methods
In this single‑center, retrospective, interventional case series, eyes that underwent MP-TLT and had prior glaucoma aqueous tube shunt surgeries were included. The Cyclo Glaucoma Laser System (IRIDEX Corporation, Mountain View, CA, USA) with the MicroPulse P3 probe (version 1) was used. Post‑operative data were collected at day 1, week 1, and months 1, 3, 6, 12, 18, 24, 30 and 36.
Results
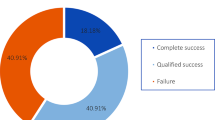
A total of 84 eyes (84 patients) with mean age of 65.8 ± 15.2 years and with advanced glaucoma (baseline mean deviation -16.25 ± 6.80 dB and best-corrected visual acuity 0.82 ± 0.83 logMar) were included in the study. Baseline mean IOP was 19.95 ± 5.6 mm Hg with a mean number of medications 3.39 ± 1.02. There were statistically significant differences in IOP between baseline and all follow-up visits (p < 0.01 for all). The mean percentage of IOP reduction between baseline and different follow-up visits ranged from 23.4% to 35.5% (p < 0.01). There was a significant reduction of visual acuity (≥ 2-lines) at 1 year (30.3%) and 2 years (76.78%). There was a statistically significant reduction in the number of glaucoma medications between baseline and all follow-up visits after postoperative week 1 (p < 0.05 for all). No severe complications including persistent hypotony and related complications were observed. At the last follow-up visit, only 24 (28%) eyes out of 84 eyes remained in the study.
Conclusion
MP-TLT is an effective treatment for reducing IOP and decreasing the number of medications in patients with advanced glaucoma and prior glaucoma aqueous tube shunt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
The treatment of glaucoma aims to reduce the intraocular pressure (IOP), by decreasing the production of aqueous humor, increasing the aqueous outflow or both [1]. Traditional transscleral cyclophotocoagulation targets the melanin in the ciliary body, leads to photocoagulative thermal damage and thereby reduces aqueous humor production [2]. The infrared MicroPulse® transscleral laser therapy (MP-TLT) (IRIDEX Corp., Mountain View, CA) uses repetitive pulses of active diode laser 810 nm of 0.5 ms (‘‘On’’ cycles), which is followed by rest intervals of 1.1 ms (‘‘Off’’ cycles). These ‘‘on’’ and ‘‘off’’ cycles allow energy to escalate in the target tissue during the ‘‘on’’ cycle and permits the adjacent nonpigmented structures to dissipate it during the ‘‘off’’ cycles to reduce collateral damage and adverse effects [3,4,5,6]. The mechanisms of action are proposed to be 1) reduction of aqueous humor production, 2) increase in uveoscleral outflow, and 3) increase in trabecular outflow. The decreased photothermal side effects have allowed MP-TLT to be used not only in cases of refractory glaucoma but also for the treatment of eyes with mild or moderate glaucoma and good visual potential [6,7,8,9,10,11,12,13].
In patients that have undergone prior glaucoma aqueous tube shunt surgery and still have uncontrolled IOP with medical therapy, it seems logical to add a procedure that can decrease aqueous production and/or enhance outflow via other pathways. In this capacity, many clinicians have adopted cyclophotocoagulation procedures in patients with prior aqueous tube shunt [14, 15]. In this retrospective interventional case series, our aim is to report outcomes of MP-TLT in IOP control in patients with advanced glaucoma with a history of prior glaucoma aqueous tube shunt surgeries.
Methods
This was a single‑center, retrospective, interventional case series of patients who were treated at UCLA Doheny Eye Institute, Los Angeles, CA, USA. The institutional review board at UCLA approved the study protocol for retrospective data collection. All adult patients (age 18 years or older) who underwent MP-TLT at UCLA Doheny Eye Institute between January 2016 and December 2019 were included. All subjects had prior glaucoma aqueous tube shunt surgeries, and only one eye of each person was included in the study. In cases where both eyes were eligible, the eye that was treated first was included. Eyes with post‑operative follow‑up of less than three months and those with prior cyclodestructive procedures were excluded from the study. Eyes with no light perception visual acuity were not included in the study. The following pre‑and post‑operative data were collected from the patients’ electronic medical records: age, ethnicity, gender, type of glaucoma, IOP (mm Hg), best‑corrected visual acuity (BCVA, LogMAR), number and type of glaucoma medications, and previous glaucoma tube and filtering surgeries (i.e., trabeculectomy, ExPRESS shunt, Xen implant, Baerveldt Glaucoma Implant, Ahmed Glaucoma Valve, or a combination of those). All patients were followed up for 36 months unless they were lost to follow-up or underwent another surgical glaucoma procedure. Post‑operative data were collected at day 1, week 1, and months 1, 3, 6, 12, 18, 24, 30 and 36.
