Abstract
Background
Excessive angiogenesis of the retina is a key component of irreversible causes of blindness in many ocular diseases. Pitavastatin is a cholesterol-lowering drug used to reduce the risk of cardiovascular diseases. Various studies have shown the effects of pitavastatin on angiogenesis but the conclusions are contradictory. The effects of pitavastatin on retinal angiogenesis have not been revealed. This study investigated the effects of pitavastatin at clinically relevant concentrations on retinal angiogenesis and its underlying mechanisms using retinal microvascular endothelial cells (RMECs).
Methods
The effects of pitavastatin on retinal angiogenesis were determined using in vitro model of retinal angiogenesis, endothelial cell migration, adhesion, proliferation, and apoptosis assays. The mechanism studies were conducted using immunoblotting and stress fiber staining.
Results
Pitavastatin stimulated capillary network formation of RMECs in a similar manner as vascular endothelial growth factor (VEGF) and lipopolysaccharide (LPS). Pitavastatin also increased RMEC migration, adhesion to Matrigel, growth, and survival. The combination of pitavastatin with VEGF or LPS was more effective than VEGF or LPS alone in stimulating biological activities of RMECs, suggesting that pitavastatin can enhance the stimulatory effects of VEGF and LPS on retinal angiogenesis. Pitavastatin acted on RMECs in a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase-independent manner. In contrast, pitavastatin activated pro-angiogenic microenvironment via promoting the secretion of VEGF and stimulated retinal angiogenesis via multiple mechanisms including activation of RhoA-mediated pathways, induction of focal adhesion complex formation, and activation of ERK pathway.
Conclusion
Our work provides a preclinical evidence on the pro-angiogenic effect of pitavastatin in retina via multiple mechanisms that are irrelevant to mevalonate pathway.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis, the formation of blood vessels from the pre-existing ones, is regulated by an interplay of stimulators and inhibitors. Excessive angiogenesis can lead to many vision-threatening diseases, including proliferative diabetic retinopathy, age-related macular degeneration, and retinopathy of prematurity [1, 2]. The main endogenous stimulators of angiogenesis are growth factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [3]. Lipopolysaccharide (LPS), a major component of inflammation, also plays an essential role in the excessive angiogenesis in proliferative diabetic retinopathy [4]. In addition, retinal endothelial cell plays an important role in excessive angiogenesis and is a key participant in retinal ischemic vasculopathies [5].
HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase is a key enzyme of mevalonate pathway that catalyzes the synthesis of cholesterol as well as mevalonate. As competitive inhibitors of HMG-CoA reductase, statins are lipid-lowering drugs and frequently used for the prevention of cardiovascular disease [6,7,8]. Pitavastatin is a new generation of statins with stronger effect on cholesterol reduction than conventional ones [9]. Apart from cholesterol lowering, the pleiotropic effects of pitavastatin have been reported, including improving endothelial function, increasing thrombomodulin, and decreasing inflammation [10,11,12]. Interestingly, pitavastatin inhibits angiogenesis in murine tumor model, whereas it induces angiogenesis in a murine hindlimb ischemia model [11, 13], suggesting the differential effects of pitavastatin on angiogenesis. Given the important association between angiogenesis and ocular diseases, our work was designed to examine what is the effect of pitavastatin on retinal angiogenesis using human retinal microvascular endothelial cells (RMECs), and to determine whether pitavastatin affects RMEC biological functions as well as what is the underlying mechanism of pitavastatin’s action.
Materials and methods
Endothelial cells, compounds, and antibodies
Primary human RMECs (Cat. No. H-6065) were purchased from Cell Biologics, Inc. and expanded using the complete human endothelial cell medium (Cat. No. H1168) as provided by the manufacturer. Cells were starved in basal endothelial medium (Cell Systems, Inc.) for 2 h prior to drug treatment. Recombinant human VEGF165 (R&D Systems, Inc.) was reconstituted in water and kept in − 20 °C. LPS was freshly prepared by dissolving in cell culture medium. Pitavastatin, mevalonate, and cholesterol (Selleckchem, Inc.) were reconstituted in dimethyl sulfoxide (DMSO). Antibodies for phospho- and total myosin phosphatase targeting subunit 1 (MYPT1), myosin light chain (MLC), VEGF receptor 2 (VEGFR2), and focal adhesion kinase (FAK) were from Cell Signaling Technology, Inc. Antibodies for phospho- and total paxillin, extracellular-signal-regulated kinase (ERK), and Akt were from Abcam, Inc. Antibody for β-actin was from Santa Cruz Technology, Inc. Western blot was performed using the standard protocol as described in our previous studies [14]. The band density was quantified using ImageJ 1.32 software.
