Abstract
Purpose
The aim of this study was to evaluate subfoveal choroidal thickness (SFCT) as a marker of outcome in real-world treatment of diabetic macular edema (DME) and to correlate it with choroidal thicknesses (CT) collected around the fovea.
Methods
Prospective interventional case series included a total of 126 eyes from 126 patients with recently diagnosed DME treated with a 3-monthly loading dose of ranibizumab or aflibercept and PRN thereafter until 24 months (M). CT was manually measured in the central 3500 μm area, subfoveally (SFCT), at 1750 μm right and left from the center in the horizontal plane and at 1750 μm up and down from the center in the vertical plane, by OCT. Anatomic (10% decrease in central retinal thickness) and functional (gain ≥ 5 letters) responses were assessed using univariate and multivariate analyses. The areas under ROC curves were used to assess whether baseline SFCT was a predictor of outcome.
Results
CT significantly decreased in all follow-ups (3 months after the 3 injections’ loading dose (3M), 6 months (6M), 12 months (12M), 18 months (18M), 24 months (24M)). SFCT and other CT parameters are correlated. SFCT decrease from baseline was related with treatment (p = 0.003 to p < 0.001) but not with anatomic (3M, p = 0.858; 6M p = 0.762) or functional response (3M, p = 0.746; 6M, p = 0.156). SFCT was not found to be predictive of anatomic (AUC = 0.575, p = 0.172) or functional (AUC = 0.515, p = 0.779) outcome.
Conclusions
SFCT is a reliable marker of choroidal thickness. Baseline SFCT decreased with anti-VEGF treatment but did not predict DME outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of diabetic macular edema (DME) shifted from laser to anti-VEGF agents [1]. Most patients respond well to therapy while others do not so well [2]. Several attempts have been made to find markers of prognosis or predictors of treatment response in DME. The choroidal thickness (CT) or the subfoveal choroidal thickness (SFCT) were suggested as predictors of treatment response in DME, but the use of the SFCT as a marker of the CT needs further evidence [2]. A diabetic choroidopathy has been demonstrated [3]; therefore, the choroid has been looked out to search for markers of the pathogenesis of diabetic retinopathy (DR) or of the response to treatment. The availability of the OCT was a step forward in order to easily assess and monitor CT [4]. Despite growing evidence demonstrating alterations of the SFCT in DME and with DME treatment [5], its use as a marker of prognosis and of response to anti-VEGF therapy has not been fully elucidated. Previous studies used different approaches that originated contradictory data [4].
The present study was designed to investigate whether baseline SFCT is a predictor of anatomic or functional response to anti-VEGF therapy and whether measuring the choroid at different locations from the center correlates with SFCT.
Methods
In a prospective interventional case series, type 2 diabetic patients with non-proliferative diabetic retinopathy (NPDR), diagnosed with recent onset DME in at least one eye, naïve to intravitreal treatment, were included after approval from the Ethical Committee of the Leiria Hospital. Informed consent was given before inclusion. The study adhered to the tenets of the Declaration of Helsinki and to the standards of Good Clinical and Scientific Practice of the Faculty of Medicine of the University of Coimbra.
Diagnostic criteria for DR and DME were based on past ophthalmic history and complete ophthalmic evaluation, including a dilated fundus examination, fundus photography, OCT imaging, and fluorescein angiography in selected cases.
Data included patient age, sex, blood pressure, duration of diabetes, glycated hemoglobin level (Hb A1c), best-corrected visual acuity (BCVA), biomicroscopic examination, laser therapy, and length of follow-up.
DME was considered when clinical significant macular edema (CSME) involving the central macula (CI-CSME) [6] or a central (1 mm central subfield thickness in the OCT-modified ETDRS grid) retinal thickness (CRT) ≥ 300 μm were present. Eyes were included when baseline BCVA ranged from 24 to 78 ETDRS letters (Snellen equivalent 20/320–20/32, logMAR 1.22–0.14). Whenever DME was bilateral at presentation, the right eye was included in patients whose year of birth was an even number and the left eye was included when the year of birth was an odd number [7]. Patients were excluded if they had any other treatments related to their NPDR, except for focal laser for more than 6 months. Individuals were excluded if they had any ocular diseases aside from DME in the treated eye. Eyes with a myopic refractive error of greater than 4 diopters (D) were also excluded [4, 8].
Eyes were treated with a 3-monthly loading dose of ranibizumab or aflibercept and on a pro re nata (PRN) regimen thereafter. Eyes that developed proliferative diabetic retinopathy and needed panretinal photocoagulation, eyes that were rescued with focal/grid laser, and eyes that were switched to intravitreal steroids were discontinued from follow-up. Eyes were allowed to be switched from ranibizumab to aflibercept.
