Abstract
Purpose
To show that an immediate vitrectomy with an intravitreal injection of antibiotics can be an effective approach for the treatment of acute endophthalmitis following intravitreal injections.
Methods
We reviewed all cases of clinical endophthalmitis caused by an intravitreal injection that were treated in our department between March 2012 and November 2019. Only patients that underwent a vitrectomy within 6 h after presentation to the clinic and with a documented visual acuity shortly before the causative event were included. Baseline best-corrected visual acuity (BCVA) before the causative event was compared to BCVA measured within a follow-up period of 8 months (up to 14 months).
Results
In total, 30 eyes of 30 patients were included. The BCVA before the intraocular infection was a mean value of 0.55 logMAR, and the BCVA on the day of the endophthalmitis decreased significantly to 1.66 logMAR. Within 2 months following the pars plana vitrectomy (PPV), the mean BCVA improved to 0.83 logMAR. Eight months following PPV (mean value, 8.20 months; SD, 3.59 months), the mean BCVA was 0.63 logMAR. In the last follow-up interval most of the eyes recovered, and the BCVA did not differ significantly from baseline. Two eyes underwent further pars plana surgery during the follow-up period. No enucleation was required.
Conclusion
In this study, we have shown that an immediate vitrectomy with subsequent intravitreal injection of antibiotics is an effective option for treating post-injection endophthalmitis and frequently results in recovery of vision; thus, it should be performed as early as possible, where available.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative endophthalmitis not only is a severe sight-threatening complication but also can lead to complete loss of the affected eye. This complication occurs after intravitreal injections [1], cataract surgery [2, 3], vitrectomy [4,5,6], and after other surgeries such as filtering glaucoma surgery [7]. Unfortunately, even eyes with a good outcome after this complication may remain significantly impacted [8]. Recently, cases with an excellent visual and morphological outcome after early and aggressive intervention have been described [9, 10]. However, these reports contradict the data from the randomized controlled Endophthalmitis Vitrectomy Study (EVS) [11], which concluded that pars plana vitrectomy (PPV) was only beneficial in patients with light perception, while patients with better visual acuity did not benefit. The EVS is an excellent landmark study, but its conclusions have been continuously challenged for a number of reasons: firstly, its findings cannot be directly applied to recent cases of post-cataract endophthalmitis because both extracapsular cataract extraction and scleral tunnel phacoemulsification (the most common procedure in the past) have been replaced lately by clear corneal micro-incisional techniques [12]. Furthermore, cases of endophthalmitis related to intravitreal injections were not included in this study. Moreover, PPV as a possible intervention for the treatment of postoperative endophthalmitis has been developed over the past decades in many aspects: the incisions have become less invasive (from 20 to even 27 gauge) [13], the vitrectomy devices as well as the visualization methods have evolved, and even the surgical instruments (e.g., new two-dimensional cutters) can reduce iatrogenic damage in the inflamed setting of a posterior segment affected by endophthalmitis. It is mainly assumed that the severity of postoperative endophthalmitis is related to pathogens using the vitreous as a scaffold to proliferate; this explains the high incidence of postoperative endophthalmitis after complicated cataract surgery with a posterior capsular rupture [14] and would warrant an early removal of as much vitreous as possible as early as possible. Another critical factor is the release of damaging reactive agents following the infection within the vitreous cavity, again favoring an early and complete removal of vitreous.
After the publication of the EVS data in 1995, not only were technical advances in the operating procedure introduced but also better healthcare with broader and faster access to vitreoretinal surgery units, yielding earlier interventions and better surgical outcomes for affected patients. We are therefore in the rather peculiar situation that data from an RCT (randomized controlled trial) are considered outdated.
In the present study, a patient cohort that developed endophthalmitis after previous intravitreal injection and was subsequently treated in a specialized center was analyzed. As all patients had been instructed to present immediately to the hospital if symptoms compatible with endophthalmitis arose, the time between disease onset and presentation to our clinic was very short. From the electronic medical record of each patient, it was possible to analyze the interval between presentation to the clinic and the start of surgery. Hence, we could investigate the effect that an immediate vitrectomy has on patients with post-injection endophthalmitis.