The indications for MP-TLT were inadequate IOP control on maximal tolerated medical therapy. Treatments were performed by an attending glaucoma surgeon at UCLA Doheny Eye Institute in the operating room. To carry out the procedure, the Cyclo Glaucoma Laser System (IRIDEX Corporation, Mountain View, CA, USA) with the MicroPulse P3 probe (version 1) was used. It emits a diode laser at a wavelength of 810 nm. The device was set to micropulse mode. The laser settings were programmed as follows: laser power 2000 to 2500 mW, delivered with a duty cycle of 31.3%, equivalent to 0.5 ms of “on time” and 1.1 ms of “off time”, for a total duration of 160–200 s (80–100 s per hemisphere) and with a sweep speed of 15 s per sweep. The laser was performed 360 degrees avoiding 3 and 9 o’clock to avoid damage to the ciliary arteries and nerves; cystic blebs and areas of thin sclera or conjunctiva were avoided. Before the procedure, all patients underwent local monitored anesthesia care. During the treatment, the probe was applied perpendicularly to the scleral plane, and the fiberoptic tip of the probe was positioned 3 mm posterior to the limbal margin. The probe was held with a firm and steady pressure over the conjunctiva in a continuous, with slow back-and-forth sliding for 4.5 passes (each sweep in one direction took approximately 15–20 s) over the superior hemisphere, and was then repeated in the inferior hemisphere. Following the treatment, all eyes received topical prednisolone acetate 1% two times daily and Ketorolac Tromethamine 0.5% two times daily for a minimum of 1 week, which then could be tapered depending on the grade of inflammation. All preoperative IOP-lowering medications were continued initially and then adjusted at each follow-up visit according to the IOP level and target IOP defined by each surgeon. Decisions on retreatment or additional incisional surgery were made according to the details of each case and at the clinical discretion of the surgeon.
Statistical analyses were performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA). A P value of < 0.05 was considered statistically significant. The Kolmogorov‑Smirnov test was used to verify the normal distribution of the study outcomes. Appropriate parametric and nonparametric statistics were used to analyze the data. Kaplan–Meier survival plots were based on the time from the MP-TLT procedure to the first point at which a patient failed to meet the criteria of success. Two different criteria were used to define treatment success: (1) IOP between 6 and 18 mmHg and a reduction of IOP ≥ 20% from baseline; (2) IOP between 6 and 15 mmHg and a reduction of IOP ≥ 20% from baseline. An IOP of less than 6 mm Hg was considered to be hypotony and a treatment failure. A decrease in visual acuity of 3 or more lines by Snellen assessment was also considered a treatment failure. The Kaplan–Meier curve was used to perform a survival analysis of the success, and the Cox proportional-hazard regression analysis was conducted to estimate hazard ratios for failure to achieve treatment success.
