Abstract
Purpose
To compare serum and aqueous humor (AH) vitamin D levels between the patients with diabetic macular edema (DME) and controls.
Methods
A total of 65 subjects (30 DME, 35 control) were included. One-third of the control group had hypertension, dyslipidemia, or diabetes mellitus without diabetic retinopathy as underlying diseases. Serum and AH levels of 25-hydroxyvitamin D were measured in each subject. Multiple linear regression analysis was performed to investigate factors associated with serum and AH vitamin D levels.
Results
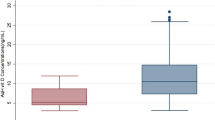
There were no significant differences in serum vitamin D levels between the DME (14.3 ± 9.1 ng/mL) and control (16.2 ± 8.0 ng/mL) groups (P = 0.374). However, eyes with DME (41.6 ± 8.0 ng/mL) had a higher AH level of vitamin D than control eyes (25.5 ± 4.1 ng/mL, P < 0.001). AH vitamin D level was significantly associated with the presence of DME (β = 0.775, P < 0.001). Serum and AH levels of vitamin D were not significantly correlated (r = − 0.157, P = 0.211).
Conclusion
Serum vitamin D levels did not significantly differ between the DME and control groups. Localized vitamin D level in the eye was independent from systemic vitamin D level and it might be another indicator of DME severity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) can develop in any stage of diabetic retinopathy (DR) and is a major cause of visual acuity decreases in patients with DR. DME is mainly caused by the breakdown of the blood-retinal barrier, induced by elevated levels of vascular endothelial growth factor (VEGF) and various other inflammatory cytokines [1].
Vitamin D is a biosynthesized secosteroid that is essential to a wide range of physiologic processes. Vitamin D has both anti-inflammatory and anti-angiogenic effects and vitamin D deficiency has been associated with various diseases, including diabetes mellitus, hypertension, cardiovascular disease, autoimmune disease, and infectious disease [2]. Vitamin D receptors and 1-α-hydroxylase, which converts vitamin D to its active form, have been found in the retina [3, 4]. It has been suggested that a vitamin D deficiency may influence the development and progression of various retinal disorders, including DR. Alcubierre et al. [5] and Patrick et al. [6] found a correlation between deficient serum vitamin D levels and more advanced DR. Additionally, He et al. [7] showed that the prevalence of sight-threatening DR doubles when serum 25-hydroxyvitamin D levels are below 15.57 ng/mL. Additionally, Millen et al. [8] showed that higher levels of serum 25-hydoxyvitamin D (> 75 nmol/L) significantly lowered the risk of developing DR. On the other hand, Alam et al. did not find a significant association between serum vitamin D deficiency and severity of DR or diabetic maculopathy, while the study was retrospective and only a small proportion of included patients had diabetic maculopathy [9].
The anti-inflammatory and anti-angiogenic effects of vitamin D may improve DME pathophysiology and be protective against DME development. Furthermore, recent studies found vitamin D metabolites in the aqueous humor (AH) and vitreous body of the eye [3, 10], while studies that have examined the association between DME and vitamin D levels are scarce. Therefore, this study examined serum and AH vitamin D levels in patients with DME. We also examined the correlation between serum and AH vitamin D levels and whether or not vitamin D can protect patients from developing DME. Vitamin D measurements were also made in a control group for comparison.
Methods
This study protocol was reviewed and approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB No. 2016-10-010; Seoul, South Korea). All study conduct adhered to the tenets of the Declaration of Helsinki and written informed consent to participate in the study was obtained from all participants.
