Abstract
Aim
To evaluate the incidence and clinical indications for which eyes were treated for retinopathy of prematurity (ROP) outside the guidelines set by International Classification of ROP (ICROP).
Methods
Medical records of the patients treated at a single tertiary care ophthalmology hospital for ROP from January 2016 to December 2019 were retrospectively analysed to evaluate the indications for which they were treated.
Results
Out of 241 eyes, 33 eyes (13.7%) were treated outside the guidelines. The reasons for the treatment outside the guidelines were structural changes (n = 24, 72.7%), persistent stage 3 ROP that did not show any sign of regression for 6 weeks (n = 7, 21.2%) and active ROP with fellow eye being treated (n = 2, 6.1%). The recorded specific structural changes were tangential traction with temporal vessel straightening concerning for macular distortion and ectopia (n = 5, 15.2%), and stage 3 neovascularisation or ridge with anteroposterior traction with risk of progression to stage 4 disease (n = 19, 57.6%). Pre-plus disease was present in 11 eyes (33.3%).After the treatment, ROP stages regressed and retinal vessels grew either until the ora or at least into zone III in all the treated eyes. None of the eyes showed worsening of structural changes after treatment. The mean follow-up of the patients was 12.4 ± 11.7 months.
Conclusion
Experts occasionally recommend treatment in eyes with disease milder than type 1 ROP. This study may help paediatric retinal practitioners in decision-making in borderline cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disorder affecting the developing retinal vasculature of premature babies. This potentially blinding disease is absent at birth and evolves with the growth of an infant. Natural history of the disease shows that it can either regress spontaneously or progress to a vision-threatening stage. Therefore, timely screening and treatment form the cornerstone of management of the disease [1,2,3]. Being one of the leading causes of childhood blindness worldwide, internationally accepted screening and treatment guidelines have been laid down [4]. The current treatment guidelines are based on the results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomised clinical trial. The guidelines recommend treatment for type 1 ROP and observation for type 2 ROP [5].
However, decision-making in real-life ROP practice is complex. The decision “to treat” or “to wait” has to be delicately balanced keeping in mind a number of factors like parents’ compliance for repeated follow-ups, fear of litigations and other logistical issues like the need for repeated long-distance travel, health of the baby, visit to the paediatrician and possible need for general anaesthesia in big babies [6,7,8,9,10,11,12,13,14,15,16,17,18]. As a delay in treatment can cause permanent loss of vision, sometimes ROP experts decide to treat even if the recommended criterion is not fulfilled [6,7,8, 19, 20]. However, over-treatment also has its own set of inherent risks. While laser photocoagulation can potentially cause peripheral visual field loss and induction of myopia, intravitreal anti-vascular endothelial growth factor (VEGF) injections can be complicated by iatrogenic lenticular or retinal damage, endophthalmitis and potential damage due to systemic absorption [21,22,23,24,25,26]. However, not much literature is available to help the ROP experts decide the management of such borderline cases.
This study was done to evaluate the incidence and clinical indications for which eyes were treated for ROP outside the guidelines set by International Classification of ROP (ICROP).
Material and methods
This retrospective study was done at Aravind Eye Hospital, Madurai, India. Records of all the infants who underwent treatment for ROP from January 2016 to December 2019 were reviewed. The study was conducted with the approval of the Institutional Review Board (Registration No. ECR/182/INST/TN/2013, dated 20 April 2013, Project Code RET201600239) and adhered to the tenets of the Declaration of Helsinki.
As per the guidelines laid by the National Neonatal Forum, ROP screening was done for babies with gestational age (GA) ≤ 34 weeks, and/or birth weight (BW) ≤ 1750 g, or 34–36 weeks gestation, or 1750–2000 g birth weight in case of presence of risk factors for ROP or if the neonatologist felt the need for evaluation [27]. Clinical examinations were performed using indirect ophthalmoscopy, and wide-angle retinal images were captured with the help of a commercially available cameras (Forus 3nethra neo, Forus Health Pvt. Ltd., Bengaluru, India, and RetCam version 2, Clarity Medical Systems, USA), whenever possible. While ROP was classified according to the revised ICROP, treatment protocol was guided by the recommendations laid down by ETROP [4, 5].
The management was decided by one of the two ROP specialists (RPR, MT), both of whom were fellowship-trained vitreoretinal specialists with more than 5 years of experience in paediatric retina. In case treatment was required, informed consent was taken from the parents of the babies after explaining the nature of the procedure being performed and its associated complications. Laser photocoagulation was done with the help of a portable diode (810 nm) laser (Iris Medical OcuLight SLx, IRIDEX Corporation, Mountain View, CA, USA) under topical anaesthesia (0.5% proparacaine hydrochloride). Near confluent laser spots were delivered to the avascular retina up to the ora serrata. The laser settings used included a duration of 200 ms and power ranging from 150 to 300 mW. The number of laser spots depended on the area of the avascular retina. Similarly, intravitreal bevacizumab (IVB) was also given under topical anaesthesia with the assistant holding the infant’s head throughout the procedure. The injection was administered under all aseptic precautions at a dose of 0.5 mg/0.02 ml with a 30-gauge needle 1 mm away from the limbus.
