Abstract
Purpose
To compare the Preceyes Surgical Robotic System (Eindhoven, Netherlands) to manual internal limiting membrane (ILM) peeling using the Eyesi surgical simulator (VRmagic, Mannheim, Germany) as the operative platform.
Methods
A comparative study was carried out with surgeons initially performing ILM peeling manually and then with the robot. Twenty-three vitreoretinal surgeons agreed to participate and all consented to the use of their surgical data from the Eyesi surgical simulator. Surgeons were given a 5-min demonstration of the devices and were allowed to practice for 10 min before attempting the membrane peel. Initially, the peel was performed manually and afterwards, this was repeated using the robot-controlled forceps. Surgical simulator outcome measures were compared between approaches.
Results
The average time required for the procedure was 5 min for the manual approach and 9 min with the robot (paired t test, p = 0.002). Intraocular instrument movement was reduced by half with the robot. On average 344 mm was required to complete the ILM peeling with the robot compared with 600 mm using the manual approach (paired t test, p = 0.002). There were fewer macular retinal hemorrhages with the robot: 53 with manual surgery, 32 with the robot (Mann-Whitney U test, p = 0.035). Retinal injuries were eliminated with the robot.
Conclusions
Intraocular robotic surgery is still in its infancy and validation work is needed to understand the potential benefits and limitations of emerging technologies. Safety enhancements over current techniques may be possible and could lead to the broader adoption of robotic intraocular surgery in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robotic surgery has advanced rapidly over the past 15 years, particularly in areas such as general surgery, urology, and gynecology [1,2,3,4,5]. Development has been slower in ophthalmology, presumably because of the existing minimally invasive nature of modern eye surgery, and also due to challenges related to engineering machines that can work safely at micrometer precision within a confined anatomic space. However, several research groups have noted benefits that automation can provide to eye surgery, including possible solutions to challenges such as vitrectomy teaching, retinal vessel cannulation, sub-retinal injections, and telesurgery [6, 7]. The Preceyes Surgical System 1.4 (Eindhoven, The Netherlands) is one such platform that has evolved to a stage that has allowed the completion of the first-in-human robotic surgical study [8, 9]. This device has been approved for use in Europe with a CE mark approval “to assist trained vitreoretinal surgeons during surgical tasks”. It has not been approved by the FDA as of August 2019.
Surgical robots generally require a significant amount of simulation training by the operator prior to use in humans [10, 11]. The same can be assumed to apply to intraocular robotic devices [12,13,14]. The Preceyes Surgical System is a one-handed, telemanipulator robot that has been designed to function in a very similar fashion to a traditional intraocular instrument. As opposed to robots such as the da Vinci System (Intuitive Surgical Inc., Sunnyvale, California), it makes use of existing visualization systems, with the surgeon seated at his/her usual surgical location. For these reasons, extensive training may not be as essential with this system. Furthermore, Preceyes can restrain the approach to the retinal surface by creating a fixed intraocular boundary beyond which instruments cannot advance. An instrument-integrated optical coherence tomography (OCT) A-scan sensor can also be used to limit the attached instruments’ distance from the retina and/or provide auditory feedback when an instrument approaches the retina. These features potentially enhance the safety of this system compared to manual surgery as once a bound is set for the retina, the surgeon can move throughout the eye trusting the instrument will not touch the retina.
We sought to study the ability of experienced vitreoretinal surgeons to adapt to the surgical robot system for undertaking the task of macular internal limiting membrane (ILM) peeling within the Eyesi surgical (VRmagic, Mannheim, Germany) simulated vitreoretinal surgical environment. We also sought to track surgical safety and efficiency outcomes for the robot compared to manual surgery.
