Abstract
Purpose
Unfolding and attachment of the posterior donor lamella may be the most challenging part in Descemet membrane endothelial keratoplasty (DMEK) procedure. We investigated the correlation of the difficulty degrees of this step to the postoperative clinical outcome 6 years after surgery.
Methods
One hundred sixty-nine consecutive DMEKs between September 2012 and August 2013 at the Charité–University Medicine Berlin were graded prospectively into 4 groups according to their grade of difficulty in unfolding and attachment of the graft lamella. Postoperative visual acuity, endothelial cell density, and rate of graft failure were measured after 1 year, after 2 years, and after 6 years and analyzed according to their grading group.
Results
Visual acuity improved significantly in all groups and did not differ significantly between the grading groups at any time point postoperatively. There was a significant decrease of endothelial cell density in all groups with a significantly higher endothelial cell loss in group IV compared with the other groups within the first 24 months after surgery. The graft failure rate was significantly higher in eyes graded III and IV than in groups I and II (p = 0.012).
Conclusion
Although the endothelial cell loss and the graft failure rate increase significantly with a more difficult graft unfolding and attachment, DMEK surgery is a promising procedure with a good long-term postoperative outcome. A direct manipulation of the graft lamella for unfolding and centering by cannula or forceps should be avoided if possible to reduce the risk of an increased endothelial cell loss and a higher graft failure rate.
Trial registration
NCT02020044
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Within the last decade, Descemet stripping-automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) have become the gold standard for disorders of the corneal endothelium. The advantages of both procedures include a good visual outcome, fast recovery, and few complications during and post-surgery, but the DMEK procedure seems superior to the DSEK procedure in terms of visual acuity and refractive results [1,2,3,4]. Basis of the advantages of DMEK procedure is an isolated transplantation of an endothelium Descemet membrane layer without corneal stroma, a microsurgical technique, which was first described by Melles in 2004 [1,2,3,4]. However, the surgery is demanding especially the main step, the unfolding of the lamella and the attachment of the graft to the posterior stroma; is even under perfect conditions; and is ambitious, and most manipulations to the graft occur in this step [5, 6]. Therefore, a DSAEK is still performed, which is not so difficult from a surgical point of view, but at the expense of the final visual acuity.
Our group investigated the impact of the donor and patient characteristics on the difficulty of unfolding and attaching the graft lamella and the impact of the difficulty of unfolding and attaching the flap on the postoperative clinical outcome [6]. We found no correlation between donor characteristics like age and gender, preoperative endothelial cell density, total storage time, storage de-swelling time or post-mortem time, and challenging surgery [6, 7].
There was also no correlation detected between patients’ characteristics like age and gender, indication for DMEK, preoperative endothelial cell density or preoperative central corneal thickness, and a demanding surgery [6].
However, we found a correlation between the patients’ preoperative visual acuity and the difficulty of unfolding and attaching the flap, which might be due to the poor visualization during surgery. Investigating the effects of a demanding surgery, we found a reduced endothelial cell density 6 months after surgery as well as a tendency for a higher re-bubbling rate in patients after demanding surgery [6, 8, 9]. However, all patients independent of the difficulty of the DMEK procedure regained a good postoperative visual acuity [6].
In this presented study, we focused on the long-term outcomes after DMEK surgery; the main question was if a more difficult graft unfolding and attachment correlates with the final outcomes of the visual acuity, the endothelial cell density, or the rate of complications during the first 72 months after surgery.
Materials and methods
Patients
One hundred sixty-nine consecutive DMEKs were performed at the Department of Ophthalmology, Charité–University Medicine Berlin, Campus Virchow Klinikum, between September 2012 and August 2013 by one experienced surgeon, who performed more than 80 DMEK surgeries before (N.T.), and were analyzed prospectively. This study followed the ethical standards of the Declaration of Helsinki. The patients gave informed consent for the treatment and the participation in the study. Institutional ethical approval was obtained by the Ethics Committee of the Charité–Universitätsmedizin Berlin (EA2/108/12) for this prospective study, and clinical trial registration was performed (http://www.clinicaltrials.gov, NCT02020044).