In vitro model of retinal angiogenesis
RMECs (104 cells per well in a 96-well plate) together with pitavastatin in the presence of LPS or VEGF were seeded onto the Corning® Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrigel and cultured for 10 h at 37 °C in 5% CO2. Tubular structures were evident and documented using a phase-contrast microscope (Leica, Inc.). The capillary networks were quantified with ImageJ 1.32 software.
Bromodeoxyuridine/5-bromo-2'-deoxyuridine (BrdU) assay
RMECs (104 cells per well in a 96-well plate) together with pitavastatin in the presence of LPS or VEGF in 2% fetal bovine serum (FBS)-containing basal endothelial medium were incubated for 24 h at 37 °C in 5% CO2. Twenty microliters of BrdU reagent was added to each well and cell proliferation was evaluated by using BrdU Cell Proliferation enzyme-linked immunosorbent assay (ELISA) Kit (Abcam, Inc.) as per the manufacturer’s protocol. Absorbance at 490 nm was measured using a Tecan Infinite M200 microplate reader (Thermo Fisher Scientific, Inc.).
Apoptosis assay
RMECs (106 cells per well in a 6-well plate) together with pitavastatin in the presence of LPS or VEGF in 2% FBS-containing basal endothelial medium were incubated for 24 h at 37 °C in 5% CO2. The apoptosis was determined by quantifying cytoplasmic histone-associated DNA fragments using the Cell Death Detection ELISA kit (Creative Diagnostics, Inc.). Absorbance at 405 nm was measured using a Tecan Infinite M200 microplate reader (Thermo Fisher Scientific, Inc.).
Boyden chamber migration assay
Migration assay was performed using the Boyden chamber as described in our previous studies [14, 15]. Briefly, RMECs (5 × 103 cells per well in a 24-well plate) in 2% FBS-containing basal endothelial medium were placed in the gelatin-coated cell culture insert. Pitavastatin together with LPS or VEGF was placed on the lower chamber. After 8-h incubation at 37 °C in 5% CO2, cells on the upper surface of the insert were swabbed. Cells moving through the pores were fixed, stained, and counted under a light microscope.
Adhesion assay
RMECs was pre-labelled with calerin using Vybrant™ Cell Adhesion Assay kit as per the manufacturer’s protocol. RMECs (5 × 103 cells per well in a 96-well plate) together with pitavastatin in the presence of LPS or VEGF in 2% FBS-containing basal endothelial medium were seeded onto a 10 × diluted Matrigel-coated 96-well plate and incubated for 1.5 h at 37 °C in 5% CO2. Medium was added to each well and gently swirled to remove the nonadherent cells. The calcine absorbance was measured on the fluorescence microplate reader (Thermo Fisher Scientific, Inc.).
ELISA
RMECs (105 cells per well in a 24-well plate) together with pitavastatin in 2% FBS-containing basal endothelial medium were incubated for 24 h at 37 °C in 5% CO2. Supernatant was collected and cells were harvested for protein lysates using a standard protocol. Cellular RhoA and Rac1 activities were determined using cell lysates and measured using kits from Cytoskeleton, Inc. VEGF and PDGF-AA levels were determined using supernatant and measured using kits from Thermo Fisher Scientific, Inc.
Phalloidin staining
RMECs (103 cells per well in a 96-well plate) together with pitavastatin in 2% FBS-containing basal endothelial medium were incubated for 6 h at 37 °C in 5% CO2. Cells were then washed with PBS, fixed with 4% paraformaldehyde, and stained with rhodamine phalloidin reagent (Abcam, Inc.) as per the manufacturer’s protocol. Images were taken under a fluorescent microscope (Leica, Inc.).