Results
A total of 84 eyes of 84 patients with mean age of 65.8 ± 15.2 (SD) years were included in the study. Clinical characteristics of the study subjects are demonstrated in Table 1. The majority of patients had POAG and had undergone Baerveldt glaucoma implant prior to MP-TLT. Study outcomes at baseline and different follow-up visits are reported in Table 2. There was a statistically significant difference in BCVA (logMAR) between baseline and all follow-up visits after postoperative month 3 (except the last follow-up visit) (p < 0.05 for all) (Table 2; Fig. 1). Baseline mean IOP was 19.95 ± 5.62 (SD) mm Hg with a mean number of medications 3.39 ± 1.02 (Table 2). There were statistically significant differences in IOP between baseline and all follow-up visits (p < 0.01 for all) (Table 2; Fig. 2). The mean percentage of IOP reduction between baseline and different follow-up visits ranged from 23.4% to 35.5% (p < 0.01) (Table 2; Fig. 3). There was a statistically significant difference in number of glaucoma medications between baseline and all follow-up visits after postoperative week 1 (p < 0.05 for all) (Table 2; Fig. 2). In our series, no patient was on oral IOP-lowering medications pre- and post-operatively at any follow-up visits. The Kaplan–Meier plots in Figs. 4 and 5 represents the cumulative probability of treatment success after MP-TLT based on criteria 1 and 2, respectively. Table 3 demonstrates cumulative probability of success at different time intervals based on criteria 1 and criteria 2. In the Cox proportional-hazard analysis, there was no significant association between failure to achieve treatment success and different factors including age, gender, type of glaucoma, surgeon, lens status, preoperative visual acuity, baseline IOP, baseline number of medications, baseline mean deviation and prior surgeries (p > 0.05 for all) (Table 5).
There were no cases with persistent hypotony and related complications (flat anterior chamber, corneal decompensation, choroidal effusion or hemorrhage), prolonged anterior chamber inflammation (defined as 1 + cell or flare for ≥ 3 months), persistent macular edema, mydriasis or loss of accommodation, phthisis bulbi and sympathetic ophthalmia. There were 20 (30.3%) and 43 (76.8%) patients who had equal to or more than a two-line reduction of visual acuity at 1 and 2 years, respectively. There was a relatively strong correlation between visual acuity at 1 (r2 = 0.75; p < 0.01) and 2 years (r2 = 0.66; p < 0.01), and baseline visual acuity (Figs. 6 and 7). Although statistically not significant, eyes with ≥ 2-line reduction of visual acuity had worse baseline visual acuity (~ 1 line) and worse baseline MD in the bivariate analysis (p > 0.05 for all) (Table 4). There was no statistically significant difference in lens status and baseline IOP between eyes with ≥ 2 and those with < 2 line-reduction in visual acuity (p > 0.05 for all) (Table 4). There was a weak correlation between baseline IOP and percentage of IOP reduction after 1 (r2 = 0.28; p < 0.05) and 2 (r2 = 0.26; p < 0.05) years (Figs. 8 and 9).
Discussion
MicroPulse transscleral laser therapy is a simple procedure that is becoming more widely used to treat a variety of glaucoma types and severities. While continuous-wave transscleral cyclophotocoagulation has often been reserved for eyes with refractory advanced glaucoma and limited visual potential, in view of the satisfactory outcomes and low complication rates, MP-TLT has been shown to be a safe and effective procedure earlier in the treatment of glaucoma or even as a primary surgical procedure for uncontrolled glaucoma in patients with good visual potential. This retrospective interventional case series reports the outcome of MP-TLT in IOP control in eyes with more advanced glaucoma and a history of prior tube shunt surgeries.
In our study, there was a significant IOP reduction in all follow-up visits with the average percentage of IOP reduction ranging between 23.4% to 35.5% with a weak correlation between baseline IOP and percentage of IOP reduction (Figs. 8 and 9). Although some studies found a similar percentage of IOP reduction [16], other studies achieved higher percentage of IOP reduction (range: 35–50%) [12, 13, 17,18,19,20]. The average baseline IOP in our study (19.95 ± 5.6 mm Hg) was considerably lower compared to other studies (~ 30 mm Hg) [17,18,19,20]. These differences between studies could reflect the significant differences in patients’ characteristics. Our study included patients with advanced glaucoma who had undergone prior and, in many cases, multiple incisional filtration surgeries. There was a significant reduction in the requirements for topical IOP-lowering medications after postoperative week 1 until the end of the study. This is similar to the majority of previous studies with varying follow-up durations [12, 13, 16,17,18,19,20]. In our series, no patient was on oral IOP-lowering medications. On Kaplan–Meier analysis, the cumulative probability of success was 81% and 80% at month 12 based on criteria 1 and 2, respectively (Table 3; Figs. 4 and 5). The success rates decreased to 66% and 64% at month 24 based on criteria 1 and 2 (Table 3; Figs. 4 and 5). On Cox proportional-hazard analysis, there was not any significant association between failure to achieve treatment success based on criteria 1 and several factors including age, gender, type of glaucoma, lens status, preoperative visual acuity, baseline IOP, baseline MD, prior surgeries and surgeons (Table 5).