Study subjects
Patients who had macular center-involving DME and were scheduled to undergo intravitreal anti-VEGF injection (bevacizumab, Avastin®, Genentech/Roche, CA, USA) between March and June 2017 were considered for inclusion. All subjects were scheduled to undergo intravitreal injection at Kangdong Sacred Heart Hospital. During the same time period, patients with no retinal disorders (including DR) and who were scheduled to undergo cataract surgery at the same hospital were considered for enrollment into the control group. One-third of the control group had hypertension, dyslipidemia, or diabetes mellitus without diabetic retinopathy as underlying diseases. The study period was limited to one season to prevent seasonal variations in serum vitamin D levels from confounding our results. Subjects who had any of the following conditions were excluded: (1) combined chorioretinal disorder other than DR and DME (e.g., age-related macular degeneration, retinal vein occlusion, and central serous chorioretinopathy); (2) history of intravitreal anti-VEGF injection within 6 months of enrollment; (3) history of intraocular surgery (except for uncomplicated cataract surgery), (4) systemic inflammatory or autoimmune disease, vitamin D absorption problems, liver disease, thyroid disease, or parathyroid disease, (5) use of medications known to influence serum vitamin D levels (e.g., anticonvulsants, rifampin, and glucocorticoids), (6) chronic kidney disease with a creatinine (Cr) level at or below 2.0 mg/dL.
Study examinations
Ophthalmic examinations and measurements
All subjects underwent a thorough ophthalmologic examination prior to any procedure, including slit lamp examination, fundus examination, optical coherence tomography (OCT, Spectralis, Heidelberg Engineering Inc., Heidelberg, Germany), and measurement of intraocular pressure and best-corrected visual acuity. Fluorescein angiography (FA) was performed (TRC-50DX, Topcon, Oakland, NJ, or Optos 200TX, Optos PLC, Dunfermline, Scotland) in patients with DME who were able to undergo contrast enhancement. Visual acuity was evaluated using a Snellen chart and all measurements were converted to the logarithm of the minimal angle of resolution (logMAR) for statistical evaluation. Cataract evaluation was done according to the Lens Opacities Classification System III [11]. Study OCT images were obtained using a 25-line scan that included 30° × 20° of the central macular area. Central macular thickness (CMT) was defined as the mean retinal thickness within the 1-mm-diameter central region of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. A subject was said to have DME if center-involving macular edema was present and CMT was greater than 250 μm (as measured with OCT). In the control group, those with diabetes also underwent OCT examination and confirmed that there was no DME. We also classified DME type into four categories according to the previous literature: (1) sponge-like diffuse retinal thickening, (2) cystoid macular edema, (3) serous retinal detachment, and (4) all patterns combined [12, 13]. Diabetic retinopathy severity was assessed by two trained ophthalmologists (KLK, Y-KK) using the modified ETDRS severity classification. Study FA images were obtained using standard 8 field views used in the Central Vein Occlusion Study [14] (TRC-50DX, Topcon, Oakland, NJ) or wide-field fundus camera (Optos 200TX, Optos PLC, Dunfermline, Scotland). The microaneurysms were counted in the central macula area within the major vascular arcades. Microaneurysm was defined as a localized hyperfluorescent dilatation arising from a capillary equal to or larger than 1.0 mm [15]. We also measured peripheral non-perfusion area by disc area (DA) [16] and categorized it as the following criteria: < 1 DA, ≥ 1 and < 10 DA, ≥ 10 and < 30 DA, and ≥ 30 DA. All measurements were performed by two trained ophthalmologists (KLK, Y-KK).
Aqueous humor collection
All AH specimens were collected using an aseptic technique in an operating room. After applying topical anesthesia eyedrops (proparacaine, Alcaine®, Alcon, Fort Worth, TX, USA), the ocular surface, eyelid, and eyebrow were disinfected with 5% povidone iodine and a sterilized eyelid speculum was inserted. Anterior chamber punctures were performed using a 1-mL syringe with a 30-gauge needle and 0.1 mL of AH was collected. All anterior chamber punctures were performed before intravitreal bevacizumab injection (1.25 mg in 0.05 mL) in the DME group and before main corneal incision creation of cataract surgery in the control group. All AH samples were immediately transferred to sterile tubes and stored in a deep freeze at − 80 °C. AH 25-hydroxyvitamin D levels were measured using an automated, competitive immunoassay (25-hydroxyvitamin D ELISA kit, Enzo, Switzerland) that relies upon chemiluminescence.