Statistical analyses
Results were obtained from minimal three independent experiments with triplicates and presented as mean ± standard deviation (SD). Statistical analyses for comparisons of two categorical variables were conducted using the Mann–Whitney U test. To ascertain each independent factor of VEGF and LPS stimulation, a one-way analysis of variance (ANOVA) was conducted. Results were deemed statistically significant when P value < 0.05.
Results
Pitavastatin stimulates retinal angiogenesis and enhances pro-angiogenic effects of VEGF and LPS
To investigate the effects of pitavastatin in retinal angiogenesis, we performed in vitro angiogenesis assay using primary human RMECs in the presence of pitavastatin under three conditions: basal, VEGF-stimulated, and LPS-stimulated. The mean serum maximal concentration is ~ 500 nM in patients after a single dose of 4 mg pitavastatin [16]. To correlate with clinical significance, we tested concentrations of pitavastatin up to 100 nM in our study. VEGF and LPS are known angiogenesis stimulators that promote endothelial cell capillary network formation [17]. In order to display the stimulating effects of VEGF and LPS on the tubular structure formation, we seeded RMECs onto growth factor-reduced basement membrane Matrigel as control. As shown in Fig. 1A, RMECs formed capillary network but with disrupted tubular structure in control whereas more well-formed branches were formed in VEGF, LPS, and pitavastatin. Extensive well-formed branches without disruption were observed in the combination of pitavastatin with VEGF or LPS. Quantification of capillary length was performed using ImageJ software and ANOVA shows significant differences among different concentrations of pitavastatin. Pitavastatin dose-dependently increased the capillary length under basal condition (Fig. 1B). In addition, there was a significant increase in capillary length in the combination compared to VEGF or LPS alone (Fig. 1C and D).
Pitavastatin stimulates retinal angiogenesis and acts synergistically with VEGF and LPS. (A) In vitro angiogenesis images using RMECs in the absence (DMSO) and presence of pitavastatin, VEGF, or LPS alone or the combination of pitavastatin with VEGF or LPS. (B) Pitavastatin dose-dependently increases capillary network formation of RMECs under basal condition. (C and D) Pitavastatin significantly further enhances VEGF- and LPS-stimulated capillary network formation. 10 ng/ml of VEGF and 1 µg/ml of LPS were used. (E) Time course analysis shows that pitavastatin significantly stimulates early stages of capillary network formation of RMECs. Pitavastatin at 100 nM was added to the medium at 0 h, 1 h, 2 h, and 4 h after RMECs were seeded to Matrigel. Results were shown as relative to control (DMSO). *P < 0.05, compared to control, VEGF alone, or LPS alone
Endothelial cell capillary network formation involves multiple steps including cell attachment to matrix, cell migration, spreading, and morphogenesis. After seeding RMECs onto Matrigel, we observed that cells attached to Matrigel and migrated during the 0–1-h period; cells then spread and elongated during 1–2-h period; capillary network appeared within 4 h and well-extensive tubular structure was formed within 8 h (data not shown). To investigate at which stage(s) pitavastatin promotes in vitro capillary network formation, pitavastatin was added to the Matrigel at the same time when RMECs were seeded (0 h), or 0.5, 1, 2, and 4 h after seeding cells. We observed a gradual loss of capillary network formation when pitavastatin was added at 1 h and onward (Fig. 1E), demonstrating that pitavastatin promotes early stages of capillary network formation.
Pitavastatin stimulates RMEC activities and acts synergistically with VEGF and LPS
To confirm the above findings, we examined the effects of pitavastatin on RMEC adhesion to Matrigel and migration using adhesion and Boyden chamber migration assays under three conditions: basal, VEGF-stimulated, and LPS-stimulated. ANOVA analyses showed all conditions were statistically different. We found that pitavastatin increased cell migration by twofold compared to control under basal condition (Fig. 2A). The combination of pitavastatin with VEGF or LPS significantly increased more cell migration compared to VEGF or LPS alone (Fig. 2B and C). Pitavastatin increased cell adhesion to diluted Matrigel under basal condition (Fig. 2D), and increased more cell adhesion to diluted Matrigel compared to VEGF or LPS alone (Fig. 2E and F).