Data from BCVA using the ETDRS standardized chart and SD-OCT were collected in every visit (baseline, 3 months after the 3 injections’ loading dose (3M), 6 months (6M), 12 months (12M), 18 months (18M), and 24 months (24 M)). The top score allowed in the ETDRS chart was 85L (20/20).
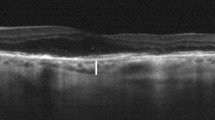
Enhanced depth imaging (EDI) optical coherence tomography mode was selected (Spectralis; Heidelberg Engineering, Heidelberg, Germany) and a 6-mm × 6-mm macular cube scan was performed using the high-resolution (HR) posterior pole scanning mode. HR star scan mode (6 scans each made up of 1536 A scans, 30° apart from each other cutting through the fovea) and HR map mode scan (comprising 61 horizontal B-scans, 120 μm apart from each other, each made up of 1536 A-scans) (1536 × 1536 pixels, lateral resolution of 6 μm/pixel) were acquired. A signal strength greater than 20 was required for all scans. CT was evaluated manually after plotting a 7.2 mm-ETDRS grid centered at the fovea and CRT was evaluated automatically using a 6-mm OCT-modified ETDRS grid. The fovea was always checked and the center of the OCT star mode was re-centered at the fovea whenever needed before performing the scans and thereafter. CT was manually measured using the digital caliper tool in the Heidelberg Eye Explorer software, from the hyperreflective line of the Bruch’s membrane to the hyperreflective line of the choroidoscleral interface, in the central 3500-μm area, subfoveally and at a distance of 1750 μm right and left from the center, in the horizontal plane, and 1750 μm up and down from the center in the vertical plane. The CT area underneath the 3500-μm central macula (square inches) in the plane defined by the horizontal line scan encompassing the fovea was calculated manually from the RPE hiperreflective line to the choroidoscleral junction by the Image J software (version 1.48, National Institutes of Health, USA) (Fig. 1).
Choroidal thickness manually measured in the central 3500-μm area underneath the RPE line, subfoveally (SFCT) and at 1750 μm nasal (CT1750n) and temporal (CT1750t) from the center, in the plane defined by the horizontal line scan encompassing the fovea (Image J software, version 1.48, National Institutes of Health, USA). A similar procedure was done in the plane defined by the vertical line encompassing the fovea to obtain the superior (CT1750s) and inferior (CT1750i) choroidal thicknesses
Two independent raters (AC, do Carmo) measured the scans masked to the subject’s outcome in a prospective way, and final measures were the mean of the two scored for each location and follow-up period. Both raters retrospectively reviewed all scans masked to subjects’ outcome at the end of the study and when the gap from one another was greater, final measures were reached by consensus. All scans were performed from 9.00 a.m. to 1.00 p.m. To evaluate whether baseline SFCT might predict clinically relevant response to treatment, we defined anatomic responders as eyes having a 10% decrease in baseline CRT (≥ 300 μm). Eyes with BCVA gains of ≥ 5 letters from baseline at 3M were defined as early functional responders, while eyes with BCVA gains of ≥ 5 letters from baseline at 6M only were defined as late functional responders. Only the eyes followed for 6M were considered for these calculations, n = 122 eyes.
Statistical analysis
Nominal data were described by absolute and relative frequencies. Quantitative data were described by using the mean, standard deviation, median, minimum, and maximum in the sample characterization. Median, minimum, and maximum were omitted in the tables for convenience. Quantitative variables were assessed for normality with the Shapiro Wilk test. Comparisons between two measures, in different time points, of the same variable, were performed resorting to paired sample t test or Wilcoxon test, taking normality requirements into account. Comparisons of quantitative variables between two groups were performed with t-Student or Mann-Whitney tests, as applicable. Intra-class correlations (ICC) were used to compare CT measurements between raters at all locations and time points. ROC analyses were undertaken to assess how accurately SFCT baseline values could be used to predict anatomic or functional response. Correlations between quantitative variables were assessed by computing Pearson or Spearman correlation coefficients, depending on whether normality requirements were met or not. The statistical analyses were performed on IBM SPSS Statistics 24 and on R 3.3.2. The level of significance adopted was 0.05.
Results
From June 2014 to November 2017, a total of 126 eyes from 126 patients were prospectively included. A total of 113 eyes were treated with a loading dose of 0.5 mg ranibizumab, 13 eyes were treated with a loading dose of 2 mg aflibercept, and 18 were switched from ranibizumab to aflibercept. Baseline demographic and ocular characteristics are outlined in Supplementary Table 1. A total of 126 eyes were followed by 3 months, 122 eyes were followed by 6 months, 60 eyes were followed by 12 months, 29 eyes were followed by 18 months, and 26 eyes were followed by 24 months.
