Abstract
Purpose
To investigate the changes in the choroidal vascularity index (CVI) of patients with multiple sclerosis (MS) using binarization on enhanced depth imaging optical coherence tomography (EDI-OCT) images and to evaluate the effect of optic neuritis (ON) attacks on these measurements.
Method
Three groups were created by including forty eyes of 20 patients diagnosed with relapsing-remitting MS and had a unilateral history of ON attack and the randomly selected eyes of 30 healthy age- and sex-matched control subjects. Group 1 (n = 20) consisted of the ON-affected eyes of the MS patients (MSON); group 2 (n = 20) included their fellow healthy eyes (MSNON); group 3 (n = 30) included the eyes of healthy controls.
Results
The mean age was 33.3 ± 9.4 years in the MS group and 33.4 ± 11.1 years in the healthy control group. Mean choroidal vascularity index (CVI) was significantly lower in the MSON group than the MSNON group (59.6 ± 3.72 % vs 61.7 ± 3.16 %, p = 0.007). The CVI values of both the MSON and MSNON groups were significantly lower when compared to the controls (63.9 ± 2.76) (p < 0.001, p = 0.030). Compared to controls, the subfoveal total choroidal area and luminal area values were significantly greater in the MSON (p = 0.009, p = 0.009, respectively) and MSNON groups (p = 0.031, p = 0.013, respectively).
Conclusion
The presented study demonstrates that, compared to healthy subjects, CVI values are lower in the affected and unaffected eyes of patients who had a history of ON in relation with their MS diagnosis. The presence of significant anatomical changes, especially in the luminal area, may suggest that ON causes vascular disorganization which contributes to MS pathophysiology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Multiple sclerosis (MS) is a chronic inflammatory and degenerative disease of the central nervous system. It is one of the most common neurological disorders, affecting an estimated 2.3 million people worldwide [1]. The disease often occurs in young adults. While some types are characterized by attacks, others are progressive [2]. The most common ocular symptom of the disease is optic neuritis (ON), which is caused by axonal degeneration of the optic nerve. This problem manifests itself as the first clinical sign of the disease in 25% of patients and is seen in 70% of patients during the disease [3].
Autoimmunity plays a prominent role in the pathogenesis of MS [4]. More specifically, demyelination and neurodegeneration have been associated with T cell-mediated inflammation in the central nervous system [5]. In the context of ocular findings, it has been shown that retinal nerve fiber layer thickness (RNFLT) is decreased due to axonal damage in patients with MS [6, 7]. Furthermore, vascular disorganization mechanisms are known to be involved in the pathophysiology of MS. Some Doppler ultrasonography studies have shown significantly decreased blood flow in the posterior ciliary artery and there have been reports of altered retrobulbar hemodynamics in the case of ON due to MS [8, 9]. Also, decreased extraocular blood flow and increased endothelin-1 plasma levels have been identified in patients with MS [10].
The dense vascular choroid layer is a structure that is affected by disorders characterized by systemic inflammation and vascular disorganization—such as MS [11]. It is possible to visualize the structure of the choroid and measure subfoveal choroidal thickness (SFCT)—an indicator of subfoveal ocular perfusion—in the EDI (enhanced depth imaging) mode of spectral-domain optical coherence tomography (OCT) devices [12, 13]. In recent years, EDI-OCT images have been binarized utilizing a software program to separate the choroid into luminal and interstitial areas in order to measure the vascularity changes in the choroid more precisely [14]. A quantitative value, called choroidal vascularity index (CVI), that can be identified in a semiautomated manner, emerged as a parameter that could be used to assess the vascular status of the choroid using binarization techniques [15].
This study presents the comparison of ON-affected eyes of MS patients, their fellow eyes, and healthy control eyes in terms of CVI values determined by the binarization of EDI-OCT images. To the best of our knowledge, this is the first study evaluating CVI values in MS patients with unilateral ON attack history.
Methods
Study population
This prospective study included 40 eyes of 20 patients who applied to the ophthalmology clinic and diagnosed with relapsing-remitting MS according to the McDonald criteria [16]. These patients had unilateral ON history (optic papillitis/retrobulbar neuritis). Additionally, 30 healthy eyes from a randomly selected group of age- and sex-matched healthy controls were included in the study. Three groups were created: group 1 (n = 20) consisted of the affected eyes (ON attack) of the MS patients (MSON group), group 2 (n = 20) included their fellow unaffected eyes (MSNON group), and group 3 (n = 30) included eyes of healthy controls. The study was conducted according to the principles put forth by the Helsinki Declaration and received ethics approval from the local clinical trials ethics committee.