Pitavastatin stimulates RMEC migration and adhesion, and acts synergistically with VEGF and LPS. (A) Pitavastatin significantly stimulates RMEC migration under basal condition. (B and C) Pitavastatin enhances more RMEC migration than VEGF or LPS alone. (D) Pitavastatin significantly increases RMEC adhesion to diluted Matrigel under basal condition. (E and F) Pitavastatin significantly increases more RMEC adhesion compared to VEGF or LPS alone. *P < 0.05, compared to control, VEGF alone, or LPS alone
We further examined the effects of pitavastatin on RMEC proliferation and apoptosis under three conditions: basal, VEGF-stimulated, and LPS-stimulated. ANOVA showed majority of factors to be significantly different. The exception was apoptosis results under VEGF-stimulated environment (p value = 0.31). We found that pitavastatin significantly stimulated RMEC proliferation and decreased apoptosis under basal condition (Fig. 3A and D). The combination of pitavastatin with VEGF or LPS resulted in significantly more cell proliferation compared to VEGF or LPS alone (Fig. 3B and C). Compared to LPS alone, pitavastatin significantly decreased more cell apoptosis (Fig. 3F). Although ANOVA showed VEGF overall apoptosis trend to be insignificant, comparing results from VEGF alone and its combination of VEGF and pitavastatin (100 nM), there was statistical significance. This may indicate that a higher concentration may be required to further decrease apoptosis compared to VEGF. Taken together, we demonstrate that (1) pitavastatin stimulates biological activities of RMECs and (2) pitavastatin enhances pro-angiogenic activities of VEGF and LPS on RMECs.
Pitavastatin stimulates RMEC proliferation and survival. (A) Pitavastatin stimulates RMEC proliferation under basal condition. (B and C) Pitavastatin significantly increases RMEC proliferation than VEGF or LPS alone. (D) Pitavastatin significantly decreases RMEC apoptosis under basal condition. (E) Pitavastatin at 100 nM significantly decreases more RMEC apoptosis compared to VEGF alone. (F) Pitavastatin significantly decreases more RMEC apoptosis compared to LPS alone. *P < 0.05, compared to control, VEGF alone, or LPS alone
Pitavastatin acts on RMECs in a HMG-CoA reductase-independent manner
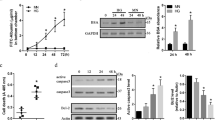
As an inhibitor of HMG-CoA reductase, pitavastatin is known to inhibit cholesterol synthesis [18] and post-translational modification prenylation [13]. We therefore attempted to reverse the effects of pitavastatin using cholesterol or mevalonate to determine whether HMG-CoA reductase is involved in pitavastatin’s action in RMECs. The concentration of cholesterol and mevalonate used in the rescue study has been shown to effectively reverse cholesterol level and prenylation inhibition [19]. We found that cholesterol or mevalonate alone did not affect capillary network formation of RMECs and failed to reverse the stimulatory effects of pitavastatin (Fig. 4A-C). This result demonstrates that pitavastatin acts on RMECs in a HMG-CoA reductase-independent manner. We further investigated the effects of pitavastatin on the release of VEGF and PDGF-AA. As assessed by ELISA using supernatant of RMECs after pitavastatin treatment, we found that pitavastatin significantly increased the supernatant level of VEGF (Fig. 4D). In contrast, we did not observe difference on PDGF-AA level in supernatant of cells exposed to pitavastatin compared to control (Fig. 4E). This suggests that pitavastatin stimulates pro-angiogenic microenvironment via upregulating specific angiogenic growth factors.