The retrospective nature of the study limited our ability to report about complications. However, there were no patients with persistent hypotony and related complications, prolonged anterior chamber inflammation (defined as 1 + cell or flare for ≥ 3 months), persistent macular edema, mydriasis or loss of accommodation, phthisis bulbi and sympathetic ophthalmia. The major complication in our study was a statistically significant decrease in visual acuity after month 3 of follow-up (Table 2 and Fig. 1). This may be due to the MP-TLT procedure, but there were no cases of chronic inflammation or prolonged hypotony and associated choroidal effusions or maculopathy. Certainly, it is also possible that the procedure itself can cause a decrease in visual acuity via mechanisms currently not recognized.
There were 20 (30.30%) and 43 (76.78%) patients who had ≥ 2-line reduction of visual acuity at 1 and 2 years, respectively. The observed slight improvement in visual acuity from month 30 to 36 could be because eyes with better outcomes remained in the study. Only 24 eyes remained in the study at the last follow-up visit. This is in contrast to the results of most studies in which there has not been any statistically significant change in postoperative visual acuity at different follow-up points [16, 17]. Williams et al. reported that 13 out of 82 patients (15.85%) had ≥ 2-line reduction in visual acuity during mean follow-up time of 7.8 ± 4.5 months. They used IRIDEX Cyclo G6 laser with settings of 2000 mW of 810 nm infrared diode laser with a duty cycle of 31.3% [13]. The laser was delivered in a “stop-and-go” pattern (i.e., it was held in place for 10 s before being moved to the adjacent section of perilimbal conjunctiva) for 120 to 360 s [13]. Lim et al. reported 14 out of 43 eyes (32.56%) had visual acuity loss more than 2 lines during 3 years of follow-up in a series of eyes with advanced glaucoma (average mean deviation: − 24.69 ± 5.13 dB; laser setting: 2000 mW of 810 nm infrared radiation over 50 to 180 s) [16].
There was a relatively strong correlation between visual acuity at 1 (r2 = 0. 75; p < 0.01) and 2 years (r2 = 0.66; p < 0.01), and baseline visual acuity (Figs. 6 and 7). In bivariate analysis, there was no statistically significant difference in baseline visual acuity, lens status, baseline IOP and baseline MD between eyes with ≥ 2 and those with < 2 line-reduction in visual acuity (Table 4). While the duration of laser in our series (160–200 s) was similar to other studies, slightly higher laser energy (up to 2500 mW) compared to other studies (~ 2000 mW) was applied [12, 13, 16,17,18,19,20,21]. Our series included only patients with advanced glaucoma with prior history of failed incisional/filtering surgeries and advanced visual field loss with an average mean deviation of worse than -16 dB. While the average baseline IOP in our series (19.95 ± 5.6 mm Hg) was considerably lower than other studies (~ 30 mm Hg), our patients needed additional interventions because of uncontrolled IOP or progression. Therefore, our series likely included more eyes with more advanced glaucoma. Thus, the decrease in visual acuity in our study could be secondary to the course of the disease rather than being induced by the procedure. Alternatively, the observed decrease in visual acuity could be due to IOP-unrelated disease progression, or factors not related to glaucoma such as corneal edema or media opacity, ocular surface disease or concomitant retinal disease. These conditions are more commonly seen in individuals with advanced glaucoma.
To our knowledge, there is still no universal agreement on laser settings especially for different stages of glaucoma and visual potential. There are some reports that even lower laser settings could achieve similar IOP reduction and success rates. Vig et al. [12] found relatively similar IOP reduction and success rate with lower laser settings (power 2000 mW and duration 90 s) compared to studies with higher laser settings (power 2000 mW and duration 100–360) [8, 10, 11]. It is important to note that our study was performed with the version 1 probe, and the laser setting may differ from standard practices for the version 2 probe [22].