Inclusion and exclusion criteria
For the MS group, the inclusion criteria were as follows: having a history of ON attack that was proven with visual field, contrasted orbital magnetic resonance imaging and examination results, attending follow-up at the Neurology and the Ophthalmology clinics, having a best-corrected visual acuity (BCVA) of ≥ 2/10, having spherical refractive errors in the range of –4 to +3 diopters and cylindrical refractive errors of ≤ 3 diopters, not having any ocular or systemic diseases other than MS, not having a history of any ocular surgery, not having a history of ON attacks as well as MS attacks for at least 3 months, and not having a history of systemic or topical cortisone use for at least 3 months.
Healthy controls were randomly selected from individuals who had applied for routine ophthalmological examination to the ophthalmology department. The inclusion criteria were as follows: the absence of any ophthalmologic pathology other than refraction errors or cataract (spherical refractive errors of –4 to +3 diopters, and cylindrical refractive errors of ≤ 3 diopters), having a BCVA of ≥ 6/10, absence of systemic diseases or a history of any ocular surgery, and not receiving any systemic or topical medications within the last 3 months.
Exclusion criteria included the presence of glaucomatous damage to the optic disc in fundus examination, having glaucoma or ocular hypertension, family history of glaucoma, having optical media opacities (dense cataracts, corneal opacity, pupillary anomaly, vitreous opacity) which could affect OCT images, having uveitis or any type of diagnosed retinal pathology, and having a history of ocular surgery or major surgery involving any other system or organ.
Ophthalmologic examination, OCT imaging, and measurement protocols
All participants underwent the Snellen BCVA test, biomicroscopic anterior segment examination, IOP measurement with Goldmann applanation tonometer (Haag–Streit, Bern, Switzerland), axial length (AXL) measurement (IOL Master, Carl Zeiss Meditec, Dublin, CA), central corneal thickness (CCT) measurement (Topcon, RK-8800, Tokyo), pupillary reflexes, eye movements, and fundus examination. All participants underwent OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) examination, and the Humphrey visual field (Humphrey Visual Field Analyzer III, Carl Zeiss Inc., Dublin, CA) was performed with 24-2 SITA standard strategy for MS patients. The OCT scans were performed from 9 AM to 11 AM in order to minimize diurnal variation of choroid thickness. All scans were performed with a target of sustaining high (> 50) signal strength index scores.
We utilized the standard settings for the EDI mode of OCT imaging: 1024 A-scans on a 6-mm horizontal line passing through the center of the fovea were obtained from all participants. Subfoveal choroidal thickness was measured with horizontal B-scans on vertical lines running from the retinal pigment epithelium’s outer border towards the choroidoscleral junction in the subfoveal region. Measurements were performed using the manual measurement tool of the OCT device by two independent observers blinded to the clinical characteristics of patients.
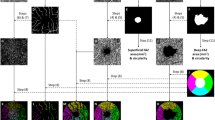
The EDI-OCT images (Fig. 1a) were binarized in the Image J software (Version 1.52i, National Institutes of Health, Bethesda, MD USA) using the protocol described by Agrawal et al. [15] (Fig. 1b). The subfoveal area from the retinal pigment epithelium to the choroidoscleral junction was marked with the polygon selection tool from 750-μm nasal and 750-μm temporal to the fovea. The marked total choroidal area image was binarized using Niblack’s autolocal thresholding method. The image was converted to 8 bits and was applied an autolocal threshold; the luminal area was highlighted by first converting the image to red-green-blue color space and then applying the color threshold. The first automatically calculated value represents total choroidal area in the ROI manager, and the second calculated value represents luminal area (Fig. 1c). Stromal area is calculated by subtracting luminal area from total choroidal area, and CVI is computed by dividing luminal area by total choroidal area. All CVI procedures were repeated three times by the same observer who was blinded to patients’ diagnosis, and the average of these three measurements was recorded and used in the analyses.