Donor characteristics
As described before, all patients received organ-cultured grafts from the Cornea Bank Berlin (University Tissue Bank, Institute of Transfusion Medicine, Charité–University Medicine Berlin) with the presented donor characteristics [6]. The minimum central endothelial density accepted for transplantation was 2000 cells/mm2.
Surgical technique
Graft was prepared as described in detail by Melles et al., and the diameter of the grafts differed between 8.0 and 9.0 mm [3, 4].
The surgical technique was performed as described by Maier et al. [6]. A combined procedure (triple DMEK) with DMEK following standard cataract surgery was performed in 54 cases of all 160 included cases.
In the following 2 weeks after surgery, an additional air bubble (re-bubbling) was injected in the eyes with detachment of the graft lamella documented by OCT and focal corneal edema over more than 2 clock hours.
Standard postoperative topical treatment included prednisolone acetate 1%, lubricants, and ofloxacin eye drops as described by Maier et al. [6].
Grading system
We used the grading system as described by Maier et al. [6]. In brief, the unfolding and attaching of the graft lamella was graded with regard to its difficulty in four grading groups using the following specification:
-
I-
Graft lamella primarily oriented correctly in anterior chamber, straight and direct unfolding and centering
-
II-
Slightly complicated indirect unfolding and centering (duration less than 5 min)
-
III-
Difficult indirect unfolding and centering (duration longer than 5 min), repeated air injection with BSS exchange necessary
-
IV-
Direct manipulation of the graft lamella for unfolding and centering by cannula or forceps
Pre- and postoperative evaluations
We performed the clinical examinations 1 day before the DMEK surgery as well as 1, 3, 6, 12, and 24 months and 72 ± 6 months after the DMEK surgery. The examinations included best corrected visual acuity (BCVA) tested with a Snellen chart, slit-lamp examination, applanation tonometry (Goldmann applanation tonometer, Haag Streit, Bern Switzerland) or pneumatic tonometry (CT20D computerized Tonometer, Topcon, Japan), endothelial cell density (NONCON-ROBO CA specular microscope, KONAN MEDICAL INC., Nishinomiya, Japan), and funduscopy. The Snellen decimal number was converted in LogMAR visual acuity using a conversion table [10]. Central corneal thickness was analyzed using the Spectralis-OCT device with an anterior segment module (Spectralis optical coherence tomography, Heidelberg Engineering GmbH, Heidelberg, Germany).
Statistical methods
Statistical analysis was performed using IBM SPSS statistics 19 (RRID:SCR_002865, SPSS Software, Munich, Germany). Descriptive statistics were expressed as mean ± standard deviation (SD). Normality was tested for all outcome measures, and the appropriate statistical test was used for analysis. Analyzing differences between the different grading groups, we used the Kruskal-Wallis test and, for post hoc analysis, the Dunnett test. We used Kaplan-Meier survival analysis to estimate graft rejection- and graft failure-free survival. Difference between the different grading groups was measured by the log-rank test for multiple groups. A p value < 0.05 was considered statistically significant.
Results
One hundred sixty-nine consecutive DMEKs were performed during the study period. Nine eyes were excluded because the grading degree was not noted during the DMEK surgery. As described before, in 63 eyes, the DMEK surgery was graded in terms of difficulty for grade I, in 47 eyes for grade II, in 32 eyes for grade III and in 18 eyes for grade IV [6]. Data of 134 patients were analyzed at the 6-month follow-up, 127 at the 12-month follow-up, and 100 at the 24-month follow-up. Seventy-two patients (45.0%) completed the follow-up time of 72 ± 6 months postoperative. Comparing both groups, group 1 with patients, which completed the follow-up visit after 72 ± 6 months, and group 2, which missed the follow-up visit after 72 ±6 months, showed that the distribution of the grading groups did not significantly differ, but the mean age (Table 1, supplement data). Reasons for a missed follow-up after 72 ±6 months are described in the following. Eight patients died; data of 30 patients were missing due to incorrect or missing contact data; and 38 patients missed the follow-up appointment due to comorbidities such as dementia, tumor disease, severe walking impairments, need of care, or logistical difficulties. Eleven patients had a graft failure during the follow-up and received re-grafting (6 Re-DMEK, 4 perforating keratoplasty, 1 no therapy). Data from these patients were included until the last visit prior to re-grafting. Data collected after the re-grafting were excluded. In 5 patients (3 patients graded II, 1 patient graded III, 1 patient graded IV), measurement of the endothelial cell density was not possible at the 6-year follow-up time point: in 3 patients due to corneal scars, in 2 patients it remained unclear why the measurement was not possible.