Several mechanisms have been proposed through which MP-TLT can reduce IOP: 1) Decreased Aqueous Humor Production: The 810 nm-wavelength diode laser is absorbed by the pigmented epithelium of the ciliary body. While the “on” cycle helps to achieve the coagulation threshold of the pigmented ciliary epithelium the “off” cycle reduces the accumulation of heat and allows thermal dissipation in the nearby cells, which results in less disruption of the non-pigmented epithelium and the ciliary stroma [23]. This limited temperature rise secondary to laser application denatures proteins faster than the natural biological cell repair mechanism, which leads to reduction of aqueous humor production without any macroscopic tissue change. There has been no major alteration in the ciliary body or anterior segment anatomy on anterior segment OCT and ultrasound biomicroscopy [24, 25]. No significant damage to the ciliary body (epithelium destruction, separation of the pigmented ciliary epithelium from the stroma and stromal coagulation) has been noticed with MP-TLT, which suggests that the IOP-lowering mechanism may be due to factors other than that of decreased aqueous production [25]. 2) Increased Uveoscleral Outflow: There is shortage of evidence on the direct effect of MP-TLT on uveoscleral outflow. However, this effect was directly observed with continuous-wave transscleral cyclophotocoagulation, both with the assistance of tracer-particle perfusion and histologically [26, 27]. In addition, an increase in uveoscleral outflow has been suggested indirectly through the observation of an increase in choroidal thickness observed in patients treated successfully with MP-TLT. 3) [28] Increased Trabecular Meshwork Outflow: This may occur through the action of the laser on the longitudinal fibers of the ciliary body and, to a lesser extent, scleral fibers. This results in contraction and displacement of the scleral spur, which leads to a change in the configuration of the trabecular meshwork and increasing the outflow through this pathway. This mechanism is similar to the effect of pilocarpine in decreasing IOP and is energy-dependent. This means that there is less recovery of the contracted ciliary muscle when higher energy is applied, which results in a more sustained pilocarpine-like effect [3, 29].
One histologic study in equine eyes has shown that MP-TLT does not cause immediate post-procedure adverse clinical effects or pronounced morphological changes to the ciliary body, except with the highest laser settings evaluated (power 3000 mW, duration 270 s, duty cycle 50%) [30]. In this setting, ocular tissues had less “cooling off” time as the duty cycle was set to 50% (instead of 31.3%), meaning the “on” and “off” time were equal. In their series, all treatment eyes showed certain histologic changes that were not noted in the negative controls, indicating a degree of microscopic tissue alterations and an effect on the ciliary body tissue [31]. However, these effects were inconsistent within and between eyes and not noted in all sections despite the constant sweeping technique of the Mi laser probe. This variation may be due to individual anatomical differences or “user error,” that is, inappropriate probe placement or inconsistent pressure against the sclera. They found that in order to cause consistent and pronounced tissue destruction the laser settings need to be maximized to 3000 mW, 270 s, duty cycle 50%. It is unknown; however, if significant tissue damage is required for the IOP-lowering effects of MP-TLT, versus subtle microscopic and ultrastructural changes [30].
The limitations of this study included its retrospective design and unavailable data for some eyes during some follow-up visits. In conclusion, MP-TLT resulted in significant IOP reduction and a decrease in the number of IOP-lowering medications in patients with advanced glaucoma and prior glaucoma aqueous tube shunt. It appears to be a viable treatment alternative to repeat higher risk filtration surgery in this group of patients. The most important complication in our series was a moderate decrease of visual acuity. This needs to be further investigated in comparative studies with control groups that include other treatment modalities. At this time, there is no universal or standardized protocol for this procedure, although recommendations have been published from an expert consensus panel, and the settings and technique may vary based on surgeon preference and experience [31]. The settings may need to be modified for different stages of glaucoma and visual potential.