Serum laboratory evaluations
A venous blood sample was obtained from each patient. The following serum levels were measured: 25-hydroxyvitamin D, glycated hemoglobin (HbA1c), total cholesterol (TC), high-density lipoprotein (HDL), triglyceride, low-density lipoprotein (LDL), and Cr. The HbA1c level was determined using turbidimetric inhibition immunoassay (Cobas Integra 400 Plus testing system, Roche Diagnostics, Indianapolis, IN, USA). Serum triglyceride, TC, HDL, and LDL were determined using enzymatic colorimetric assays (reagents obtained from Roche Diagnostics). Serum 25-hydroxyvitamin D was measured using the ADIVA Centaur XP immunoassay system (Siemens Healthcare Diagnostics, Erlangen, Germany) after sample centrifugation (2500 rpm for 10 min).
Subject data collection
Demographic, lifestyle, and medical history data were collected via medical record review and patient interview. More specifically, the following parameters were obtained: (1) presence of systemic disease (diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease), (2) vitamin D supplement intake within 1 year of enrollment (never taken, taken in the past but discontinued, currently taking), (3) degree of outdoor activity (corresponds to sunlight exposure), (4) smoking status (current smoker, former smoker, never smoked), (5) education periods (number of years of education [elementary school through university]). Outdoor activity was classified as inactive (mostly indoor activity because of poor mobility), underactive (mostly indoor, static activity [e.g., regular office worker]), active (indoor work with regular outdoor activity outside of working hours), and very active (mainly engaged in outdoor work).
Statistical analyses
Data are presented as mean ± standard deviation, where applicable. Serum and AH 25-hydroxyvitamin D levels were compared between the DME and control groups using Student’s t test. The correlation between serum and AH 25-hydroxyvitamin D levels was assessed using Pearson’s correlation test. Multiple linear regression analysis with a stepwise approach was used to examine clinical factors potentially associated with serum and AH vitamin D levels, including age, sex, body mass index (BMI), HbA1c levels, serum cholesterol (TC, HDL, LDL) and triglyceride levels, and Cr levels. Potential correlations with the degree of outdoor activity, vitamin D supplement intake, and the presence or absence of DR or DME were also investigated. Multiple linear regression analysis was also performed on DME group data to examine correlations between AH 25-hydroxyvitamin D levels and various clinical and OCT (e.g., CMT before and 1 month after intravitreal injection) parameters. We divided DME patients into tertiles according to the AH 25-hydroxyvitamin D levels and compared OCT and FA findings. Statistical analyses were performed using statistical software (Stata version 14.0; Stata Corp., College Station, TX, USA) and statistical significance was defined as P < 0.05.
Results
A total of 65 subjects were enrolled in this study. Thirty patients were included in the DME group (19 men, 11 women) and 35 patients were included in the control group (14 men, 21 women). There was no significant difference between groups in age, sex, or BMI. Both groups also had comparable levels of serum cholesterol (TC, HDL, and LDL) and triglyceride and had similar levels of outdoor activity and vitamin D supplement intake. Although we did exclude patients with chronic kidney diseases that had a serum Cr level higher than 2.0 mg/dL, serum Cr level was significantly higher in the DME group (1.4 ± 1.4 mg/dL) than in the control group (0.8 ± 0.2 mg/dL, P = 0.023). There was no significant difference in lens status between the two groups. The DME group had a higher proportion of patients with diabetes and dyslipidemia and a higher serum HbA1c level than the control group. Serum vitamin D levels were not significantly different between groups (DME 14.3 ± 9.1 ng/mL, control 16.2 ± 8.0 ng/mL; P = 0.374). A large percentage of subjects in both groups (90% in the DME group and 94% in the control group) had a serum vitamin D deficiency (serum vitamin D < 30 ng/mL). However, the DME group had a significantly higher AH vitamin D level (41.6 ± 8.0 ng/mL) than the control group (25.5 ± 4.1 ng/mL, P < 0.001; Table 1).