Materials and methods
This retrospective study was approved by the local Ethics Committee (approval number 243/14) and followed the declaration of Helsinki.
Electronic patient records were reviewed, and data on their clinical presentation, examination, microbiology results, and procedures were gathered.
We retrospectively reviewed all cases of clinical endophthalmitis caused by an intravitreal injection that were treated at Eye Clinic Sulzbach, Knappschaft Hospital Saar, Germany, between March 2012 and November 2019. We found a total of 67 cases. Only those cases that received an immediate vitrectomy were included, where “immediate” was defined as the start of surgery in less than 6 h after presentation. Eight out of 67 cases (12%) received a delayed vitrectomy after more than 6 h and were excluded.
We included only patients that had a documented visual acuity both in the period before or on the day of the causing event and in the following months. Twenty-nine out of 67 patients (43%) were referred to our clinic without reliable data and thus were excluded. For some patients, we could retrieve missing data from the referring colleagues.
Preoperative evaluation included BCVA (best-corrected visual acuity), IOP (intraocular pressure), and a dilated fundus evaluation as well as ultrasound. The diagnosis was made on clinical presentation and ultrasound. The predominant symptoms were acute decrease of visual acuity accompanied by pain, a red eye, and hypopyon with or without a fibrinous anterior chamber reaction upon slit-lamp examination. Using ultrasound, B-scan vitreous infiltrates could be demonstrated in most cases.
The primary purpose of this study was to evaluate the change in BCVA pre- and postoperatively, and postoperative complications. The baseline BCVA before injection or at the time of the injection that caused endophthalmitis was compared to the BCVA measured at the time of diagnosis and throughout two different periods within 14 months of the infective event. Because most of the patients had repeated intravitreal injections afterwards, we did not look at the long-term development of BCVA because this could be confounded by the underlying retinal condition. The first follow-up visit took place within 2 months following PPV (mean value, 24.50 days; SD, 15.02 days). The last follow-up visit took place within the following year (mean value, 8.20 months; SD, 3.59 months). The choice of the specified intervals for the follow-up visits was also linked to better availability of retrospective data and consequently the possibility to increase the size of our patient cohort.
Surgical and pharmacological protocol
Before the start of PPV, an anterior chamber irrigation was performed in all eyes, and all eyes received a vitreous tap. The vitreous tap was performed under air without dilution and without the addition of antibacterial drugs prior to the procedure. The vitreous sample was sent for microbiological examination using microscopy, bacterial culture, and molecular diagnostics (16S pan-bacterial polymerase chain reaction (PCR)) to the Institute of Medical Microbiology and Hygiene (Saarland University in Homburg/Saar, Germany).
PPV was performed under a standard ophthalmic operating microscope (Lumera 700, Carl Zeiss Meditec AG, Germany) by experienced surgeons only. Depending on the specific situation, two lenses were used, combined or alone: a 128-degree fundus lens (Resight®, Carl Zeiss Meditec AG, Germany) and a handheld contact lens (Landers, Ocular Instruments, USA). Patients were operated on either under general or peribulbar anesthesia. A standard 23-gauge, sutureless vitrectomy system was used (EVA, D.O.R.C., Zuidland, Netherlands). No excessive peripheral vitrectomy or shaving was performed. At the end of the surgery, 1 mg/0.1 mL vancomycin (Vancomycin Noridem 500, DEMO Pharmaceuticals GmbH, Germany), 2 mg/0.1 mL ceftazidime (Ceftazidim-MIP 1 g, MIP Pharma GmbH, Germany), and 1 mg/0.1 mL dexamethasone (Fortecortin Inject 4 mg, Merck Serono GmbH, Germany) were given intravitreally, after having removed all ports and the infusion cannula.
All patients received the above-mentioned antibiotics intravenously for 1 week (vancomycin, 1 g bid; ceftazidime, 2 g tid). The topical therapy included 10 mg/mL, 6×/d prednisolone acetate (Inflanefran forte, Allergan Pharmaceuticals Ireland, Ireland), 5 mg/mL qid moxifloxacin (Vigamox, Novartis Pharma GmbH, Germany), and 5 mg/mL tid cyclopentolate hydrochloride (Zyklolat EDO, Dr. Mann Pharma GmbH, Germany). This therapy was tapered subsequently based on the individual requirements of each case.