Methods
The Preceyes Surgical System has been developed for intraocular procedures such as vitrectomy, membrane peeling, as well as sub-retinal and intravascular injections. The device functions as an adjunct to traditional 3-port vitrectomy and was designed to be operated in a fashion that mimics the current use of the working vitrectomy port. Employed through a single sclerotomy, this externally controlled telemanipulation system allows tremor-free and highly precise motion-scaled instrument movements within the eye. Probe actions are controlled by one hand of the surgeon using a motion controller that, for safety purposes, requires the engagement of two-finger buttons prior to activation. Forceps, scissors, and cutters can be used interchangeably with grasping or cutting actuation via a foot pedal. Intraoperative illumination and infusion are provided in the customary manual fashion, but the former could also be managed by the robot, allowing for true bimanual intraocular surgery. Safety is potentially enhanced via the use of computer-generated distance boundaries that are provided by real-time intraocular optical coherence tomography (OCT) A-scans generated from a sensor in the tip of the instrument. This functionality can limit the movement of intraocular instruments beyond a pre-specified retinal depth, preventing accidental or deep engagement of retinal tissue. Auditory feedback (beeping with increasing frequency) is also provided as instruments move in to proximity with the retina. An augmented retraction mechanism allows for instant removal of the probe from the eye in potentially dangerous situations such as unexpected patient head movement. This mechanism is in addition to other measures for the mitigation of patient movement such as head and eye fixation.
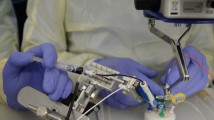
To study the safety of surgeon adaptation to the robotic system, the device was coupled to the vitreoretinal surgical simulator (see Fig. 1). The Eyesi surgical simulator is a computerized device that creates a virtual intraocular surgical environment. Instruments are introduced into a model eye. The location of the eye and the instruments are optically tracked. All relevant devices of the real surgical task have their counterparts in the simulator setup: a stereo microscope head contains high-resolution microdisplays for a realistic, three-dimensional view of the computer-generated surgical situation; a BIOM® mimic serves as a wide-field viewing system for the fundus; foot pedals control the surgical microscope and the OR machine; real instrument handpieces can assume functions such as vitreous cutter, light source, microforceps, or endolaser probe. The Eyesi surgical software simulates a variety of vitreoretinal procedures (Fig. 2) by calculating tissue interactions and their 3D visualization in real-time. An integrated step-by-step curriculum allows for structured learning and reproducible training results. A modified version of the simulator was developed collaboratively with Preceyes to allow integration of the surgical robot.
Original (left) and modified (right) vitreoretinal surgical simulator Eyesi surgical. The modified Eyesi has a third foot pedal and a second touchscreen for the control of the attached Preceyes Surgical Robotic System. The robot is mounted on the right side of the “operating table” from where it moves an instrument tip within the simulator’s eye model
The modified simulator software mimics the robot’s OCT-guided instrument boundary limiter by calculating the virtual retina-instrument distance and providing a feedback loop to the robotic system. The robot then gives auditory feedback and limits instrument movement within a certain proximity to the virtual retina. The surgical instrument boundaries of the robotic system are programmable to limit the reach of an instrument within the virtual eye. Bounds can be placed a certain distance in front of or behind the retina surface. For the simulator set-up in this study, the posterior bound of the system was set to − 50 μm (50 μm deep to the surface of the retina) for the initiation of the ILM peel after which the bound was lifted to + 50 μm for the subsequent ILM rhexis and removal. Retinal hemorrhages were observed with instrument penetration of the retina beyond a depth of − 10 μm.
Twenty-three (23) vitreoretinal surgeons were offered the opportunity to use the robotic system in a virtual surgical setting. As this system has yet to enter the commercial arena, none had prior experience with the device in the simulation or operating room environment. All were given a 5-min demonstration of the robotic device and were allowed to practice manipulating the robotic intraocular forceps on the simulator for 10 min. Initially, a peel was performed manually with the simulator’s intraocular forceps, and afterwards, this was repeated using the robot-controlled forceps.