Long-term outcome and grading system
Visual acuity
The data for the visual acuity are presented in Fig. 1. Visual acuity improved significantly after surgery (p < 0.001, preoperative 0.73 ± 0.43 LogMAR versus 0.18 ± 0.31 LogMAR after 12 months, 0.13 ± 0.24 after 24 months, and 0.14 ± 0.27 LogMAR after 72 months). In 11 cases, a retinal disease with macular involvement and, in 4 cases, a pre-existing amblyopia limited the results of visual acuity as presented before [6]. Preoperative visual acuity was significantly different between the grading groups (p = 0.017). However, postoperatively, the visual acuity did not differ significantly between the grading groups at any time point (after 1 month: p = 0.789, after 3 months p = 0.437, after 6 months: p = 0.910, after 12 months p = 0.374, after 24 months: p = 0.835, after 72 months p = 0.733).
Endothelial cell density
Endothelial cell density decreased within the first month statistically significantly (p < 0.001) (preoperative: 2295.5 ± 201.8 cells/mm2 versus after 1 month: 1796.0 ± 463.3 cells/mm2). Between the first and 24th month after surgery, no further statistically significant differences in endothelial cell density were observed (after 1 month: 1796.0 ± 463.3 cells/mm2 versus after 24 months: 1763.7 ± 412.9 cells/mm2, p = 0.892). Between the 24th and 72th month after surgery, the endothelial cell density decreased significantly (after 24 months: 1763.7 ± 412.9 cells/mm2 versus after 72 months: 1383.0 ± 434.9 cells/mm2, p < 0.001). After 72 months, the mean loss rate of endothelial cell density was 39.8%.
Mean endothelial cell density differs significantly between the grading groups at any time point postoperatively. Grading group IV developed a significantly higher endothelial cell loss after 1 month, after 3, after 6, after 12, and after 24 months compared with the other groups (after 1 month: p = 0.039, after 3 months: p = 0.006, after 6 months: p = 0.034, after 12 months: p = 0.001, after 24 months: p = 0.008) (Fig. 2).
The mean and the standard deviation (SD) of endothelial cell density (cells/mm2) obtained at the different time points for the four grading groups. Endothelial cell density decreased postoperatively (p < 0.001) and stabilized over time. Significant differences between the grading groups were marked with an asterisk (*) (p < 0.05)
Complications
Graft rejection
Graft rejection occurred in 8 patients during the follow-up period. Six of these 8 patients showed only a mild graft rejection with corneal precipitates but no corneal edema or visual acuity worsening. These patients were successfully treated with topical steroid eye drops. The other two patients presented with a severe graft rejection with visual acuity worsening, corneal edema, and corneal precipitates. One of these patients was treated successfully with topical steroid eye drops, and visual acuity recovered and reached 0.0 LogMAR. Unfortunately, the other patient developed a graft failure despite an adequate systemic and topical treatment with steroids. There was no significant difference of graft rejection rate between the different grading groups (p = 0.975). Graft rejection-free survival curve is shown in Fig. 3.
Graft failure
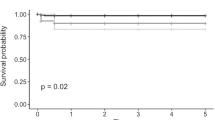
Graft failure occurred in eleven cases. Three patients developed an early graft failure during the first 2 weeks after DMEK and received an early re-DMEK. In eight patients, graft failure occurred later than 2 weeks. Treatment of the graft failure included a re-DMEK in three patients and a perforating keratoplasty in four patients. In one case, an additional treatment was not desired. Graft survival curve is shown in Fig. 4. In eyes graded III and IV, significantly more graft failure occurred than in eyes graded I and II (p = 0.012) (Fig. 4).
Other complications
Seven eyes (3.8%) developed a cystoid macular edema after surgery, which regressed and completely declined in all cases.