Statistical analysis
To compare trends in BCVA over the four measurement points (before endophthalmitis, at time of diagnosis, at the first and last follow-up visit), we applied a mixed-model approach that has been increasingly adopted in medical research [15, 16]. Beyond this, mixed models are better suited to analyze repeated-measurement designs than often-used repeated-measurement analysis of variance or non-parametric tests (see [17, 18] for a further discussion of mixed models). We expected higher visual acuity before endophthalmitis compared to the time of diagnosis and increasing visual acuity after treatment (more specifically, a quadratic trend over time). To test this assumption, we used a mixed model with patients as random effects and point in time as the fixed effect. The model was fitted with R (Version 3.6.3) and the lme4 package (Version 1.1-23). Due to the small sample size, we based the estimate calculation on 1000 bootstrap samples. To further investigate these results, we derived estimated marginal means from the mixed model and conducted post hoc tests to compare points in time with each other.
Results
In total, 30 eyes of 30 patients matched the inclusion criteria and were included in the study. The mean age of the patients was 73 years (range 59–101) at the time of surgery. Eighteen patients were female and 12 were male. Seven eyes were phakic, 23 pseudophakic, and none were aphakic.
All patients were diagnosed with endophthalmitis following an intravitreal injection. Two eyes received a Dexamethasone implant (Ozurdex, Allergan Pharmaceutics, Ireland), one eye was treated with Ocriplasmin (Jetrea, Oxurion N.V., Leuven, Belgium), and the remaining eyes received intravitreal injections with VEGF inhibitors. Underlying conditions for which the patients were treated intravitreally were age-related macular degeneration (AMD) in 15 eyes, diabetic macular edema (DME) in nine eyes, and macular edema following retinal vein occlusion (MEfRVO) in three eyes. The remaining three cases received intravitreal drug delivery on the grounds of chronic central serous chorioretinopathy (CSCR) complicated with secondary choroidal neovascularization (CNV), pseudophakic cystoid macular edema (Irvine–Gass syndrome), or vitreomacular traction syndrome (VMTS).
The mean time from causative intravitreal injection to the diagnosis of endophthalmitis was 3.88 days (range 1.96–8.07 days).
The mean time between presentation (either the documented time when endophthalmitis was diagnosed by the consulting resident or the time the emergency ward reception scanned the letter of referral containing the term “endophthalmitis”) and the start of the vitrectomy was 3:26 h (range 1:35–5:33 h). All 30 eyes underwent an urgent PPV. Only two eyes received an air tamponade at the end of surgery. Nineteen patients underwent surgery under general anesthesia while eleven were given a peribulbar anesthesia. The mean IOP was 14.4 mmHg and 14.0 mmHg before and after surgery, respectively.
The BCVA before the intraocular infection represented a mean value of 0.55 logMAR. At diagnosis, the patients presented with a mean BCVA of 1.66 logMAR. Within 2 months following the PPV, the mean BCVA increased to 0.83 logMAR. Two to 14 months following PPV, the mean BCVA achieved was 0.63 logMAR. One eye (3.3%) underwent further pars plana surgery during the follow-up period because of a retinal detachment and another eye (3.3%) due to epiretinal gliosis. Two other eyes (6.6%) resulted in an unsatisfying BCVA (hand movements and counting fingers). No enucleation was required.
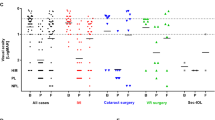
The mixed model yielded no linear trend of points in time (b = − 0.11, 95% CI [− 0.28, 0.05], p = 0.176), but a quadratic trend (b = − 0.60, 95% CI [− 0.75, − 0.45], p < 0.001) and a cubic trend (b = 0.52, 95% CI [0.37, 0.69], p < 0.001) were observed. As expected, the negative quadratic trend indicated higher BCVA logMAR values at the time of diagnosis and comparatively lower BCVA logMAR values before endophthalmitis and after treatment. Figure 1 illustrates this time course.