Illustration of binarized EDI-OCT images in an eye affected with optic neuritis attack in a patient with multiple sclerosis. a Enhanced depth imaging optical coherence tomography (EDI-OCT). b Binarized EDI-OCT image showing the segmentation of choroidal structures. c In the ROI manager, the first automatically calculated value represents total choroidal area and the second calculated value represents luminal area. Stromal area was calculated by subtracting luminal area from total choroidal area, and CVI was computed by dividing luminal area by total choroidal area
The peripapillary RNFLT was measured around the disc with 16 averaged circular B-scans (12° in diameter) and automatically segmented using the SPECTRALIS software. Then, patients’ average peripapillary RNFLT was recorded.
Central macular thickness and volume were measured at 1-mm diameter fovea area of the topographic thickness map in 9 zone maps defined by the Early Treatment Diabetic Retinopathy Study.
Statistical method
Statistical analysis was performed using the Number Cruncher Statistical System (NCSS) 2007 (Kaysville, UT, USA) program. Descriptive statistical methods (mean, standard deviation, frequency, percentage, minimum, maximum) were used when depicting study data. The Shapiro-Wilk test and graphical examinations were used to test the suitability of quantitative data to normal distribution. Generalized linear mixed models were used for comparisons between groups and to determine the factors affecting the presence of ON. Pearson correlation analysis was used to evaluate the relationships between quantitative variables. Univariate and multivariate regression models were used to determine the effect of the evaluated parameters on the presence of ON and the effects of CVI values. Inter-examiner and intra-examiner intraclass correlation coefficient (ICC) was used to determine the agreement between observers. Statistical significance was accepted as p < 0.05.
Results
There were 20 patients (13 women, 7 men; average age: 33.25 ± 9.44 years) in the MS group and 30 patients (18 women, 12 men; average age: 33.4 ± 11.14 years) in the healthy subject group. There was no statistically significant difference between subjects with MS and healthy controls regarding age and gender distribution (p = 0.999 and p = 0.958, respectively). The mean disease duration was 53.15 ± 50.38 (12–240) months in patients with MS. The mean time that had surpassed after the last ON attack was 24.60 ± 18.16 (8–72) months before OCT shots. There were also no statistically significant differences between the groups with regard to refraction value, IOP, CCT, and AXL values. The distribution of demographic characteristics by group is given in detail in Table 1.
Mean SFCT was 281.75 ± 81.89 μm in the MSON group, 308.3 ± 101.15 μm in the MSNON group, and 286.23 ± 57.14 μm (p =0.322) in the control group. The mean CVI value was 59.6 ± 3.72 in the MSON group, 61.7 ± 3.16 in the MSNON group, and 63.9 ± 2.76 in the control group. The mean CVI was found to be significantly lower in both the MSON and MSNON groups when compared with the controls (p < 0.001 and p = 0.030, respectively). In the MSON group, mean CVI was significantly lower compared to the MSNON group (p = 0.007). In terms of total choroidal area, both of the MSON and MSNON groups had significantly higher values compared with controls (p = 0.009 and p = 0.031, respectively). Additionally, mean total choroidal area was significantly greater in the MSON group compared to that of the MSNON group (p = 0.047). Mean luminal area was 1.21 ± 0.34 mm2 in the MSON group and 1.20 ± 0.35 mm2 in the MSNON group (p = 0.105), while it was found to be significantly lower in controls (0.92 ± 0.34 mm2) compared to both of these groups (p = 0.009 and p = 0.013, respectively) (Table 1).
The mean peripapillary RNFLT value was found to be lowest in the MSON group (97.3 ± 17.91 μm); however, a significant difference was not observed between the three groups (p = 0.261) (Table 1).
Pearson correlation analysis results in patients with MS are shown in Table 2 and Table 3. It was determined that there is a moderate significant negative correlation between CVI values and EDSS score (r = −0.407, p = 0.002) in the MSON group. A univariate regression analysis was performed by including the parameters evaluated in the study to determine those that had an effect on CVI value. The results showed that total choroidal area and EDSS score in the MSON group (Table 4) and the total choroidal area in the MSNON group (Table 5) were predictive parameters associated with lower CVI values. In the MSON group, multivariable regression showed that, when controlled for gender and age, an increase of 1 unit in the EDSS score would cause a decrease of 7.074 units in CVI value [Beta (95% CI) = −7.074 (−12.867, −1.282), p=0.020]. The univariate evaluations showed that none of the variables had a statistically significant effect on the presence of ON in the MSON group (p > 0.05).