Twenty-seven eyes presented with intraocular pressure (IOP) elevation during the follow-up period. In 4 patients, a surgical intervention was performed to control the intraocular eye pressure (2 patients: trabeculectomy, 2 patients: cyclodestructive surgery). All other patients were treated successfully with antiglaucomatous topical medication. In seventeen eyes, steroid-induced IOP elevation has been suggested as possible reason, as IOP normalized when topical steroids were stopped.
Three patients were treated with phototherapeutic keratotomy because of additional subepithelial corneal scars.
In three cases, complications of the intraocular lens occurred; in one case, the intraocular lens dislocated in the vitreous and a lens salvage with implantation of an iris-fixed intraocular lens was necessary; in another case, an intraocular lens exchange was performed because of a intraocular lens clouding; in the last case, an IOL reposition was necessary.
Discussion
This prospective consecutive study investigated the long-term impact of difficult unfolding and attachment of the posterior lamella in DMEK surgery regarding visual acuity, endothelial cell density, graft rejection, graft failure, and clinical course.
In some challenging DMEK procedures, direct manipulation of the flap like described for grading group IV may be necessary and raises the question if this kind of surgery may still lead to success for the patient.
We already showed that our patients achieved a good visual acuity 6 months after surgery regardless of the difficulty of the surgery, but those patients in grading group IV tended to have more re-bubblings in the early weeks (although not statically significant) and had a significantly higher endothelial cell loss [6]. Therefore, the question was, if these patients had an impaired visual acuity or higher rates of graft failure in the long-term follow-up.
Firstly, we found no significant difference of mean visual acuity between the grading groups 6 years after surgery. The visual acuity improved in all groups. This result is even more enjoyable as this might be the most important goal for our patients. Additionally, this result is also very motivating for surgeons to continue performing DMEKs under difficult conditions.
When analyzing the visual acuity results, one must take into account that only a limited number of patients could be included 6 years after DMEK. This is in accordance to other long-term studies analyzing DMEK patients [9, 11, 12]. The difficulty analyzing this patient collective is the high average age as shown by our data. The high average age increases the risk of missing long-term results due to patient’s death or serious illness.
As we have shown previously, it is not possible to predict by donor characteristics if unfolding or attaching of the flap will be difficult [6, 13]. But we found that a distinctly impaired previous visual acuity is associated with an increased risk of a complicated unfolding and attaching procedure probably due to a reduced intraoperative visualization [6, 8]. Patients with a distinctly reduced visual acuity due to endothelial dysfunction therefore may often face a perforating keratoplasty as even experienced surgeons fear the difficult flap unfolding and attaching may result in poor visual outcome. Although understandable, our long-term results show that even in the group with direct manipulation, visual acuity outcome is good.
As we have shown before for the first 6 months post-DMEK surgery, we found a statistically significant correlation regarding the endothelial cell density between grading group IV and the other groups at any time within the first 24 months post-surgery [6]. In consequence, a demanding surgery with direct manipulation of the flap leads to a reduced endothelial cell density. However, our data showed that the endothelial cell loss was detectable in the short-term follow-up with the greatest difference 1 month after surgery. During further follow-up visits up to 6 years after surgery, patients showed in all groups a stable decrease of the endothelial cell density. In our previous performed study, we found a slightly higher re-bubbling rate in group IV with a demanding DMEK surgery, although this effect was not statistically significant [6]. Some studies showed that a higher re-bubbling rate is correlated with a higher endothelial cell loss [7, 14]. The higher re-bubbling rate due to more difficulties in unfolding and attachment of the graft lamella may therefore also have an impact on the endothelial cell loss. Therefore, it can hypothesized that the main loss seems to be due to the direct manipulation to the flap and the slightly higher re-bubbling rate in the early postoperative period, and is not an ongoing process.
We did not detect a significant difference of endothelial cell density 6 years after DMEK surgery between the grading groups. This might be due to the small number of patients in group IV, as some patients missed follow-up visits or died and the fact that more patients in group IV than in the other groups did not reach the last follow-up time point due to graft failure (Fig. 4).
Analyzing correlation between difficulty grade and postoperative complications, we found significantly higher graft failure rates in the groups III and IV than in groups I and II, which is probably due to the increased endothelial cell loss in these groups. From these 11 patients with graft failure, 6 patients were treated successfully with a re-DMEK, and 4 with a perforating keratoplasty; only one patient refused treatment. Therefore, we recommend that in patients with a more demanding DMEK surgery, follow-up visits should be carried out regularly.