Line plots displaying the BCVA before endophthalmitis (a), at the time of diagnosis (b), at 1st follow-up (c), and at 2nd follow-up (d). Black points indicate the mean BCVA at a certain point in time, and vertical lines represent the standard error of the corresponding mean. The upper plot depicts the BCVA on a logMAR scale, whereas the lower plot depicts BCVA on the ETDRS scale
A further investigation of these trends using post hoc tests of estimated marginal means indicated that the BCVA was significantly worse at the time of diagnosis compared to before the endophthalmitis (p < 0.001) and compared to the follow-up measurements (all p < 0.001). Comparisons of other points in time with each other were not statistically significant (all p > 0.05), except for the comparison of the BCVA before the infective event with the BCVA in the first follow-up period (p = 0.042). These results show that the BCVA in the last follow-up period does not statistically differ from the BCVA before endophthalmitis (p = 0.891). Results of post hoc tests are shown in Table 1.
Microbiological test results of vitreous taps were available for 29/30 cases. Bacterial pathogens were found in 21 specimens, owing to a positivity rate of 72%. Except for one Gram-negative bacterium (i.e., Citrobacter koseri), all infections were caused by Gram-positive pathogens (97%), with Staphylococcus epidermidis being the most frequently detected (17 of 21 cases, 81%). Other bacteria included Staphylococcus aureus (n = 1), Streptococcus mitis (n = 1), and Enterococcus faecalis (n = 1; co-detection together with S. epidermidis in one sample). In one case, Gram-positive bacteria were observed using microscopy, but the culture and PCR examinations remained negative; hence, no species identification was achieved. All Gram-positive pathogens were susceptible to vancomycin, while C. koseri was susceptible to ceftazidime.
The particular BCVA evolution in the eye that tested positive for Streptococcus mitis is remarkable. Even though the time from diagnosis to PPV was only 1:56 h, the patient could not achieve more than 0.1 ETDRS BCVA after our treatment (from an ETDRS BCVA of 0.5 before the intravitreal injection). Correspondingly, the surgeon of this case mentioned a poor visual outcome prognosis in his report due to massive central retinal necrosis exceeding the vascular arcades, which was associated with multiple retinal bleedings.
Discussion
Our results show that an immediate vitrectomy can restore BCVA in patients suffering from endophthalmitis following intravitreal injections. No severe postoperative complications such as phthisis or need for enucleation were observed. To the best of our knowledge, this is the first study showing pronounced visual recovery with a prompt approach.
In our opinion, these results are important because of the increasing number of intravitreal injections and thus postoperative endophthalmitis cases: the incidence of post-injection endophthalmitis is as high as 0.095%, according to a 2011 study by a group from Japan [19]. Because the number of intravitreal injections worldwide is still continuously rising, and although prophylactic measures such as minimizing oral flora exposure (by using surgical masks and adhering to a strict no-talking policy [20, 21]) or applying povidone-iodine after the placement of the lid speculum [22] are able to significantly reduce the incidence of this devastating iatrogenic complication, the absolute number of post-injection endophthalmitis cases is expected to grow due to demographic changes on the one hand and increasingly available intravitreal therapy on the other hand. Therefore, we believe it is important to establish an adequate protocol in order to maximize the chance of visual recovery in the case of post-injection endophthalmitis.
The positivity rate of our results for bacterial pathogens was 72%, which is slightly higher compared to previous studies [21, 23, 24]. However, we believe that even in the absence of a positive culture or PCR result there is still a high chance of biased results; possible sources of error can arise from the vitreous tap itself, the transportation or conservation of the probe, and lastly from the microbiological analysis.