There was a good agreement between intra-observer and inter-observer measurements of SFCT values (ICC = 0.999, p <0.001). Figure 2 shows color fundus photo (a), OCT image of RNFLT map (b), and 24-2 SITA Humphrey visual field (c) for the left affected eye (ON attack, 18 months prior to scan) in a patient with MS.
Color fundus photo, OCT image of RNFLT map, and 24-2 SITA Humphrey visual field for the left affected eye (ON attack, 18 months prior to scan) in a patient with MS. a The fundus examination showed atrophic optic disc in the temporal, superior temporal, and superior nasal quadrants on the affected eye with ON attack of the MS patient. b The retinal nerve fiber layers were observed to be thinned in the temporal, superior temporal, and superior nasal quadrants on the same eye. c Showing the altitudinal inferior visual field loss
Discussion
In this study, CVI values were found to be significantly lower (and total choroidal area was expanded) in the ON-affected eyes of MS patients, when compared to their unaffected eyes and healthy control eyes. Additionally, the unaffected eyes of patients with MS also demonstrated significantly lower mean CVI values and significantly higher total choroidal area and luminal area values compared to the control group. Nevertheless, there was no significant difference between the three groups regarding mean SFCT.
The dense vascular choroid layer is affected by disorders that cause systemic inflammation and vascular disorganization, such as MS [11]. The choroid supplies blood flow to the retinal pigmented epithelium, photoreceptors, and the prelaminar portion of the optic nerve [17,18,19]. Since the choroid demonstrates considerable changes in several diseases, choroidal imaging has become essential to understand the pathophysiology of such diseases and their effects in the eye. The EDI-OCT technique introduced by Spaide et al. has helped advance the understanding of choroidal structure [20]. This imaging technique has made it possible to divide the choroid into different subregions by using binarization techniques on EDI-OCT images. Through these methods, the luminal (vascular) and stromal (interstitial) areas can be differentiated, and automated determination of quantitative values can be performed to identify vascularity changes.
Dogan et al. found that the mean SFCT value was significantly different between the MSON and MSNON groups (p = 0.004). However, they reported no significant differences in the MSON versus control and MSNON versus control comparisons [21]. Esen et al. reported there were no significant differences between the MSON and MSNON groups, in terms of mean SFCT values [22]. In the present study, although mean SFCT did not show a significant difference between the three groups, the CVI values were found to be significantly lower in the ON-affected eyes of MS patients compared to their unaffected eyes and control eyes. It was also interesting to note that CVI values were also significantly lower in the unaffected eyes of MS patients when compared to the control group (p = 0.030). We thought that evaluating CVI values through the utilization of a semiautomated program which provides quantitative values may be more reliable than the assessment of SFCT in terms of assessing choroidal changes.
It should be noted that, until recently, MS was thought to be a myelin disorder; however, postmortem studies showed neuronal and axonal loss [5]. Gordon-Lipkin et al. suggested the presence of neuronal damage mechanisms other than demyelination in MS and reported a correlation between reduced RNFLT and brain atrophy, as measured by magnetic resonance imaging [23]. Besides, vascular disorganization mechanisms are known to be involved in the pathophysiology of MS. Recently, sensitive perfusion-weighted magnetic resonance imaging techniques have demonstrated decreased cerebral perfusion in patients with MS [24, 25]. It has been reported that there is an elevation in the plasma levels of endothelin-1, a potent vasoconstrictor peptide that could cause cerebral hypoperfusion if it was elevated in the spinal fluid. It has been suggested that the elevation of endothelin-1 could contribute to neurodegeneration [26, 27]. Hypoperfusion causes axonal degeneration through disruption of mitochondrial oxygenation, decreased energy metabolism, and increased oxidative stress [28, 29]. Endothelin-1 is mainly secreted from reactive astrocytes [30]. Endothelin-1 not only acts as a potent vasoconstrictor but also augments the sensitivity of vessels to other vasoconstrictors, such as norepinephrine and angiotensin II [26]. As mentioned before, CVI was lower and total choroidal area and luminal area values were higher in patients with MS when compared to controls. Therefore, we hypothesized that such vascular disorganization in the MS could be disproportionately affecting the choroidal layer of the eye—especially the vascular area of the choroid rather than the stromal area. The significant anatomical changes seen, especially in the luminal area, may prove that vascular disorganization (which is