Surgical safety and efficiency were tracked by the Eyesi surgical assessment software. The following outcome variables were obtained during both the manual and robotic procedures: surgical time, induced retinal hemorrhages (macular or extramacular), instrument to retina touch injuries (macular or extramacular), and the distance (odometer) the surgical instrument tip moved in performing the tasks. All surgical outcome measures are summarized and described in more detail in Table 1.
The simulator’s software also generates outcome measures in surgical categories such as efficiency, instrument handling, and tissue treatment. These scores group data from multiple parts of the procedure and are used to grade surgeons from “failing” to “good.” Given the complexities of interpreting this multifactorial outcome data, these measures were not included in the analysis.
Statistical analyses were performed on Stata™ 15 software (www.stata.com) using parametric and non-parametric one or two sample tests as appropriate (paired t test for continuous variables, and Mann-Whitney U tests for ordinal comparisons). Adjustments of p values were not made for multiple comparisons. As this study was exploratory, p values of 0.05 were considered significant. All surgeons consented to the use of their surgical data for research purposes. This study conforms to the Declaration of Helsinki; IRB/Ethics Committee approval was not required for this study. Exemption has been confirmed by the Central Committee on Research Involving Human Subjects (CCMO), The Hague, Netherlands.
Pre-testing outlier assessments were performed for values beyond upper and lower limits of 1.5 times the interquartile value. Videos of surgeries were reviewed when significant outlier data was encountered to determine if it was appropriate to exclude data points (for example, if a surgeon was generating hemorrhages by attempting other actions with the simulator not related to assigned task of ILM peeling). In total, only 1 data point was censored and in this case censoring did not change the significance of the statistical test or appreciably alter the p value.
Results
Twenty-three surgeons completed both the manual and robotic internal limiting membrane peels. The average time required for the procedure was 5 min for the manual approach and 9 min with the robot (paired t test, p = 0.002). Forceps engagements (tracked by number of instrument closings) of the ILM were comparable between surgical approaches with an average of 31 during the manual peeling and 36 during the robotic peeling (paired t test, p = 0.45). Interestingly, the extent of intraocular instrument movement (odometer) was reduced by half with the robot compared to manual surgery. The average distance the intraocular forceps moved during the manual surgery exceeded 600 mm, while on average, only 344 mm was required to complete the ILM peeling with the robot (paired t test, p = 0.002).
Of particular note was the elimination of retinal injuries with the robot. Injuries are prevented by the virtual OCT-A bound not allowing instruments to deeply penetrate the retina. Amongst all surgeons, twenty (20) extramacular and seven [7] macular injuries were induced in the manual surgeries. None occurred when the robot was used (Wilcoxon Single Sample Test, p = 0.008 for extramacular, p = 0.03 for macular injuries). There were also fewer macular retinal hemorrhages induced by the surgeons with the robot: 53 with manual surgery, 32 with the robot (Mann-Whitney U test, p = 0.035).
The number of extramacular retinal hemorrhages were similar between groups (34 with manual surgery, 32 with the robot; Mann-Whitney U test, p = 0.69). The vast majority of these hemorrhages occurred with the first ILM engagement at the initiation of the peel. Only two surgeons avoided initiation hemorrhages. All outcomes are summarized in Table 2.
Discussion
This study compares robot-assisted macular surgery with manual surgery in a simulated surgical environment. It provides evidence that the Preceyes system, and its close reproduction of the hand movements of traditional vitreoretinal surgery, can be learned to a basic level of competency in a short period of time. For participating surgeons, this was the first robot-assisted ILM peeling any had performed. While surgical times were significantly longer for the robotic surgeries, one would expect this to become less of an issue as experience with the system increases. This expectation is substantiated by the large distribution in surgical times for the robot cases, indicating that short surgical times are possible with the robot and that improvement is possible for surgeons who have not fully adapted to the system.
Robotic devices that incorporate boundaries to limit instruments from penetrating the retina beyond a pre-determined depth may improve the safety of intraocular, and in particular, macular surgery. The use of instrument boundaries in this simulated surgical environment significantly reduced the number of macular hemorrhages during ILM peeling (compared to manual surgery) and also completely eliminated instrument-induced retinal injuries in both the macular and extramacular regions. Overall, the robot appeared to be markedly safer than manual surgery.