Our results challenge authors like Chaudhary et al. [25], who did not show any clear benefits of a vitrectomy compared to vitreous tap and injection alone if the endophthalmitis was caused by an intravitreal injection. However, it is important to mention that patients in this study underwent a vitrectomy from 1 day to 21 days after the initial tap. Colleagues from Australia [26] could not show a clear benefit or evidence of improvement. We attribute this observation to the significantly different time to surgery from our study. Kuhn and Gini [27] reported better outcomes if the vitrectomy was complete and included induced posterior vitreous detachment, yet this was not the case in our patient collective. Yospaiboon et al. [28] and Durand [29] indicated that the timing is important in cases of a progressive clinical development (e.g., in streptococcal endophthalmitis). These findings are reflected in newer guidelines for endophthalmitis treatment following cataract surgery, as advocated by the ESCRS [30], which recommends vitreous tap combined with an injection of antibiotics as second-line therapy, but only if vitrectomy is not possible to perform.
The exact timeline from presentation in the clinic until surgery is worth discussing. All patients were treated within 6 h. This information is based on electronic medical records containing automated time stamps marking the time of admission and the start of surgery; therefore, these data should be considered very reliable. Because the majority of the patients were operated on under general anesthesia, the successful and timely coordination between different medical specialties should be emphasized. The results of this study should encourage clinicians to prioritize the immediate surgical treatment of endophthalmitis patients in their clinical workflow. However, it must be mentioned that the time of presentation is not the time of disease onset. Unfortunately, there is no way to assess the exact time of disease onset because of highly interindividual variances. We strongly believe that this time frame can be reduced to a minimum if one invests in patient education prior to an intravitreal injection. The physician should give a clear recommendation of immediate presentation in case symptoms consistent with endophthalmitis arise.
The visual prognosis after post-injection endophthalmitis or the severity of the infections also depends on factors like the anatomical locus of the infection and the virulence of the causing agent. Involvement of the macula or the optic nerve and the presence of a virulent bacterium like Escherichia coli, for example, usually have a worse prognosis, even if treated promptly with a vitrectomy. This could also be seen in one case of our patient cohort, where the microbiological result was positive for Streptococcus mitis; the evolution of BCVA was relatively poor in this particular case. However, upon clinical presentation, it is not possible to clearly distinguish more virulent from less virulent microorganisms. Besides, a precise assessment on central retinal involvement is not possible in most cases due to reduced fundus insight.
It must be mentioned that there is an ongoing discussion on whether a vitrectomy is necessary in less severe cases. Conversely, especially in the case of less virulent organisms, it must be stated that the disease could be resolved with intravitreal injection of antibiotics alone. Nevertheless, we believe that our results, especially the low rate of complications in our patient cohort, demonstrate that it is worth taking the risk of “overtreatment.”
Our study is limited by the small sample size of 30 patients, the retrospective approach, and the lack of any control group. It could be argued that the mean follow-up period of 8 months is problematic; this could negatively influence visual acuity but more so due to the underlying condition (e.g., age-related macular degeneration). Therefore, the last follow-up on visual acuity should be regarded critically.
Other important questions about the surgical approach also remain open; we cannot answer the questions regarding the importance of inducing a posterior vitreous detachment and systemic antibiotics. It is assumed that after vitrectomy, the antibiotic concentration decreases rapidly compared to non-vitrectomized eyes [31]. Therefore, not performing a complete vitrectomy and adding a systemic therapy might be advantageous. However, the vitreous body can be considered a scaffold for bacterial proliferation, and the intraocular penetration of systemic antibiotics is under debate, which favors a complete and early vitrectomy [32]. Although the ESCRS recommends additional systemic antibiotics in severe cases, the EVS could not show a clear advantage [11, 30, 32]. However, the antibiotics used for intravenous application in the EVS study were ceftazidime plus amikacin or ciprofloxacin plus amikacin, all of which have no activity (ceftazidime) or only negligible activity for staphylococci and other Gram-positive pathogens, which account for the vast majority of endophthalmitis cases following intraocular injections. Moreover, different antibiotics were used intravitreally (vancomycin and amikacin) in the EVS study. Hence, there is a need for further studies to characterize the causative agents of endophthalmitis, their respective antimicrobial susceptibility patterns, and the effects of carefully selected antibiotic regimens on patient outcome and visual acuity.