In difficult cases (according grade III or grade IV procedures), performing a DSAEK instead of DMEK could be considered, as DSAEK is less demanding from a surgical point of view, shows a lower re-bubbling rate, and seems to have less often early graft failure [6, 15, 16]. It is therefore still an option especially in eyes with pecularities like absence of an intact iris diaphragm, glaucoma surgery before, or vitrectomized eyes [17,18,19,20]. However, one problem is the missing predictability of the grade of difficulty of the DMEK in some cases. In our previous performed study, we found no correlation between any corneal donor tissue characteristic and the difficulty grade of the surgery and between the preoperative patient’s characteristics on the difficulty of unfolding and attaching the graft lamella; only the patients preoperative visual acuity as a sign of the opacity of the cornea was correlated to the grade of difficulty of the surgery [6]. Therefore, it is difficult to decide who would benefit from the advantages of a DSAEK. However, we should consider that the possible advantages of DSAEK are at the expense of final visual acuity, and that in our study, most patients in groups III and IV benefited from the advantages of DMEK with a very good long-term visual acuity.
The most important limitation of our study is the sample size at the follow-up after 6 years. Due to various reasons, we were only able to examine a limited number of patients after 6 years according to other studies analyzing long-term data after DMEK [9, 11, 12]. Despite the limited sample size, we found a significant difference according to the graft failure rate and a confirmation of the tendency towards the higher endothelial cell loss in group IV. Additionally, the distribution of the grading groups did not differ significantly between both groups (completed or missed follow-up after 6 years), so that one can assume that the data of the patients who missed the follow-up after 6 years are approximately similar. Another limitation is the statistical analysis accounting for multiple comparisons. We used statistical tests for multiple groups and analyzed the data by post hoc tests. In addition, our findings are in line with our previous findings and the literature. Also, the findings are clinically coherent which is why we believe that we are not subject to an alpha error.
To conclude, a demanding DMEK surgery may still lead to a good postoperative outcome. In our study, visual acuity in all groups improved significantly with no difference between the grading groups. However, patients after demanding DMEK surgery showed a significantly increased endothelial cell loss compared with patients after an uneventful DMEK surgery. Additionally, in patients with a difficult surgery, significantly more graft failures occurred. Therefore, we recommend avoiding a direct manipulation of the graft lamella if possible. If direct manipulation is not avoidable, we recommend close follow-up examinations after DMEK surgery.
Data availability
The authors have full control of all primary data and agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review data upon request.
References
Melles GR, Wijdh RH, Nieuwendaal CP (2004) A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis). Cornea 23(3):286–288
Price FW Jr, Price MO (2006) Descemet’s stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg 32:411–418
Melles GR, Ong TS, Ververs B, van der Wees J (2006) Descemet membrane endothelial keratoplasty (DMEK). Cornea 25:987–990
Melles GR, Ong TS, Ververs B, van der Wees J (2008) Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol 145(2):222–227
Heinzelmann S, Hüther S, Böhringer D, Eberwein P, Reinhard T, Maier P (2014) Influence of donor characteristics on Descemet membrane endothelial keratoplasty. Cornea 33(6):644–648
Maier AK, Gundlach E, Schroeter J, Klamann MK, Gonnermann J, Riechardt AI, Bertelmann E, Joussen AM, Torun N (2015) Influence of the difficulty of graft unfolding and attachment on the outcome in Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol 253(6):895–900
Gerber-Hollbach N, Baydoun L, López EF, Frank LE, Dapena I, Liarakos VS, Schaal SC, Ham L, Oellerich S, Melles GRJ (2017) Clinical outcome of rebubbling for graft detachment after Descemet membrane endothelial keratoplasty. Cornea 36(7):771–776