References
Weinreb RN, Khaw PT (2004) Primary open-angle glaucoma. Lancet 363:1711–1720. https://doi.org/10.1016/S0140-6736(04)16257-0
Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ (2001) Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma 10:294–301. https://doi.org/10.1097/00061198-200108000-00009
Sanchez FG, Peirano-Bonomi JC, Grippo TM (2018) Micropulse transscleral cyclophotocoagulation: a hypothesis for the ideal parameters. Med Hypothesis Discov Innov Ophthalmol 7:94–100
Sarrafpour S, Saleh D, Ayoub S, Radcliffe NM (2019) MicroPulse® transscleral cyclophotocoagulation: a look at long-term effectiveness and outcomes. Ophthalmol Glaucoma 2:167–171. https://doi.org/10.1016/j.ogla.2019.02.002
Varikuti VNV, Shah P, Rai O, Chaves AC, Miranda A, Lim BA, Dorairaj SK, Sieminski S (2019) Outcomes of micropulse® transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma 28:901–905. https://doi.org/10.1097/IJG.0000000000001339
Yelenskiy A, Gillette TB, Arosemena A, Stern AG, Garris WJ, Young CT, Hoyt M, Worley N, Zurakowski D, Ayyala RS (2018) Patient outcomes following micropulse® transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma 27:920–925. https://doi.org/10.1097/IJG.0000000000001023
Fili S, Kontopoulou K, Vastardis I, Perdikakis G, Papadonta SA, Armeni EZ, Kohlhaas M (2021) Transscleral cyclophotocoagulation with MicroPulse® laser versus Ahmed valve implantation in patients with advanced primary open-angle glaucoma. Int Ophthalmol 41:1271–1282. https://doi.org/10.1007/s10792-020-01682-0
Aquino MCD, Barton K, Tan AM, Sng C, Li X, Loon SC, Chew PT (2015) Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol 43:40–46. https://doi.org/10.1111/ceo.12360
Emanuel ME, Grover DS, Fellman RL, Godfrey DG, Smith O, Butler MR, Kornmann HL, Feuer WJ, Goyal S (2017) Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma 26:726–729. https://doi.org/10.1097/IJG.0000000000000715
Kuchar S, Moster MR, Reamer CB, Waisbourd M (2016) Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci 31:393–396. https://doi.org/10.1007/s10103-015-1856-9
Zaarour K, Abdelmassih Y, Arej N, Cherfan G, Tomey KF, Khoueir Z (2018) Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma 28:270–275. https://doi.org/10.1097/IJG.0000000000001174
Vig N, Ameen S, Bloom P, Crawley L, Normando E, Porteous A, Ahmed F (2020) Micropulse transscleral cyclophotocoagulation: initial results using a reduced energy protocol in refractory glaucoma. Graefes Arch Clin Exp Ophthalmol 258:1073–1079. https://doi.org/10.1007/s00417-020-04611-0
Williams AL, Moster MR, Rahmatnejad K, Resende AF, Horan T, Reynolds M, Yung E, Abramowitz B, Kuchar S, Waisbourd M (2018) Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma. J Glaucoma 27:445–449. https://doi.org/10.1097/IJG.0000000000000934
Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V (2011) Endoscopic cyclophotocoagulation (ECP) in the management of uncontrolled glaucoma with prior aqueous tube shunt. J Glaucoma 20:523–527. https://doi.org/10.1097/IJG.0b013e3181f46337
Murakami Y, Akil H, Chahal J, Dustin L, Tan J, Chopra V, Francis B (2017) Endoscopic cyclophotocoagulation versus second glaucoma drainage device after prior aqueous tube shunt surgery. Clin Exp Ophthalmol 45:241–246. https://doi.org/10.1111/ceo.12828
Lim EJY, Aquino CM, Lun KWX, Lim DKA, Sng C, Loon SC, Chew PTK, Koh VTC (2021) Efficacy and Safety of Repeated Micropulse Transscleral Diode Cyclophotocoagulation in Advanced Glaucoma. J Glaucoma 30:566–574. https://doi.org/10.1097/IJG.0000000000001862
Ariga M, Nivean NPD, Madanagopalan VG, Mohan S (2021) Micropulse trans-scleral diode laser cyclophotocoagulation in refractory glaucoma: an initial experience in Indian eyes. Int Ophthalmol 41:2639–2645. https://doi.org/10.1007/s10792-021-01697-1