The frequency of extramacular hemorrhages was similar between groups, most likely due to the challenge of initiating engagement of the ILM in the simulated surgical environment. The simulator records a retinal hemorrhage with instrument penetration deeper than 10 μm from the surface. With the robot internal bound set at − 50 μm for this study, the robot was not actively protecting against this risk. As noted, only two surgeons avoided creating a hemorrhage at the start of the ILM peels.
Less extensive intraocular forceps movements (odometer) were recorded when using the robot. This could be explained by the robotic telemanipulator allowing surgeons to freeze the movements of the instrument by releasing a button on the motion controller. This allows the surgeon to instantaneously pause the procedure prior to moving the instrument again. In this particular implementation, motion scaling allowed more rapid forceps movements when further away from the retina and slower movements when in proximity to the retina. However, even when the robot-controlled instrument was in the anterior or mid-vitreous, probe movements remained relatively restricted—especially when compared to the manipulation velocities and accelerations possible with manual surgery. Essentially, movements with the robot were intentionally constrained and demanded a more parsimonious approach to the membrane peeling. This feature of the Preceyes system likely also contributed to the longer robotic surgical times. The robotic system allows the surgeon to change motion scaling settings for each procedural task separately, optimizing the trade-off between surgical time and precision or safety.
One important limitation of this study was that the robot was not tested in a live human surgical environment, but rather in a simulated one. The validated Eyesi surgical system [15] provides a good representation of vitreoretinal surgery, but does not entirely replicate the experience of operating on human tissue. Specific surgical maneuvers, such as ILM engagement at the start of a peel, while very representative, cannot exactly match the actual task. Similarly, the OCT functionality of the robotic system was programmed into the simulator and was not generated from an OCT sensor in the instrument tip. However, validation work on the robot-integrated OCT has been performed for membrane peeling surgery in an ex vivo model and an in vivo human surgical setting [16, 17].
Conclusion
Intraocular robotic surgery is still in its infancy and validation work is needed to understand the potential benefits and limitations of emerging technologies. Besides issues of cost, training, and surgical productivity, the fundamental safety of robotic devices needs to be demonstrated. As this study suggests, safety enhancements over current techniques can be expected, and very well may be a leading reason for the broad adoption of robotic intraocular surgery in the near future. In addition, it appears that robotic systems that augment, but do not fundamentally change existing surgical techniques, may be learned in a very efficient manner.
Abbreviations
- ILM:
-
Internal limiting membrane
- OCT:
-
Optical coherence tomography
References
Cheng-maw H, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T (2013) Systematic review of robotic liver resection. Surg Endosc 27:732–739. https://doi.org/10.1007/s00464-012-2547-2
Ramsay C, Pickard R, Robertson C, et al. (2012) Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess (Rockv). ;16(41)
Lane T (2018) A short history of robotic surgery. Ann R Coll Surg Engl 100:5–7. https://doi.org/10.1308/rcsann.supp1.5
Matanes E, Lauterbach R, Boulus S, Amit A, Lowenstein L (2018) European Journal of Obstetrics & Gynecology and reproductive biology robotic laparoendoscopic single-site surgery in gynecology : a systematic review. Eur J Obstet Gynecol 231:1–7. https://doi.org/10.1016/j.ejogrb.2018.10.006
Morelli L, Guadagni S, Franco G Di, Palmeri M, Candio G Di. (2016) Da Vinci single site © surgical platform in clinical practice : a systematic review. Int J Medial Robot Comput Assist Surg. ;(November 2015):724–734. doi:https://doi.org/10.1002/rcs