A controversial point in the management of acute infectious endophthalmitis is the adjuvant intravitreal use of steroids. An extensive literature review from 2018 concludes that the burden of proof is on those who recommend the use of intravitreal steroids while stating that any definitive recommendations must await further trials [33]. Manning et al. [34] showed that intravitreal dexamethasone as an adjuvant to intravitreal antibiotics does not improve visual acuity in patients treated for presumed bacterial endophthalmitis after cataract surgery. If this is also the case, post-injection endophthalmitis still needs to be clarified. Despite the lack of evidence showing clear benefits, some literature reports conclude that its use is safe [33, 35] and may even be associated with a trend toward a lower need for recurrent intravitreal antibiotic therapy [36]. All patients in our cohort received adjunctive intravitreal dexamethasone at the end of PPV. We could not correlate any type of complications related to this; hence, we support the use of adjuvant intravitreal steroids in presumed infectious endophthalmitis. The usage of vancomycin should be regarded critically because of the risk of hemorrhagic occlusive retinal vasculitis (HORV) [37].
We have demonstrated the effectiveness of an immediate vitrectomy combined with both intravitreal and systemic antibiotics at the end of surgery. These findings were consistent with a recent study from Australia [10]. Our study suggests that it is worth investing in patient information and logistics that can yield a fast transit between diagnosis and surgical treatment.
As a result of the shift in management possibilities from 1995 to current options and the change in the etiology of postoperative endophthalmitis, we strongly suggest that indications for an early vitrectomy in the case of postoperative endophthalmitis, especially following intravitreal injection, should be reassessed in a new multicenter, prospective, and randomized controlled trial.
Change history
18 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00417-021-05179-z
References
Forooghian F, Albiani DA, Kirker AW, Merkur AB (2017) Comparison of endophthalmitis rates following intravitreal injection of compounded bevacizumab, ranibizumab, and aflibercept. Can J Ophthalmol 52:616–619. https://doi.org/10.1016/j.jcjo.2017.04.016
Garg P, Roy A, Sharma S (2017) Endophthalmitis after cataract surgery: epidemiology, risk factors, and evidence on protection. Curr Opin Ophthalmol 28:67–72. https://doi.org/10.1097/ICU.0000000000000326
Gower EW, Keay LJ, Stare DE, Arora P, Cassard SD, Behrens A, Tielsch JM, Schein OD (2015) Characteristics of Endophthalmitis after cataract surgery in the United States Medicare population. Ophthalmology 122:1625–1632. https://doi.org/10.1016/j.ophtha.2015.04.036
Czajka MP, Byhr E, Olivestedt G, Olofsson EM (2016) Endophthalmitis after small-gauge vitrectomy: a retrospective case series from Sweden. Acta Ophthalmol 94:829–835. https://doi.org/10.1111/aos.13121
Bhende M, Raman R, Jain M, Shah PK, Sharma T, Gopal L, Bhende PS, Srinivasan S, Jambulingam M, Sankara Nethralaya Vitreoretinal Study G (2018) Incidence, microbiology, and outcomes of endophthalmitis after 111,876 pars plana vitrectomies at a single, tertiary eye care hospital. PLoS One 13:e0191173. https://doi.org/10.1371/journal.pone.0191173
Cohen SM, Flynn HW Jr, Murray TG, Smiddy WE (1995) Endophthalmitis after pars plana vitrectomy. The Postvitrectomy Endophthalmitis Study Group. Ophthalmology 102:705–712. https://doi.org/10.1016/s0161-6420(95)30965-7