Multiple linear regression analysis revealed that serum vitamin D level was significantly correlated with older age (standardized β-coefficient = 0.382, P = 0.001) and marginally associated with the amount of vitamin D supplement intake (standardized β-coefficient = 0.248, P = 0.056; Table 2). The AH vitamin D level was associated with the presence of DME (standardized β-coefficient = 0.775, P < 0.001) and higher BMI (standardized β-coefficient = 0.198, P = 0.009; Table 2). Serum and AH vitamin D levels were not correlated (Pearson’s r = − 0.157, P = 0.211). In the DME group, AH vitamin D level was associated with higher BMI (standardized β-coefficient = 0.708, P < 0.001), thicker CMT 1 month after intravitreal injection (standardized β-coefficient = 0.554, P = 0.001), and lower serum triglyceride level (standardized β-coefficient = − 0.463, P = 0.009; Table 2).
When we divided DME patients into tertiles according to the AH vitamin D level, the highest AH vitamin D level tertile group showed wider non-perfusion area in FA. There were no significant differences in baseline CMT among three groups; however, those with the highest AH vitamin D level showed the thickest 1-month CMT. There were no significant differences in terms of the diabetic retinopathy severity, the number of microaneurysms, or the macular edema type among the three groups (Table 3).
Discussion
This study examined and compared serum and AH vitamin D levels in eyes with DME and in eyes of controls. Unlike our initial expectation, no significant serum vitamin D level differences between groups were observed. Moreover, AH vitamin D level was higher in the DME group than in the control group and serum and AH vitamin D levels were not significantly correlated. However, in eyes with DME, AH vitamin D level was positively correlated with BMI and CMT 1 month after intravitreal anti-VEGF injection, and those with high level of AH vitamin D showed wider area of non-perfusion in FA.
Some prior studies have demonstrated an inverse relationship between serum vitamin D level and DR severity [5,6,7,8, 17]. However, other studies did not [9, 18]. Bonakdaran et al. [18] did not find a significant difference in serum vitamin D level among patients with no DR, nonproliferative DR, and proliferative DR. Additionally, vitamin D level was not significantly correlated with other known risk factors of DR, including diabetes duration, poor glycemic control, hypertension, inflammation, and insulin growth factor. Alam et al. [9] also found no association between serum vitamin D level and DR or diabetic maculopathy severity. Both studies included a population with a high incidence of serum vitamin D deficiency and both investigative groups theorized that this explained why there was no correlation between serum vitamin D level and DR severity. The population examined in the current study also had a very high proportion of patients with a serum vitamin D deficiency (60 [92.3%] of 65 subjects). This was not surprising because serum vitamin D deficiency is more common in Korea than in other countries, likely because Koreans are reluctant to expose their skin to sunlight (except for their faces) and the traditional Korean diet is low in vitamin D [19]. Therefore, it is possible that our ability to examine the relationship between serum vitamin D levels and DR severity was limited by a low range of subject vitamin D levels.
Subjects with DME, on average, surprisingly had a higher AH vitamin D level than control subjects. Even after adjusting for several clinical factors, multiple regression analysis revealed that DME presence was still significantly associated with the higher AH vitamin D levels. When the same analysis was performed on data from DME subjects only, CMT measured 1 month after intravitreal anti-VEGF injection was significantly associated with AH vitamin D level, and those with higher level of AH vitamin D showed wider area of non-perfusion in FA. Therefore, it may be that localized levels of vitamin D represent the degree of organ ischemia. It has been reported that the active form of vitamin D (1,25(OH)2D3) increases VEGF expression and release in vascular smooth muscle cells via direct binding of the vitamin D receptor (as a transcription factor) to a VEGF promoter [20,21,22]. This finding has not yet been investigated in retinal or retinal pigment epithelial cells. However, this finding makes it possible for AH vitamin D levels to be correlated with ocular ischemia severity. Further research is needed to investigate whether ischemia-driven VEGF production is preceded by local vitamin D synthesis.
The current study did not show a significant correlation between serum and AH vitamin D levels. Previous studies have examined the relationship between serum vitamin D level and clinical parameters in DR patients. However, this is the first study to measure ocular vitamin D levels in eyes with DME. Interestingly, we found that systemic and ocular vitamin D levels were not correlated to each other. This may have occurred because the eye is capable of producing vitamin D when exposed to UV light [10] and the blood-retinal barrier likely restricts free movement of vitamin D between the systemic circulation and the eye [23,24,25,26]. Further studies are needed to better understand how systemic and local organ-specific vitamin D levels are correlated.