Pitavastatin acts on RMECs in a prenylation-independent manner. (A) In vitro angiogenesis images using RMECs in the absence (DMSO) and presence of pitavastatin, mevalonate, or cholesterol alone or the combination of pitavastatin with mevalonate or cholesterol. (B and C) Mevalonate and cholesterol do not reverse the stimulating effect of pitavastatin in RMEC capillary network formation. Mevalonate at 50 µM, cholesterol at 1 µM, and pitavastatin at 100 nM were used. Pitavastatin significantly increases the secretion of VEGF (D) but not PDGF-AA (E) in RMEC cells. VEGF and PDGF-AA protein in supernatant were measured by ELISA. *P < 0.05, compared to control. ns, not significant
Pitavastatin stimulates RhoA-mediated signaling pathways and focal adhesion complex in RMECs
Rho GTPase family is known to regulate several processes critical for endothelial cell migration, growth, and maintenance [20]. We investigated the activities of RhoA and Rac1 in RMECs after pitavastatin treatment. We found that pitavastatin potently increased RhoA activity without affecting Rac1 (Fig. 5A), suggesting the specific stimulatory effect of pitavastatin on RhoA activity. RhoA regulates stress fibers which are contractile actomyosin bundles and have a central role in endothelial cell adhesion and morphogenesis [21]. Consistent with RhoA activation, we found that pitavastatin induced stress fiber formation in RMECs (Fig. 5B). We further performed immunoblotting analysis of molecules downstream of RhoA pathway. As expected, pitavastatin significantly increased phosphorylation of MYPT1 and MLC (Fig. 5C and D). Consistent with RhoA activation and stress fiber formation, we observed the increased phosphorylation of FAK and paxillin in pitavastatin-treated cells (Fig. 5C and D), suggesting that pitavastatin stimulates focal adhesion. Pitavastatin did not affect phosphorylation of VEGFR but increased phosphorylation of Akt and ERK (Fig. 5C and D). Collectively, our results demonstrate that pitavastatin stimulates RhoA-mediated pathways, induces focal adhesion complex formation, and activates ERK in RMECs.
Pitavastatin stimulates multiple pro-angiogenic signaling pathways. (A) Pitavastatin significantly increases RhoA but not Rac1 activity in RMECs. (B) Pitavastatin increases actin stress fiber formation in RMECs. Actin stress fibers were visualized with phalloidin (red) and the cell nucleus were stained with DAPI (blue). Increased stress fiber bundles were indicated by white arrows. Images shown are representative of photomicrographs captured at 400 × magnification. Representative image (C) and quantification (D and E) of western blotting show the increased phosphorylation of MYPT1, MLC, FAK, paxillin, ERK, and Akt but not VEGFR. *P < 0.05, compared to control
Discussion
Many studies have highlighted that the beneficial effects of statins in cardiovascular disease may be attributed to their pleiotropic effects on endothelial cells [22, 23]. Statins improve function of endothelial cells, augment number of endothelial progenitor cells, and enhance repair and maintenance of a functioning endothelium, via multiple mechanisms independent of cholesterol lowering [23, 24]. Different from other statins that are well known for their effects on endothelial cells, only few studies revealed the possible effects of pitavastatin on angiogenesis and endothelial cells. In this work, we demonstrated that pitavastatin has a stimulatory effect on retinal angiogenesis and RMECs.
In order to demonstrate clinical significance, the concentrations of pitavastatin tested in in vitro retinal angiogenesis models are clinically relevant. Pitavastatin at low nanomolar concentrations stimulated early stages of retinal angiogenesis, most likely via activating RMEC migration and adhesion (Figs. 1 and 2). In addition, pitavastatin enhanced RMEC growth and survival in a similar manner as growth factors (Fig. 3). These findings are in line with Kikuchi et al.’s work that pitavastatin augmented endothelial proliferation and tube formation on Matrigel [11]. In contrast, pitavastatin inhibited capillary network formation and proliferation, and induced apoptosis of human lung cancer-associated endothelial cells [13]. The reason behind this discrepancy is likely to be a result of differences on concentrations of pitavastatin and endothelial cell model systems. Similar to other statins [25, 26], pitavastatin has a biphasic effect on endothelial cells and angiogenesis [27]. Pitavastatin at high concentration inhibits migration and proliferation of endothelial cells, whereas at low concentration, it protects endothelial cell viability and stimulates migration and proliferation. We did not test high concentration of pitavastatin in our study because such concentrations are not clinically achievable.
A significant finding of our work is that pitavastatin acts synergistically with VEGF and LPS on RMECs (Figs. 2 and 3). The combination of pitavastatin with VEGF or LPS was more effective than VEGF or LPS alone in stimulating RMEC tubular structure formation, migration, adhesion, and proliferation (Figs. 1, 2, and 3). VEGF- and LPS-induced inflammation play a major role in stimulating angiogenesis and are important features of ocular diseases caused by excessive angiogenesis [5, 28]. Our findings suggest that pitavastatin can further enhance the retinal angiogenesis induced by pathological conditions, such as proliferative diabetic retinopathy and age-related macular degeneration.