Dirisamer M, van Dijk K, Dapena I, Ham L, Oganes O, Frank LE, Melles GR (2012) Prevention and management of graft detachment in Descemet membrane endothelial keratoplasty. Arch Ophthalmol 130(3):280–291
Feng MT, Price MO, Miller JM, Price FW Jr (2014) Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: five-year follow-up. J Cataract Refract Surg 40(7):1116–1121
Joussen AM, Heussen FM, Joeres S, Llacer H, Prinz B, Rohrschneider K, Maaijwee KJ, van Meurs J, Kirchhof B (2006) Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol 142(1):17–30
Schlögl A, Tourtas T, Kruse FE, Weller JM (2016) Long-term clinical outcome after descemet membrane endothelial keratoplasty. Am J Ophthalmol 169:218–226
Vasiliauskaitė I, Oellerich S, Ham L, Dapena I, Baydoun L, van Dijk K, Melles GRJ (2020) Descemet membrane endothelial keratoplasty: ten-year graft survival and clinical outcomes [published online ahead of print, 2020 Apr 10]. Am J Ophthalmol S0002-9394(20)30172-0. https://doi.org/10.1016/j.ajo.2020.04.005
Heindl LM, Bucher F, Caramoy A, Hos D, Matthaei M, Cursiefen C (2014) Safety of donor tissue preparation and use of descemetoschisis and torn tissue in Descemet membrane endothelial keratoplasty. Cornea 33(10):e7–e9
Lazaridis A, Fydanaki O, Giallouros E, Georgalas I, Kymionis G, Sekundo W, Droutsas K (2018) Recovery of corneal clarity after DMEK followed by rebubbling versus uneventful DMEK. Cornea 37(7):840–847
Marques RE, Guerra PS, Sousa DC, Gonçalves AI, Quintas AM, Rodrigues W (2019) DMEK versus DSAEK for Fuchs’ endothelial dystrophy: a meta-analysis. Eur J Ophthalmol 29(1):15–22
Stuart AJ, Romano V, Virgili G, Shortt AJ (2018) Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev 25(6):CD012097
Esquenazi S, Rand W (2010) Safety of DSAEK in patients with previous glaucoma filtering surgery. J Glaucoma 19:219–220
Schoenberg ED, Levin KH, Savetsky MJ et al (2013) Surgical outcomes of DSAEK in patients with prior Ahmed glaucoma drainage device placement. Eur J Ophthalmol 23:807–813
Karadag R, Aykut V, Esen F, Oguz H, Demirok A (2020) Descemet’s membrane endothelial keratoplasty in aphakic and vitrectomized eye. GMS Ophthalmol Cases 14(10):Doc02. https://doi.org/10.3205/oc000129
Chiang CC, Lin JM, Tsai YY (2013) Descemet’s stripping automated endothelial keratoplasty in abnormal anterior segment: scleral indentation technique to enhance donor adherence. Graefes Arch Clin Exp Ophthalmol 251(6):1557–1563
Acknowledgments
The authors thank Simone Baar and Dirk Scharf for technical support.
Funding
Anna-Karina B. Maier: Financial support was provided by the “Friedrich C. Luft” Clinical Scientist Pilot Program funded by Volkswagen Foundation and Charité Foundation. Enken Gundlach: Financial support was provided by the “Ernst und Bertha Grimmke Stiftung.” Daniel Pilger is a participant in the BIH-Charité Clinician Scientist Program funded by the Charité–University Medicine Berlin and the Berlin Institute of Health.
Author information
Authors and Affiliations
Contributions
Anna-Karina B. Maier, Necip Torun, and Antonia M. Joussen contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Enken Gundlach, Nadiya Spiller, Daniel Pilger, and Anna-Karina B. Maier. The first draft of the manuscript was written by Enken Gundlach and Anna-Karina B. Maier and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study followed the ethical standards of the Declaration of Helsinki. Institutional ethical approval was obtained by the Ethics Committee of the Charité–Universitätsmedizin Berlin (EA2/108/12) for this prospective study and clinical trial registration was performed (http://www.clinicaltrials.gov, NCT02020044).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Code availability
(Software application or custom code) SPSS, RRID:SCR_002865
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12 kb).
Rights and permissions
About this article
Cite this article
Gundlach, E., Spiller, N., Pilger, D. et al. Impact of difficult unfolding and attachment of the graft lamella on the long-term outcome after Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol 258, 2459–2465 (2020). https://doi.org/10.1007/s00417-020-04852-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04852-z