suggested to be involved in MS pathophysiology) may also be effective in the eye. Chen et al. documented thinner peripapillary choroid in patients with acute ON and suggested a relationship with endothelin-1 [31]. Also, decreased extraocular blood flow and increased endothelin-1 plasma levels have been reported in patients with MS [10]. Wang et al. studied the microcirculation of the optic nerve head in MS patients with and without ON history by using OCT angiography. They found that the mean optic nerve head flow index in the MSON group was significantly lower compared to healthy controls and the unaffected eyes of patients with MS. They hypothesized that ON attack could affect the perfusion of the vessel supplying the optic nerve. In the same study, no significant difference was reported between healthy control and MSNON groups in the optic nerve head flow index and parafoveal flow index among the three groups [32]. Interestingly, in the current study, the CVI, total choroidal area, and luminal area values that were studied in the subfoveal region of the choroid were observed to be significantly affected in MSNON eyes, as comparisons with the healthy subjects group demonstrated statistical significance (p = 0.030, p = 0.031, and p = 0.013, respectively). According to these results, choroidal vascular pathologies also appear to occur in eyes without ON history in patients with MS. Choroid thickness has been shown to be affected by many factors in past studies [33]. Although only patients with no history of cortisone use for at least 3 months were included in our study, it is possible that the systemic treatments used for MS (especially steroids) might affect these choroidal regions. As such, it is evident that we cannot reliably conclude that the changes demonstrated in this study are solely caused by the presence of MS and ON attacks; however, the differences shown in the comparison of the affected and unaffected eyes of MS patients provides evidence that this region is affected to a greater degree in the presence of ON history. We believe that these results strongly support the hypothesis that ON attacks have significant effects on vascular organization in the choroidal region.
Previous studies have shown the loss of retinal ganglion cells and axons in MS [34,35,36,37]. The presented study showed that, although retinal nerve fiber layer was thinnest in the MSON group, a significant difference was not observed when these three groups were compared (p = 0.261). Trip et al. reported decreased RNFLT in eyes with MSON compared to healthy eyes [38]. It was suggested to be because the eyes with MSNON were subclinical affected by retrograde trans-synaptic degeneration during attacks, which might have caused chronic thinning in retinal nerve fiber layers [36, 39, 40]. However, there is no clear evidence to support this conclusion. Differences between studies may be related to differences in disease duration, age groups, use of corticosteroids and systemic drugs, and ON attack time (time surpassed since attack). Many studies have demonstrated that retinal nerve fiber layer loss is correlated with visual acuity, color vision, contrast sensitivity, multifocal evoked visual potentials, magnetic resonance imaging findings, and EDSS score [23, 41, 42].
Disease duration and EDSS scores are some of the parameters associated with progressive axonal loss in MS [43]. This study found a moderate negative correlation between CVI values and the EDSS score in the MSON group (r = −0.407 and p = 0.002). Furthermore, in the MSON group, it was determined that an increase of 1 unit in the EDSS score would cause a decrease of 7.074 units in CVI value [Beta (95% CI) = −7.074 (−12.867, −1.282), p=0.020]. According to these results, the degree of physical disability appears to be inversely correlated with CVI value, even though no significant reduction can be observed in RNFLT values.
This study has some limitations: the number of patients was limited and the mean duration with disease was not long (3.12 ± 7.16 years). The study population was heterogeneous with regard to race and ethnic origin, which could alter results. Studies to be conducted with larger populations and longer follow-up may contribute to better evaluation of the choroid.
Conclusion
The current study demonstrated, to the best of our knowledge for the first time, CVI values were lower in the ON-affected and ON-unaffected eyes of patients with MS when compared to controls. Furthermore, the choroid was influenced to a greater degree in the affected eyes with ON attacks of MS patients, compared to their unaffected eyes. The significant anatomical changes (especially with regard to luminal area values) determined with EDI-OCT imaging may prove that vascular disorganization—which is argued to be involved in MS pathophysiology—also affects the eyes of patients with MS, especially in their affected eyes with ON. The CVI value, which is an indicator of the quantitative structural changes seen in the choroidal layer, might be also a useful prognostic factor in patients with MS.