De Smet MD, Meenink TCM, Janssens T, et al. (2016) Robotic assisted cannulation of occluded retinal veins. PLoS One. ;(September 27, 2016):1–17. doi:https://doi.org/10.1371/journal.pone.0162037
Molaei A, Abedloo E, de Smet M et al (2017) Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res 12(2):2120218
De Smet MD, Naus GJL, Faridpooya K, Mura M (2018) Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol 29(3):248–253. https://doi.org/10.1097/ICU.0000000000000476
Edwards TL, Xue K, Meenink HCM et al (2018) First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng 2:649–656. https://doi.org/10.1038/s41551-018-0248-4
Nezhat C, Lakhi N (2016) Learning experiences in robotic-assisted laparoscopic surgery. Best Pract Res Clin Obstet Gynaecol 35:20–29. https://doi.org/10.1016/j.bpobgyn.2015.11.009
Moglia A, Ferrari V, Morelli L, Ferrari M, Mosca F, Cuschieri A (2016) A systematic review of virtual reality simulators for robot-assisted surgery. Eur Urol 69(6):1065–1080. https://doi.org/10.1016/j.eururo.2015.09.021
Bourcier T, Chammas J, Becmeur PH et al (2017) Robot-assisted simulated cataract surgery. J Cataract Refract Surg 43(4):552–557. https://doi.org/10.1016/j.jcrs.2017.02.020
Deuchler S, Wagner C, Singh P et al (2016) Clinical efficacy of simulated vitreoretinal surgery to prepare surgeons for the upcoming intervention in the operating room. PLoS One 11(3):1–12. https://doi.org/10.1371/journal.pone.0150690
Vergmann AS, Vestergaard AH, Grauslund J (2017) Virtual vitreoretinal surgery: validation of a training programme. Acta Ophthalmol 2:60–65. https://doi.org/10.1111/aos.13209
Rossi JV, Verma D, Fujii GY et al (2004) Virtual vitreoretinal surgical simulator as a training tool. Retina. 24(2):231–236. https://doi.org/10.1097/00006982-200404000-00007
van Romunde S, Faridpooya K, Vermeer KA et al (2018) Evaluation of OCT versus surgeon guided robotic manipulation in a simulated vitreoretinal model. Invest Ophthalmol Vis Sci 59(9):5930
Cereda MG, Faridpooya K, van Meurs JC, et al. (2018) First in-human clinical evaluation of a robot-controlled instrument with a real-time distance sensor in the vitreous cavity. In: Annual Meeting of the American Academy of Ophthalmology. American Academy of Ophthalmology; :PO224
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Maberley has recently conducted phase 3 trials for Alcon, Roche, Abvie, and Ophthotech and declares no other conflicts of interest related to this study. Maarten Beelen, Jorrit Smit, Thijs Meenink, and Gerrit Naus are employees of Preceyes B.V., Eindhoven, The Netherlands, and declare no other conflicts of interest. Clemens Wagner is an employee of VRmagic Holding AG, Mannhein, Germany, and declares no other conflict of interest. Marc de Smet is Chief Medical Officer of Preceys B.V., Eindhoven, The Netherlands, and of Oxular Ltd.; he has received honoraria from Allergan, has been a part of advisory boards for Abbvie, Allergan, and Janssen and declares no other relevant conflicts of interest.
Ethical approval
All surgeons consented to the use their surgical data for research purposes. This study conforms to the 1964 Declaration of Helsinki and its later amendments. IRB/Ethics Committee approval was not required for this study as there were no human or animal subjects. Exemption has been confirmed by the Central Committee on Research Involving Human Subjects (CCMO), The Hague, Netherlands.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting presentation
1. Oral presentation at FLORetina meeting – Florence, Italy (June 7, 2019)
2. ePoster theatre presentation at American Academy of Ophthalmology 2019 – San Francisco (October 13, 2019)
Rights and permissions
About this article
Cite this article
Maberley, D.A.L., Beelen, M., Smit, J. et al. A comparison of robotic and manual surgery for internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol 258, 773–778 (2020). https://doi.org/10.1007/s00417-020-04613-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04613-y