Masayuki et al.’s work demonstrated that pitavastatin augmented function of human epidermal microvessel endothelial cell via mevalonate pathway [27]. Kikuchi et al.’s work showed that Notch signaling was responsible for pitavastatin’s stimulatory activity in human umbilical vein endothelial cells [11]. Interestingly, our study found that pitavastatin acted on RMECs in a HMG-CoA reductase-independent manner as neither mevalonate nor cholesterol reversed the stimulatory effects of pitavastatin (Fig. 4A-C). In line with Frick et al.’s work that statins augment VEGF synthesis in human umbilical vein endothelial cells [29], we showed that pitavastatin specifically increased the release of VEGF in RMECs (Fig. 4D and E). However, pitavastatin did not affect VEGF-mediated signaling as phosphorylation level of VEGFR2 was unchanged (Fig. 5C and E). We found that pitavastatin increased RhoA activity and activated RhoA-mediated signaling pathways as shown by the increased phosphorylation of MYPT1 and MLC (Fig. 5A, C, and D). The increased phosphorylation of Akt further confirms the role of pitavastatin on RhoA signaling. Although previous studies demonstrated that pitavastatin regulated RhoA activity via prenylation [30, 31], our findings suggest that prenylation is not involved in RhoA activation by pitavastatin in RMECs. Pitavastatin increases stress fiber formation and phosphorylation of FAK and paxillin (Fig. 5B, C, and D), demonstrating that pitavastatin induces focal adhesion formation. This correlates well with the increased migration and adhesion by pitavastatin.
A recent work highlights the therapeutic potential of pitavastatin for peripheral arterial disease [32]. In a murine hindlimb ischemia model, pitavastatin stimulates ischemia-induced neovascularization via stimulating endothelial nitric oxide synthase (eNOS), Akt, and Notch1 [11, 33]. Our findings agree with and extend the previous work by showing that pitavastatin not only activates retinal endothelial cells under growth factor-reduced conditions but also further enhances VEGF- and LPS-induced excessive angiogenesis. Our work and others suggest the pro-angiogenesis effect of pitavastatin under various pathological angiogenesis-induced disease models. It is worthy of investigating how pitavastatin acts as a therapeutic agent for repair of endothelial function under ischemic situations and endothelial damages, particularly for comprehensive ophthalmologists. We speculate that pitavastatin might repair endothelial function under ischemic situations via mediating eNOS-related signals (e.g., eNOS/Akt) and Notch.
In conclusion, our work demonstrates the stimulatory effect of pitavastatin on retinal angiogenesis and RMECs via HMG-CoA reductase-independent RhoA-mediated signaling pathway and focal adhesion formation. Our work provides preclinical evidence on the possible deleterious effect of pitavastatin on angiogenesis-related ocular diseases.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Puliafito CA, Wykoff CC (2020) New frontiers in retina: highlights of the 2020 angiogenesis, exudation and degeneration symposium. Int J Retina Vitreous 6:18. https://doi.org/10.1186/s40942-020-00221-4
Rubio RG, Adamis AP (2016) Ocular angiogenesis: vascular endothelial growth factor and other factors. Dev Ophthalmol 55:28–37. https://doi.org/10.1159/000431129
Eelen G, Treps L, Li X, Carmeliet P (2020) Basic and therapeutic aspects of angiogenesis updated. Circ Res 127:310–329. https://doi.org/10.1161/CIRCRESAHA.120.316851
Rezzola S, Loda A, Corsini M, Semeraro F, Annese T, Presta M, Ribatti D (2020) Angiogenesis-inflammation cross talk in diabetic retinopathy: novel insights from the chick embryo chorioallantoic membrane/human vitreous platform. Front Immunol 11:581288. https://doi.org/10.3389/fimmu.2020.581288
Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, Benedetti EE, Zamora DO, Choi D, David LL, Smith JR (2013) Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res 32:102–180. https://doi.org/10.1016/j.preteyeres.2012.08.004
Bansal AB, Cassagnol M (2020) HMG-CoA reductase inhibitors StatPearls, Treasure Island (FL).