The records were analysed to evaluate the characteristics of eyes undergoing treatment for ROP, demographic features of the patients and the treatment received. The eyes which were treated outside the ICROP guidelines i.e. milder than type 1 ROP were identified and further analysed to determine the indication for treatment.
Results
During the study period, 241 eyes of 124 infants received treatment, out of which 33 eyes (13.7%) of 20 infants were treated outside the guidelines. Retinal images were available for 15 eyes, while the rest of the eyes were graded as per the carefully maintained electronically medical records. The mean GA and BW of these babies were 31.2 ± 3.0 weeks and 1241 ± 284.7 g respectively. The average postmenstrual age (PMA) at which these babies received treatment was 42 ± 3.9 weeks (range, 36–51 weeks).
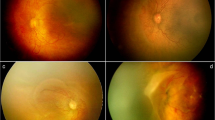
While 13 babies received bilateral treatment, seven babies received treatment in one eye only. The reasons for the treatment of eyes outside the guidelines were structural changes in 24 eyes (72.7%), persistent stage 3 ROP that showed no evidence of regression for 6 weeks in 7 eyes (21.2%) and active ROP with fellow eye being treated in 2 eyes (6.1%). The reported structural changes were tangential traction with temporal vessel straightening concerning for macular distortion and ectopia (Fig. 1) in 5 eyes (15.2%), and stage 3 neovascularisation or ridge with anteroposterior traction with risk of progression to stage 4 disease (Figs. 2, 3 and 4) in 19 eyes (57.6%). Pre-plus disease was present in 11 eyes (33.3%).
Thirty-two eyes received photocoagulation while one eye received IVB. The baby who was treated with IVB had a BW of 750 g and a GA of 33 weeks. At the time of treatment (42 weeks), his weight was 940 g only and ocular examination showed zone II posterior stage 3 ROP with pre-plus. As there was no sign of regression and the parents were finding it difficult to come for repeated examinations, we decided to treat the baby with IVB.
After the treatment, ROP stages regressed and retinal vessels grew either until the ora or at least into zone III in all the treated eyes. None of the eyes required repeat treatment. Worsening of structural changes after the treatment was not noted in any of the eyes. The mean follow-up of the patients was 12.4 ± 11.7 months.
Discussion
The multicentric trial of cryotherapy for ROP (CRYO-ROP) first laid the treatment criterion for ROP. The eyes which had a 50% risk of retinal detachment i.e. threshold ROP were advised treatment, while the eyes with milder disease were advised observation [28,29,30]. Later, the ETROP trial recommended an early treatment i.e. any stage in zone I with plus disease, stage 3 in zone I without plus disease and stages 2 or 3 in zone II with plus disease [5]. Babies with a milder disease are recommended observation with close follow-up until they either spontaneous regress or progress to the stage of treatment.
Currently, the practising environment for experts providing care for neonates with ROP is in grave crisis due to the increasing number of liability claims and judgements being passed against the concerned ophthalmologists. A large number of these cases are related to incorrect diagnosis and incorrect follow-up period [9,10,11,12,13,14,15,16]. Given the fear of litigations, many ophthalmologists are giving up ROP practice. The practising ROP specialists also tend to either over-diagnose or over-treat the disease [6,7,8]. Another challenge faced by the ROP care providers is the poor compliance of patients for follow-up [17, 18]. Repeated examinations to look for progression of disease have another drawback. Beyond a certain age, general anaesthesia is required for examining the babies, which is associated with adverse neuro-developmental outcomes in children [31, 32]. As a result of various medicolegal and logistical reasons, sometimes ROP experts tend to treat this dynamic disease at an earlier stage.
It has been reported that 9.5–27% eyes were treated despite a clinical diagnosis milder than type 1 ROP [6,7,8]. Darlow et al. reported that the number of infants in Japan, Spain and Finland treated for ROP exceeded those documented as having stage 3 ROP by 8.1%, 2.7% and 0.7% respectively [7]. Sekeroglu et al. in their nationwide survey in Turkey reported that 11.1% ROP specialist were treating type 2 ROP [20]. In our study also, 13.7% of the treated eyes had disease milder than type 1 ROP.