References
National Multiple Sclerosis Society (2020) New York: Who gest MS (epidemiology); [cited 2020 March 20]. Available from: http://www.nationalmssociety.org/What-is-MS/Who-Gets-MS
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46(4):907–911. https://doi.org/10.1212/wnl.46.4.907
Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up (2008) Arch Neurol 65(6):727-732. doi:10.1001/archneur.65.6.727
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8(9):913–919. https://doi.org/10.1038/ni1507.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338(5):278–285. https://doi.org/10.1056/nejm199801293380502.
Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH (2008) Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 14(7):893–905. https://doi.org/10.1177/1352458508091367.
Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C et al (2013) Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 80(1):47–54. https://doi.org/10.1212/WNL.0b013e31827b1a1c.
Akarsu C, Tan FU, Kendi T (2004) Color Doppler imaging in optic neuritis with multiple sclerosis. Graefes Arch Clin Exp Ophthalmol 242(12):990–994. https://doi.org/10.1007/s00417-004-0948-1.
Modrzejewska M, Karczewicz D, Wilk G (2007) Assessment of blood flow velocity in eyeball arteries in multiple sclerosis patients with past retrobulbar optic neuritis in color Doppler ultrasonography. Klin Ocz 109(4-6):183–186
Pache M, Kaiser HJ, Akhalbedashvili N, Lienert C, Dubler B, Kappos L, Flammer J (2003) Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol 49(3):164–168. https://doi.org/10.1159/000069085.
Ingegnoli F, Gualtierotti R, Pierro L, Del Turco C, Miserocchi E, Schioppo T, Meroni PL (2015) Choroidal impairment and macular thinning in patients with systemic sclerosis: the acute study. Microvasc Res 97:31–36. https://doi.org/10.1016/j.mvr.2014.08.008.
Kim M, Kim SS, Kwon HJ, Koh HJ, Lee SC (2012) Association between choroidal thickness and ocular perfusion pressure in young, healthy subjects: enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci 53(12):7710–7717
Novais EA, Badaró E, Allemann N, Morales MS, Rodrigues EB, de Souza Lima R, Regatieri CV, Belfort R Jr (2015) Correlation Between Choroidal Thickness and Ciliary Artery Blood Flow Velocity in Normal Subjects. Ophthalmic Surg Lasers Imaging Retina 46(9):920–924. https://doi.org/10.3928/23258160-20151008-04.
Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, Tomita M (2014) Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci 55(6):3893–3899. https://doi.org/10.1167/iovs.14-14447
Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY (2016) Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep 6:21090. https://doi.org/10.1038/srep21090.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Hayreh SS (1969) Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol 53(11):721–748. https://doi.org/10.1136/bjo.53.11.721.
Kur J, Newman EA, Chan-Ling T (2012) Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res 31(5):377–406. https://doi.org/10.1016/j.preteyeres.2012.04.004
Linsenmeier RA, Padnick-Silver L (2000) Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 41(10):3117–3123
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146(4):496–500. https://doi.org/10.1016/j.ajo.2008.05.032.
Doğan Ü, Ulaş F, Türkoğlu ŞA, Ögün MN, Ağca S (2019) Eyes are mirror of the brain: comparison of multiple sclerosis patients and healthy controls using OCT. Int J Neurosci 129(9):848–855. https://doi.org/10.1080/00207454.2019.1576660
Esen E, Sizmaz S, Demir T, Demirkiran M, Unal I, Demircan N (2016) Evaluation of Choroidal Vascular Changes in Patients with Multiple Sclerosis Using Enhanced Depth Imaging Optical Coherence Tomography. Ophthalmologica 235(2):65–71. https://doi.org/10.1159/000441152.
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69(16):1603–1609. https://doi.org/10.1212/01.wnl.0000295995.46586.ae
Adhya S, Johnson G, Herbert J, Jaggi H, Babb JS, Grossman RI, Inglese M (2006) Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3. 0 T Neuroimage 33(4):1029–1035
Law M, Saindane AM, Ge Y, Babb JS, Johnson G, Mannon LJ, Herbert J, Grossman RI (2004) Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology 231(3):645–652. https://doi.org/10.1148/radiol.2313030996.