Chou R, Dana T, Blazina I, Daeges M, Jeanne TL (2016) Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 316:2008–2024. https://doi.org/10.1001/jama.2015.15629
Vaughan CJ, Gotto AM Jr, Basson CT (2000) The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 35:1–10. https://doi.org/10.1016/s0735-1097(99)00525-2
Kajinami K, Koizumi J, Ueda K, Miyamoto S, Takegoshi T, Mabuchi H (2000) Effects of NK-104, a new hydroxymethylglutaryl-coenzyme reductase inhibitor, on low-density lipoprotein cholesterol in heterozygous familial hypercholesterolemia. Hokuriku NK-104 Study Group. Am J Cardiol 85:178–183. https://doi.org/10.1016/s0002-9149(99)00656-6
Markle RA, Han J, Summers BD, Yokoyama T, Hajjar KA, Hajjar DP, Gotto AM Jr, Nicholson AC (2003) Pitavastatin alters the expression of thrombotic and fibrinolytic proteins in human vascular cells. J Cell Biochem 90:23–32. https://doi.org/10.1002/jcb.10602
Kikuchi R, Takeshita K, Uchida Y, Kondo M, Cheng XW, Nakayama T, Yamamoto K, Matsushita T, Liao JK, Murohara T (2011) Pitavastatin-induced angiogenesis and arteriogenesis is mediated by Notch1 in a murine hindlimb ischemia model without induction of VEGF. Lab Invest 91:691–703. https://doi.org/10.1038/labinvest.2011.5
Chen LW, Lin CS, Tsai MC, Shih SF, Lim ZW, Chen SJ, Tsui PF, Ho LJ, Lai JH, Liou JT (2019) Pitavastatin exerts potent anti-inflammatory and immunomodulatory effects via the suppression of AP-1 signal transduction in human T cells. International journal of molecular sciences 20 https://doi.org/10.3390/ijms20143534
Hu T, Shen H, Huang H, Yang Z, Zhou Y, Zhao G (2020) Cholesterol-lowering drug pitavastatin targets lung cancer and angiogenesis via suppressing prenylation-dependent Ras/Raf/MEK and PI3K/Akt/mTOR signaling. Anticancer Drugs 31:377–384. https://doi.org/10.1097/CAD.0000000000000885
Li Z, Li Q, Wang G, Huang Y, Mao X, Zhang Y, Wang X (2017) Inhibition of Wnt/beta-catenin by anthelmintic drug niclosamide effectively targets growth, survival, and angiogenesis of retinoblastoma. American journal of translational research 9:3776–3786
Wang G, Li Z, Li Z, Huang Y, Mao X, Xu C, Cui S (2017) Targeting eIF4E inhibits growth, survival and angiogenesis in retinoblastoma and enhances efficacy of chemotherapy. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 96: 750–756 https://doi.org/10.1016/j.biopha.2017.10.034
Luo Z, Zhang Y, Gu J, Feng P, Wang Y (2015) Pharmacokinetic properties of single- and multiple-dose pitavastatin calcium tablets in healthy Chinese volunteers. Curr Ther Res Clin Exp 77:52–57. https://doi.org/10.1016/j.curtheres.2015.02.001
Melincovici CS, Bosca AB, Susman S, Marginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM (2018) Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 59: 455–467
Kajinami K, Takekoshi N, Saito Y (2003) Pitavastatin: efficacy and safety profiles of a novel synthetic HMG-CoA reductase inhibitor. Cardiovasc Drug Rev 21:199–215. https://doi.org/10.1111/j.1527-3466.2003.tb00116.x
Tan Q, Yu D, Song L (2020) Atorvastatin disrupts primary human brain microvascular endothelial cell functions via prenylation-dependent mitochondrial inhibition and oxidative stress. Fundamental & clinical pharmacology https://doi.org/10.1111/fcp.12615
Barlow HR, Cleaver O (2019) Building blood vessels-one Rho GTPase at a time. Cells 8 https://doi.org/10.3390/cells8060545
Tojkander S, Gateva G, Lappalainen P (2012) Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci 125:1855–1864. https://doi.org/10.1242/jcs.098087