Some indications for treating milder disease, other than the guidelines set by ICROP, have been reported in literature. Gupta et al. (incidence, 9.5%) reported structural changes as the most common indication (69.2%) followed by persistent ROP at an advanced postmenstrual age (> 41 weeks) in 30.8%, vitreous haemorrhage in 23.1% and active ROP with the fellow eye being treated for type 1 ROP in 15.4% eyes [6]. Similarly, the most common reason for the treatment in our study was structural changes (72.7%) concerning for future anatomical complications. However, the most common indication for treatment in the study by Liu et al. (incidence, 12.5%) was type 1 ROP in the fellow eye in 43% eyes followed by stage 3 ROP with pre-plus disease in 30%, structural changes in 7%, persistent stage 3 ROP for > 6 weeks in 6%, stage 3 ROP with no plus disease in 5%, stage 3 zone III ROP with plus disease in 3%, logistical considerations in 3% and stage 2 disease in 2% eyes [8].
Sometimes, ROP experts tend to treat eyes with stage 3 ROP which is milder than the advised guidelines in case they do not show any evidence of regression beyond a certain time point. While Gupta et al. treated at 41 weeks PMA, Liu et al. preferred treating in case no regression was documented for 6 weeks [6, 8]. Similarly, we also treated stage 3 ROP (without plus) that did not show any evidence of regression for 6 weeks. ROP experts also prefer treating an eye with active ROP in case the fellow is being treated. This is to avoid the possible need for repeated visits required to treat both the eyes separately as there is a high degree of concordance between the two eyes and the other eye is also expected to require treatment in near future [33,34,35,36]. Treating both the eyes also prevents the chances of anisometropia as myopia is usually induced in the treated eye and can make amblyopia treatment difficult [21, 37,38,39].
The treatment of choice for ROP has changed over time. The initial treatment was cryotherapy, which was later replaced by laser due to favourable anatomical and functional outcome. The latest weapon in the armamentarium of the ROP specialist is intravitreal anti-VEGF injection. Gupta et al. and Liu et al. reported treatment with laser only [6, 8]. This was mainly because they reported results from studies which were completed prior to 2015 and 2012 respectively. On the contrary, in our study, one baby was treated with IVB. Decision to treat the baby with injection instead of laser was taken based on the systemic status of the baby and its failure to gain weight which is one of the prognostic markers for ROP remission [40]. After the treatment, ROP regressed and no further treatment was required. We believe that with time, the use of intravitreal anti-VEGF injections for ROP will gain more popularity.
This study adds to the literature that experts sometimes recommend treatment of eyes with ROP, even outside the international guidelines in certain special conditions. This study is expected to help the ophthalmologists in decision-making in case of borderline cases, especially with regard to the medicolegal implications.
This study is limited by its retrospective nature and inclusion of cases from a single centre, which can have a biased treatment protocol. Although both the graders in our study were highly experience, clinical diagnosis is subjective and ophthalmologists tend to diagnose borderline cases as being more severe. It is possible that the actual number of eyes treated for non-type 1 ROP may be higher and the incidence in our study may be an underestimation. Also, this study does not comment on the beneficial role of early treatment of such eyes as it was a non-comparative study. Further prospective studies are required to investigate the potential role of treatment, in comparison with observation, of non-type 1 disease in special cases like pre-plus disease, non-regressing stage 3 disease and those have structural changes with risk of anatomical complication.
References
Kim SJ, Port AD, Swan R et al (2018) Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol 63(5):618–637
Stenkuller PG, Du L, Gilbert C et al (1999) Childhood blindness. J AAPOS 3(1):26–32
Shah PK, Prabhu V, Karandikar SS et al (2016) Retinopathy of prematurity: past, present and future. World J Clin Pediatr 5(1):35–46
International Committee for the Classification of Retinopathy of Prematurity (2005) The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 123(7):991–999
Early treatment for retinopathy of prematurity cooperative group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121(12):1684–1694
Gupta MP, Chan RVP, Anzures R et al (2016) Practice patterns in retinopathy of prematurity treatment for disease milder than recommended by guidelines. Am J Ophthalmol 163:1–10
Darlow BA, Lui K, Kusuda S et al (2017) International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol 101(10):1399–1404
Liu T, Tomlinson LA, Ying GS et al (2019) Treatment of non-type 1 retinopathy of prematurity in the postnatal growth and retinopathy of prematurity (G-ROP) study. J AAPOS 23(6):332.e1–332.e6
Moshfeghi DM (2018) Top five legal pitfalls in retinopathy of prematurity. Curr Opin Ophthalmol 29(3):206–209
Bettman JW (1985) The retinopathy of prematurity: medicolegal aspects. Surv Ophthalmol 29(5):371–373