Zheng CX, Moster MR, Khan MA, Chiang A, Garg SJ, Dai Y, Waisbourd M (2017) Infectious endophthalmitis after glaucoma drainage implant surgery: clinical features, microbial spectrum, and outcomes. Retina 37:1160–1167. https://doi.org/10.1097/IAE.0000000000001329
Solborg Bjerrum S, Prause JU, Fuchs J, la Cour M, Kiilgaard JF (2016) Morphological features in eyes with endophthalmitis after cataract surgery - histopathology and optical coherence tomography assessment. Acta Ophthalmol 94:26–30. https://doi.org/10.1111/aos.12858
Damm LJ, Boden KT, Januschowski K (2019) Stellenwert der Vitrektomie bei Endophthalmitis: Wie eine prompte Vitrektomie den Visus wiederherstellen kann. Ophthalmologe 116:569–571. https://doi.org/10.1007/s00347-018-0768-z
Ho IV, Fernandez-Sanz G, Levasseur S, Ting E, Liew G, Playfair J, Downie J, Gorbatov M, Hunyor AP, Chang AA (2019) Early pars plana vitrectomy for treatment of acute infective Endophthalmitis. Asia Pac J Ophthalmol (Phila) 8:3–7. https://doi.org/10.22608/APO.2018414
Group EVS (1995) Results of the Endophthalmitis Vitrectomy Study: a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 113:1479–1496. https://doi.org/10.1001/archopht.1995.01100120009001
Al Mahmood AM, Al-Swailem SA, Behrens A (2014) Clear corneal incision in cataract surgery. Middle East Afr J Ophthalmol 21:25–31. https://doi.org/10.4103/0974-9233.124084
Kasi SK, Hariprasad SM, Hsu J (2017) Making the jump to 27-gauge vitrectomy: perspectives. Ophthalmic Surg Lasers Imaging Retin 48:450–456. https://doi.org/10.3928/23258160-20170601-02
Wong TY, Chee SP (2004) Risk factors of acute endophthalmitis after cataract extraction: a case-control study in Asian eyes. Br J Ophthalmol 88:29–31. https://doi.org/10.1136/bjo.88.1.29
Boisgontier MP, Cheval B (2016) The ANOVA to mixed model transition. Neurosci Biobehav Rev 68:1004–1005. https://doi.org/10.1016/j.neubiorev.2016.05.034
Casals M, Girabent-Farres M, Carrasco JL (2014) Methodological quality and reporting of generalized linear mixed models in clinical medicine (2000-2012): a systematic review. PLoS One 9:e112653. https://doi.org/10.1371/journal.pone.0112653
Detry MA, Ma Y (2016) Analyzing repeated measurements using mixed models. JAMA 315:407–408. https://doi.org/10.1001/jama.2015.19394
McGlothlin AE, Viele K (2018) Bayesian hierarchical models. JAMA 320:2365–2366. https://doi.org/10.1001/jama.2018.17977
Inoue M, Kobayakawa S, Sotozono C, Komori H, Tanaka K, Suda Y, Matsushima H, Kinoshita S, Senoo T, Tochikubo T, Kadonosono K (2011) Evaluation of the incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmologica 226:145–150. https://doi.org/10.1159/000329863
Garg SJ, Dollin M, Hsu J, Storey P, Vander JF (2015) Effect of a strict ‘no-talking’ policy during intravitreal injection on post-injection endophthalmitis. Ophthalmic Surg Lasers Imaging Retin 46:1028–1034. https://doi.org/10.3928/23258160-20151027-07
McCannel CA (2011) Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 31:654–661. https://doi.org/10.1097/IAE.0b013e31820a67e4
Levinson JD, Garfinkel RA, Berinstein DM, Flory M, Spellman FA (2018) Timing of povidone-iodine application to reduce the risk of endophthalmitis after intravitreal injections. Ophthalmol Retin 2:654–658. https://doi.org/10.1016/j.oret.2017.06.004
Moshfeghi AA, Rosenfeld PJ, Flynn HW Jr, Schwartz SG, Davis JL, Murray TG, Smiddy WE, Berrocal AM, Dubovy SR, Lee WH, Albini TA, Lalwani GA, Kovach JL, Puliafito CA (2011) Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina 31:662–668. https://doi.org/10.1097/IAE.0b013e31821067c4
Lyall DA, Tey A, Foot B, Roxburgh ST, Virdi M, Robertson C, MacEwen CJ (2012) Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond) 26:1517–1526. https://doi.org/10.1038/eye.2012.199