This study showed an increase in serum vitamin D levels with age. Older age is generally considered to be a risk factor for vitamin D deficiency because of an age-related decline in cutaneous vitamin D synthesis [27,28,29]. According to the 2008 Korea National Health and Nutrition Examination Survey, the Korean people have a high prevalence of vitamin D insufficiency (47.3% in males, 64.5% in females) [30]. That study found that vitamin D insufficiency in Korean adults was associated with younger age, spring and winter seasons, living in an urban area, and having an indoor occupation. Even after adjusting for other confounders (e.g., occupation), being in a younger age group remained an independent predictor of vitamin D insufficiency. This finding may be related to behavioral factors, including indoor lifestyle, sunscreen use, and dietary habits. In agreement, the current study found an association between older age and higher serum vitamin D levels. However, some behavioral factors that were not accounted for in this study may have affected our findings.
Our study had several limitations. First, our relatively small sample size may not have been large enough to adequately power the study. Second, the control and DME groups were not fully matched in terms of clinical characteristic (e.g., serum Cr was higher in the DME group even though patients with Cr ≥ 2.0 mg/dL were excluded). Third, measurement errors may have been introduced into the obtained serum and AH vitamin D levels. Fourth, we suggested that AH vitamin D may represent the degree of organ ischemia; however, our data lack intraocular level of VEGF or other inflammatory cytokines related to DME. Last, our study did not account for dietary habits and restrictions that influence systemic vitamin D levels. However, vitamin D is mainly biosynthesized in humans with UV light exposure and only small amounts are added to the human body via dietary intake.
In conclusion, this is the first study to examine both serum and AH vitamin D levels in DME patients. Serum vitamin D levels were not significantly different between the DME and control groups. Additionally, there was no significant correlation between serum and AH levels of vitamin D. Interestingly, the DME group had a higher AH vitamin D level than the control group. Furthermore, DME subjects with a higher level of AH vitamin D had poorer clinical outcomes following intravitreal anti-VEGF injection (e.g., greater CMT 1 month after injection) and showed wider area of capillary non-perfusion in FA. These results suggest that localized vitamin D levels in the eye are independent of systemic vitamin D levels and that vitamin D measurements may be an indicator of an organ ischemia and DME severity. Further studies are needed to confirm our findings and clarify the role of vitamin D in the development and progression of DME.
References
Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R (2016) Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res 2016:2156273. https://doi.org/10.1155/2016/2156273
Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371
Lin Y, Ubels JL, Schotanus MP, Yin Z, Pintea V, Hammock BD, Watsky MA (2012) Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr Eye Res 37:871–878. https://doi.org/10.3109/02713683.2012.688235
Albert DM, Scheef EA, Wang S, Mehraein F, Darjatmoko SR, Sorenson CM, Sheibani N (2007) Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci 48:2327–2334. https://doi.org/10.1167/iovs.06-1210
Alcubierre N, Valls J, Rubinat E, Cao G, Esquerda A, Traveset A, Granado-Casas M, Jurjo C, Mauricio D (2015) Vitamin D deficiency is associated with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Res 2015:374178. https://doi.org/10.1155/2015/374178
Patrick PA, Visintainer PF, Shi Q, Weiss IA, Brand DA (2012) Vitamin D and retinopathy in adults with diabetes mellitus. Arch Ophthalmol 130:756–760. https://doi.org/10.1001/archophthalmol.2011.2749
He R, Shen J, Liu F, Zeng H, Li L, Yu H, Lu H, Lu F, Wu Q, Jia W (2014) Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet Med 31:1657–1664. https://doi.org/10.1111/dme.12581
Millen AE, Sahli MW, Nie J, LaMonte MJ, Lutsey PL, Klein BE, Mares JA, Meyers KJ, Andrews CA, Klein R (2016) Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc Diabetol 15:128. https://doi.org/10.1186/s12933-016-0434-1