Oesterle A, Laufs U, Liao JK (2017) Pleiotropic effects of statins on the cardiovascular system. Circ Res 120:229–243. https://doi.org/10.1161/CIRCRESAHA.116.308537
Sandhu K, Mamas M, Butler R (2017) Endothelial progenitor cells: exploring the pleiotropic effects of statins. World J Cardiol 9:1–13. https://doi.org/10.4330/wjc.v9.i1.1
Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, Manes C, Fischer D, de Groot K, Fliser D, Fauler G, Marz W, Drexler H (2005) Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 111:2356–2363. https://doi.org/10.1161/01.CIR.0000164260.82417.3F
Weis M, Heeschen C, Glassford AJ, Cooke JP (2002) Statins have biphasic effects on angiogenesis. Circulation 105:739–745
Hu K, Wan Q (2019) Biphasic influence of pravastatin on human cardiac microvascular endothelial cell functions under pathological and physiological conditions. Biochem Biophys Res Commun 511:476–481. https://doi.org/10.1016/j.bbrc.2019.02.090
Katsumoto M, Shingu T, Kuwashima R, Nakata A, Nomura S, Chayama K (2005) Biphasic effect of HMG-CoA reductase inhibitor, pitavastatin, on vascular endothelial cells and angiogenesis. Circ J 69:1547–1555
Dayang EZ, Plantinga J, Ter Ellen B, van Meurs M, Molema G, Moser J (2019) Identification of LPS-activated endothelial subpopulations with distinct inflammatory phenotypes and regulatory signaling mechanisms. Front Immunol 10:1169. https://doi.org/10.3389/fimmu.2019.01169
Frick M, Dulak J, Cisowski J, Jozkowicz A, Zwick R, Alber H, Dichtl W, Schwarzacher SP, Pachinger O, Weidinger F (2003) Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis 170:229–236. https://doi.org/10.1016/s0021-9150(03)00299-5
Abdullah MI, Abed MN, Richardson A (2017) Inhibition of the mevalonate pathway augments the activity of pitavastatin against ovarian cancer cells. Sci Rep 7:8090. https://doi.org/10.1038/s41598-017-08649-9
Kojima Y, Ishida T, Sun L, Yasuda T, Toh R, Rikitake Y, Fukuda A, Kume N, Koshiyama H, Taniguchi A, Hirata K (2010) Pitavastatin decreases the expression of endothelial lipase both in vitro and in vivo. Cardiovasc Res 87:385–393. https://doi.org/10.1093/cvr/cvp419
Matsumoto T, Yamashita S, Yoshino S, Kurose S, Morisaki K, Nakano K, Koga JI, Furuyama T, Mori M, Egashira K (2020) Therapeutic arteriogenesis/angiogenesis for peripheral arterial disease by nanoparticle-mediated delivery of pitavastatin into vascular endothelial cells. Ann Vasc Dis 13:4–12. https://doi.org/10.3400/avd.ra.19-00130
Kubo M, Egashira K, Inoue T, Koga J, Oda S, Chen L, Nakano K, Matoba T, Kawashima Y, Hara K, Tsujimoto H, Sueishi K, Tominaga R, Sunagawa K (2009) Therapeutic neovascularization by nanotechnology-mediated cell-selective delivery of pitavastatin into the vascular endothelium. Arterioscler Thromb Vasc Biol 29:796–801. https://doi.org/10.1161/ATVBAHA.108.182584
Funding
Hubei Provincial Health and Family Planning Commission Youth Talent Project (WJ2017Q039) provided financial support in the form of research funding. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
This article does not contain any studies with human participants performed by any of the authors.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhi Li, Jing Zhang and Yanni Xue have contributed equally to this work and are co-first authors
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, J., Xue, Y. et al. Pitavastatin stimulates retinal angiogenesis via HMG-CoA reductase-independent activation of RhoA-mediated pathways and focal adhesion. Graefes Arch Clin Exp Ophthalmol 259, 2707–2716 (2021). https://doi.org/10.1007/s00417-021-05328-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05328-4