Demorest BH (1996) Retinopathy of prematurity requires diligent follow-up care. Surv Ophthalmol 41(2):175–178
Day S, Menke AM, Abbott RL (2009) Retinopathy of prematurity malpractice claims: the ophthalmic mutual insurance company experience. Arch Ophthalmol 127(6):794–798
Mills MD (2009) Retinopathy of prematurity malpractice claims. Arch Ophthalmol 127(6):803–804
Reynolds JD (2007) Malpractice and the quality of care in retinopathy of prematurity (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 105:461–480
Wiggins RE Jr, Gold RS, Menke AM (2015) Twenty-five years of professional liability in pediatric ophthalmology and strabismus: the OMIC experience. J AAPOS 19(6):535–540
Engelhard SB, Collins M, Shah C et al (2016) Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol 134(11):1230–1235
Vinekar A, Jayadev C, Dogra MR, Shetty B (2016) Improving follow-up of infants during retinopathy of prematurity screening in rural areas. Indian Paediatr 53:S151–S154
Vinekar A, Avadhani K, Dogra M et al (2012) A novel, low-cost method of enrolling infants at risk for retinopathy of prematurity in centers with no screening program: the REDROP study. Ophthalmic Epidemiol 19(5):317–321
Adams GG, Bunce C, Xing W et al (2017) Treatment trends for retinopathy of prematurity in the UK: active surveillance study of infants at risk. BMJ Open 7(3):e013366
Sekeroglu MA, Hekimoglu E, Sekeroglu HT, Arslan U (2013) Retinopathy of prematurity: a nationwide survey to evaluate current practices and preferences of ophthalmologists. Eur J Ophthalmol 23(4):546–552
Dhawan A, Dogra M, Vinekar A et al (2008) Structural sequelae and refractive outcome after successful laser treatment for threshold retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 45(6):356–361
Axer-Siegel R, Maharshak I, Snir M et al (2008) Diode laser treatment of retinopathy of prematurity: anatomical and refractive outcomes. Retina. 28(6):839–846
Wu WC, Lien R, Liao PJ et al (2015) Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol 133(4):391–397
Kong L, Bhatt AR, Demny AB et al (2015) Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 56(2):956–961
Sato T, Wada K, Arahori H et al (2012) Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol 153(2):327–333
Hong YR, Kim YH, Kim SY et al (2015) Plasma concentrations of vascular endothelial growth factor in retinopathy of prematurity after intravitreal bevacizumab injection. Retina. 35(9):1772–1777
Shukla R, Murthy GVS, Gilbert C et al (2020) Operational guidelines for ROP in India: a summary. Indian J Ophthalmol 68:S108–S114
Multicenter trial of cryotherapy for retinopathy of prematurity (1988) Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 106(4):471–479
Multicenter trial of cryotherapy for retinopathy of prematurity (1993) 3 1/2- year outcome-structure and function. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 111(3):339–344
Cryotherapy for Retinopathy of Prematurity Cooperative Group (2001) Multicenter trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol 119(8):1110–1118
Backeljauw B, Holland SK, Altaye M, Loepke AW (2015) Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 136:e1–e2
Wang X, Xu Z, Miao C-H (2014) Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PLoS One 9:e85760
Quinn GE, Dobson V, Biglan A et al (1995) Correlation of retinopathy of prematurity in fellow eyes in the cryotherapy for retinopathy of prematurity study. Arch Ophthalmol 113(4):469–473
Fielder AR, Shaw DE, Robinson J, Ng YK (1992) Natural history of retinopathy of prematurity: a prospective study. Eye. 6(3):233–242
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group (2004) Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 102:233–248 discussion 248-50
Ying GS, Pan W, Quinn GE et al (2017) Inter eye agreement of retinopathy of prematurity from image evaluation in the Telemedicine Approaches to Evaluating of Acute-Phase ROP (e-ROP) study. Ophthalmol Retina 1:347–354
Geloneck MM, Chuang AZ, Clark WL et al (2014) Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 132(11):1327–1333
Roohipoor R, Karkhaneh R, Riazi-Esfahani M et al (2018) Comparison of intravitreal bevacizumab and laser photocoagulation in the treatment of retinopathy of prematurity. Ophthalmol Retina 2(9):942–948
Anilkumar SE, Anandi V, Shah PK et al (2019) Refractive, sensory, and biometric outcome among retinopathy of prematurity children with a history of laser therapy: a retrospective review from a tertiary care center in South India. Indian J Ophthalmol 67(6):871–876
Bal S, Ying GS, Tomlinson L, Binenbaum G (2019) Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study Group. Association of weight gain acceleration with risk of retinopathy of prematurity. JAMA Ophthalmol 137(11):1301–1305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Aravind Medical Research Foundation Institutional Ethics Committee, 1, Anna Nagar, Madurai, Tamil Nadu, India (Registration No. ECR/182/INST/TN/ 2013, dated 20 April 2013), institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from parents of all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajan, R.P., Kohli, P., Babu, N. et al. Treatment of retinopathy of prematurity (ROP) outside International Classification of ROP (ICROP) guidelines. Graefes Arch Clin Exp Ophthalmol 258, 1205–1210 (2020). https://doi.org/10.1007/s00417-020-04706-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04706-8