Alam U, Amjad Y, Chan AW, Asghar O, Petropoulos IN, Malik RA (2016) Vitamin D deficiency is not associated with diabetic retinopathy or maculopathy. J Diabetes Res 2016:6156217. https://doi.org/10.1155/2016/6156217
Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA (2011) Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci 52:7359–7364. https://doi.org/10.1167/iovs.11-7605
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY (1993) The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 111:831–836
Otani T, Kishi S, Maruyama Y (1999) Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 127:688–693
Ichiyama Y, Sawada O, Mori T, Fujikawa M, Kawamura H, Ohji M (2016) The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol 254:1545–1551. https://doi.org/10.1007/s00417-015-3251-4
(1995) A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 102:1434–1444
Baudoin C, Maneschi F, Quentel G, Soubrane G, Hayes T, Jones G, Coscas G, Kohner EM (1983) Quantitative evaluation of fluorescein angiograms: microaneurysm counts. Diabetes 32(Suppl 2):8–13
Clarkson JG (1994) Central Vein Occlusion Study: photographic protocol and early natural history. Trans Am Ophthalmol Soc 92:203–213 discussion 213-205
Payne JF, Ray R, Watson DG, Delille C, Rimler E, Cleveland J, Lynn MJ, Tangpricha V, Srivastava SK (2012) Vitamin D insufficiency in diabetic retinopathy. Endocr Pract 18:185–193. https://doi.org/10.4158/ep11147.or
Bonakdaran S, Shoeibi N (2015) Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int J Ophthalmol 8:326–331. https://doi.org/10.3980/j.issn.2222-3959.2015.02.20
Choi HS (2013) Vitamin D status in Korea. Endocrinol Metab (Seoul) 28:12–16. https://doi.org/10.3803/EnM.2013.28.1.12
Cardus A, Panizo S, Encinas M, Dolcet X, Gallego C, Aldea M, Fernandez E, Valdivielso JM (2009) 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 204:85–89. https://doi.org/10.1016/j.atherosclerosis.2008.08.020
Cardus A, Parisi E, Gallego C, Aldea M, Fernandez E, Valdivielso JM (2006) 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int 69:1377–1384. https://doi.org/10.1038/sj.ki.5000304
Yamamoto T, Kozawa O, Tanabe K, Akamatsu S, Matsuno H, Dohi S, Hirose H, Uematsu T (2002) 1,25-dihydroxyvitamin D3 stimulates vascular endothelial growth factor release in aortic smooth muscle cells: role of p38 mitogen-activated protein kinase. Arch Biochem Biophys 398:1–6. https://doi.org/10.1006/abbi.2001.2632
Shen J, Durairaj C, Lin T, Liu Y, Burke J (2014) Ocular pharmacokinetics of intravitreally administered brimonidine and dexamethasone in animal models with and without blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci 55:1056–1066. https://doi.org/10.1167/iovs.13-13650
Jordan J, Ruiz-Moreno JM (2013) Advances in the understanding of retinal drug disposition and the role of blood-ocular barrier transporters. Expert Opin Drug Metab Toxicol 9:1181–1192. https://doi.org/10.1517/17425255.2013.796928
Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y (2014) Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res 127:104–116. https://doi.org/10.1016/j.exer.2014.05.004
Fauser S, Viebahn U, Muether PS (2015) Intraocular and systemic inflammation-related cytokines during one year of ranibizumab treatment for neovascular age-related macular degeneration. Acta Ophthalmol 93:734–738. https://doi.org/10.1111/aos.12770
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281. https://doi.org/10.1056/NEJMra070553
MacLaughlin J, Holick MF (1985) Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 76:1536–1538. https://doi.org/10.1172/jci112134
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820. https://doi.org/10.1007/s00198-009-0954-6
Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK (2011) Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 96:643–651. https://doi.org/10.1210/jc.2010-2133
Funding
This research was supported by Hallym University Research Fund 2016 (HURF-2016-59).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, K.L., Moon, S.Y., Noh, HM. et al. Serum and aqueous humor vitamin D levels in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 257, 1191–1198 (2019). https://doi.org/10.1007/s00417-019-04305-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04305-2