Chaudhary KM, Romero JM, Ezon I, Fastenberg DM, Deramo VA (2013) Pars plana vitrectomy in the management of patients diagnosed with endophthalmitis following intravitreal anti-vascular endothelial growth factor injection. Retina 33:1407–1416. https://doi.org/10.1097/IAE.0b013e3182807659
Ng JQ, Morlet N, Pearman JW, Constable IJ, McAllister IL, Kennedy CJ, Isaacs T, Semmens JB, Team E (2005) Management and outcomes of postoperative endophthalmitis since the endophthalmitis vitrectomy study: the Endophthalmitis Population Study of Western Australia (EPSWA)’s fifth report. Ophthalmology 112:1199–1206. https://doi.org/10.1016/j.ophtha.2005.01.050
Kuhn FGG (2007) Complete and early vitrectomy for endophthalmitis vitreo-retinal surgery (part of the essentials in ophthalmology book series (ESSENTIALS)), p 16
Yospaiboon Y, Meethongkam K, Sinawat S, Laovirojjanakul W, Ratanapakorn T, Sanguansak T, Bhoomibunchoo C (2018) Predictive factors in the treatment of streptococcal endophthalmitis. Clin Ophthalmol 12:859–864. https://doi.org/10.2147/OPTH.S161217
Durand ML (2013) Endophthalmitis. Clin Microbiol Infect 19:227–234. https://doi.org/10.1111/1469-0691.12118
Barry P, Cordovés L; Gardner, S (2013) ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery data, Dilemmas and Conclusions. ESCRS
Pflugfelder SC, Hernandez E, Fliesler SJ, Alvarez J, Pflugfelder ME, Forster RK (1987) Intravitreal vancomycin. Retinal toxicity, clearance, and interaction with gentamicin. Arch Ophthalmol 105:831–837. https://doi.org/10.1001/archopht.1987.01060060117045
Grzybowski A, Turczynowska M, Kuhn F (2018) The treatment of postoperative endophthalmitis: should we still follow the endophthalmitis vitrectomy study more than two decades after its publication? Acta Ophthalmol 96:e651–e654. https://doi.org/10.1111/aos.13623
Ching Wen Ho D, Agarwal A, Lee CS, Chhablani J, Gupta V, Khatri M, Nirmal J, Pavesio C, Agrawal R (2018) A review of the role of intravitreal corticosteroids as an adjuvant to antibiotics in infectious endophthalmitis. Ocul Immunol Inflamm 26:461–468. https://doi.org/10.1080/09273948.2016.1245758
Manning S, Ugahary LC, Lindstedt EW, Wubbels RJ, van Dissel JT, Jansen JTG, Gan I, van Goor AT, Bennebroek CA, van der Werf DJ, Ossewaarde-van Norel A, Mayland Nielsen CC, Tilanus M, van den Biesen PR, Schellekens PA, La Heij E, Faridpooya K, van Overdam K, Veckeneer M, van Meurs JC (2018) A prospective multicentre randomized placebo-controlled superiority trial in patients with suspected bacterial endophthalmitis after cataract surgery on the adjuvant use of intravitreal dexamethasone to intravitreal antibiotics. Acta Ophthalmol 96:348–355. https://doi.org/10.1111/aos.13610
Albrecht E, Richards JC, Pollock T, Cook C, Myers L (2011) Adjunctive use of intravitreal dexamethasone in presumed bacterial endophthalmitis: a randomised trial. Br J Ophthalmol 95:1385–1388. https://doi.org/10.1136/bjo.2010.187963
Moisseiev E, Abbassi S, Park SS (2017) Intravitreal dexamethasone in the management of acute endophthalmitis: a comparative retrospective study. Eur J Ophthalmol 27:67–73. https://doi.org/10.5301/ejo.5000866
Witkin AJ, Chang DF, Jumper JM, Charles S, Eliott D, Hoffman RS, Mamalis N, Miller KM, Wykoff CC (2017) Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology 124:583–595. https://doi.org/10.1016/j.ophtha.2016.11.042
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Saarland Medical Association (approval number 243/14) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Januschowski, K., Boden, K., Szurman, P. et al. Effectiveness of immediate vitrectomy and intravitreal antibiotics for post-injection endophthalmitis. Graefes Arch Clin Exp Ophthalmol 259, 1609–1615 (2021). https://doi.org/10.1007/s00417-021-05071-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05071-w