Haufschild T, Shaw SG, Kesselring J, Flammer J (2001) Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol 21(1):37–38. https://doi.org/10.1097/00041327-200103000-00011.
D'Haeseleer M, Beelen R, Fierens Y, Cambron M, Vanbinst AM, Verborgh C, Demey J, De Keyser J (2013) Cerebral hypoperfusion in multiple sclerosis is reversible and mediated by endothelin-1. Proc Natl Acad Sci U S A 110(14):5654–5658. https://doi.org/10.1073/pnas.1222560110
Cambron M, D'haeseleer M, Laureys G, Clinckers R, Debruyne J, De Keyser J (2012) White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab 32(3):413–424
Trapp BD, Stys PK (2009) Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol 8(3):280–291. https://doi.org/10.1016/s1474-4422(09)70043-2.
Blomstrand F, Giaume C (2006) Kinetics of endothelin-induced inhibition and glucose permeability of astrocyte gap junctions. J Neurosci Res 83(6):996–1003. https://doi.org/10.1002/jnr.20801.
Chen TC, Yeh CY, Lin CW, Yang CM, Yang CH, Lin IH, Chen PY, Cheng JY, Hu FR (2017) Vascular hypoperfusion in acute optic neuritis is a potentially new neurovascular model for demyelinating diseases. PLoS One 12(9):e0184927. https://doi.org/10.1371/journal.pone.0184927.
Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, Hornegger J, Fujimoto JG, Wu Q, Huang D (2014) Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 98(10):1368–1373. https://doi.org/10.1136/bjophthalmol-2013-304547
Han JM, Hwang JM, Kim JS, Park KH, Woo SJ (2014) Changes in choroidal thickness after systemic administration of high-dose corticosteroids: a pilot study. Invest Ophthalmol Vis Sci 55(1):440–445. https://doi.org/10.1167/iovs.13-12854.
Balk LJ, Petzold A (2014) Current and future potential of retinal optical coherence tomography in multiple sclerosis with and without optic neuritis. Neurodegener Dis Manag 4(2):165–176. https://doi.org/10.2217/nmt.14.10.
Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ (2012) Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One 7(5):e36847. https://doi.org/10.1371/journal.pone.0036847.
Alonso R, Gonzalez-Moron D, Garcea O (2018) Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: A review. Mult Scler Relat Disord 22:77–82. https://doi.org/10.1016/j.msard.2018.03.007.
Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB et al (2012) Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 119(6):1250–1257. https://doi.org/10.1016/j.ophtha.2011.11.032
Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, Plant GT, Miller DH (2005) Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol 58(3):383–391. https://doi.org/10.1002/ana.20575
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ (2008) Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 4(12):664–675. https://doi.org/10.1038/ncpneuro0950.
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML et al (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113(2):324–332. https://doi.org/10.1016/j.ophtha.2005.10.040
Balcer LJ, Miller DH, Reingold SC, Cohen JA (2015) Vision and vision-related outcome measures in multiple sclerosis. Brain 138(1):11–27. https://doi.org/10.1093/brain/awu335.
Klistorner A, Arvind H, Nguyen T, Garrick R, Paine M, Graham S, O'Day J, Yiannikas C (2009) Multifocal VEP and OCT in optic neuritis: a topographical study of the structure-function relationship. Doc Ophthalmol 118(2):129–137. https://doi.org/10.1007/s10633-008-9147-4.
Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T et al (2016) Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 15(6):574–584. https://doi.org/10.1016/s1474-4422(16)00068-5.
Acknowledgments
We want to thank Dr. Devran Suer from Neurology Department, Haydarpaşa Numune Training and Research Hospital, for evaluating the patients and EDSS scores.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sevcan Balci; Methodology: Sevcan Balci; Formal analysis and investigation: Sevcan Balci, Alev Ozcelik Kose; Writing (original draft preparation): Sevcan Balci, Alev Ozcelik Kose; Writing (review and editing): Sevcan Balci, Alev Ozcelik Kose, Nursal Melda Yenerel.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balci, S., Ozcelik Kose, A. & Yenerel, N.M. The effect of optic neuritis attacks on choroidal vascularity index in patients with multiple sclerosis. Graefes Arch Clin Exp Ophthalmol 259, 2413–2424 (2021). https://doi.org/10.1007/s00417-021-05143-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05143-x