Tekeli O, Köse HC (2021) Comparative efficacy and safety of micropulse transscleral laser cyclophotocoagulation using different duration protocols in eyes with good visual acuity. Graefes Arch Clin Exp Ophthalmol 259:3359–3369. https://doi.org/10.1007/s00417-021-05265-2
Chen HS, Yeh PH, Yeh CT, Su WW, Lee YS, Chuang LH, Shen SC, Wu WC (2022) Micropulse transscleral cyclophotocoagulation in a Taiwanese population: 2-year clinical outcomes and prognostic factors. Graefes Arch Clin Exp Ophthalmol 260:1265–1273. https://doi.org/10.1007/s00417-021-05468-7
ELGwaily AM, Khedr SA, Assaf AH, Latif MAMAL, Elsayed HA, Latif AAMAL (2021) MicroPulse® transscleral laser therapy in the management of glaucoma patients. Arch Soc Esp Oftalmol (Engl Ed) 96:640–648. https://doi.org/10.1016/j.oftale.2020.12.005
Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT (2010) Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Experiment Ophthalmol 38:266–272. https://doi.org/10.1111/j.1442-9071.2010.02238.x
An international consensus panel of ten glaucoma experts discusses best practices for MicroPulseTransscleral Laser Therapy. Available at: https://www.iridex.com/consensus.aspx. Accessed 25 May 2023
Dastiridou AI, Katsanos A, Denis P, Francis BA, Mikropoulos DG, Teus MA, Konstas AG (2018) Cyclodestructive procedures in glaucoma: a review of current and emerging options. Adv Ther 35:2103–2127. https://doi.org/10.1007/s12325-018-0837-3
Amoozgar B, Phan EN, Lin SC, Han Y (2017) Update on ciliary body laser procedures. Curr Opin Ophthalmol 28:181–186. https://doi.org/10.1097/ICU.0000000000000351
Moussa K, Feinstein M, Pekmezci M, Lee JH, Bloomer M, Oldenburg C, Sun Z, Lee RK, Ying GS, Han Y (2020) Histologic changes following continuous wave and micropulse transscleral cyclophotocoagulation: a randomized comparative study. Transl Vis Sci Technol 9:22–22. https://doi.org/10.1167/tvst.9.5.22
Liu GJ, Mizukawa A, Okisaka S (1994) Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res 26:65–79. https://doi.org/10.1159/000267395
Schubert HD, Agarwala A (1990) Quantitative CW Nd:YAG pars plana transscleral photocoagulation in postmortem eyes. Ophthalmic Surg 21:835–839
Barac R, Vuzitas M, Balta F (2018) Choroidal thickness increase after micropulse transscleral cyclophotocoagulation. Romanian J Ophthalmol 62:144–148
Abdelmassih Y, Tomey K, Khoueir Z (2021) Micropulse Transscleral Cyclophotocoagulation. J Curr Glaucoma Pract 15:1–7. https://doi.org/10.5005/jp-journals-10078-1298
Foote BC, Smith JD, Allbaugh RA, Sebbag L (2021) Histologic effects of MicroPulse™ transscleral cyclophotocoagulation in normal equine eyes. Vet Ophthalmol 24:59–70. https://doi.org/10.1111/vop.12846
Grippo TM, de Crom RMPC, Giovingo M, Töteberg-Harms M, Francis BA, Jerkins B, Brubaker JW, Radcliffe N, An J, Noecker R (2022) Evidence-Based Consensus Guidelines Series for MicroPulse Transscleral Laser Therapy: Dosimetry and Patient Selection. Clin Ophthalmol 16:1837–1846. https://doi.org/10.2147/OPTH.S365647
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
All procedures performed in studies involving human participants were in accordance with the ethical standards of University of California at Los Angeles (UCLA) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study before the procedures.
Conflict of interest
No conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The study data has not been presented in a meeting.
Brian Francis is a consultant for Iridex, Inc, the makers of the CycloGx Micropulse Laser Therapy system. All other authors have no financial or proprietary interest in a product, method, or material described herein.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nassiri, N., Tseng, V.L., Kim, C. et al. Outcomes of microPulse transscleral laser therapy in eyes with prior glaucoma aqueous tube shunt. Graefes Arch Clin Exp Ophthalmol 261, 2935–2944 (2023). https://doi.org/10.1007/s00417-023-06119-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06119-9