The mean number of injections given was 3.0 at 3M, 4.6 ± 1.3 (3–7, median 4) at 6M, 5.3 ± 2.0 (3–10, median 6) at 12M, 7.6 ± 2.5 (3–12, median 8) at 18M, and 8.0 ± 4.0 (3–15, median 7.5) at 24M. The mean baseline BCVA improved significantly, the mean baseline CRT and the mean baseline SFCT decreased significantly (Table 1 and Fig. 2). The distribution of baseline SFCT showed a wide range of variability although it decreased by 25.45 μm per each decade of life (Fig. 3). The mean CT decreased at all locations in all follow-up intervals (Supplementary Fig. 1). The percent decrease in the SFCT and in the other CT parameters were correlated in all follow-up intervals, more prominently between the SFCT and CT areas until 6M (Fig. 4). ICC between raters was 0.98, ranging from 0.94 to 0.98, according to the locations. The mean inter-observer difference was 6.8 μm.
Evolution of mean central retinal thickness (CRT), mean subfoveal choroidal thickness (SFCT), and mean best-corrected visual acuity (BCVA) scored in ETDRS letters collected from ETDRS charts with time in eyes with DME under anti-VEGF treatment. Note that the evolution of the SFCT curve does not have the same profile as those of the CRT and BCVA. Until 6M when the stratification by outcome was done, the slopes of the BCVA curve and that of the CRT curve are also different, expressing a poor correlation between anatomic and functional outcome as depicted in Tables 2 and 3
Scatterplot depicting the negative weak correlation between age and baseline SFCT (μm), r = −0.328, p < 0.001. Though a wide inter-individual variability can be observed, the average decrease of SFCT per decade was seen to be 25.45 μm. Variability in SFCT may introduce bias when dealing with small samples
Radar chart displaying the percentage of participants with decrease in the CT parameters at the different time points. SFCT subfoveal choroidal thickness (dotted black line); CT temp choroidal thickness 1750 μm temporal to the fovea, in the plane defined by the horizontal line scan encompassing the fovea (orange line); CT nasal same as the previous but 1750 μm nasal to the fovea (purple line); CT sup choroidal thickness 1750 μm superior to the fovea, in the plane defined by the vertical line scan encompassing the fovea (red line); CT inf same as previous but 1750 μm inferior to the fovea (green line); CT area area of choroidal thickness from 1750 μm nasal to 1750 μm temporal to the fovea, in the plane defined by the horizontal line scan encompassing the fovea (blue line) (Image J software, version 1.48, National Institutes of Health, USA). 3M 3-month endpoint, 6M 6-month endpoint, 12M 12-month endpoint, 18M 18-month endpoint, 24M 24-month endpoint. Results expressed as percentage of eyes that decreased at each endpoint considered. SFCT shows basically an identical behavior along the timeline with similar percentages of decrease in all follow-up intervals. However, data from area measurements show that the percentage of decrease is lower at 12M and 18M
From the 126 eyes enrolled, 64 eyes (50.8%) had past history of macular photocoagulation, but past laser history did not significantly affect the CT parameters at any follow-up interval (Supplementary Fig. 2). A total of 98 eyes from the 119 eyes with baseline CRT ≥ 300 μm decreased CRT 10% from baseline (82.4%), and were considered anatomic responders. A total of 74 eyes (60.7%) at 3M and an additional 22 eyes (18.0%) at 6M were functional responders (96 eyes out of 122, 78.7%). SFCT was not significantly different when comparing either anatomic responders versus nonresponders (Table 2) or functional responders versus nonresponders (Table 3). The ROC analyses showed that baseline SFCT was not found to be a statistically significant predictor of being an anatomic responder, (area under the curve, AUC = 0.575, p = 0.172) nor of being a functional responder (sorting out early functional responders from nonresponders) (AUC = 0.515, p = 0.779). Since baseline SFCT was not found to be a marker of outcome at 6M but changed more prominently in the first 3 months, a new ROC curve was calculated to correlate the early SFCT decrease and early functional outcome (Fig. 5). The baseline SFCT was not found to be a marker of early outcome either (AUC = 0.529, p = 0.589). This absence of a significant difference between SFCT decreases from baseline to 3M between functional responders at 3M and nonresponders is depicted in a boxplot (Fig. 6).
ROC curve analysis comparing the decrease in SFCT from baseline to 3M with a ≥ 5 L gain at EM (early functional response). Dif T0_T1 is the difference between mean baseline SFCT and mean 3M SFCT; Sig is the P value. The Area (area under the curve, AUC) of 0.529 means that the change in baseline SFCT at 3M does not predict visual gain
Boxplot or figure of extremes and quartiles of the difference found in SFCT from baseline to 3M in functional responders at 3M (gain of 5 L or more) and nonresponders. T0-T1 is the difference between baseline SFCT and 3M SFCT; NR + LR is the group of nonresponders at 3M (nonresponders at 6M and late functional responders); ER is the group of responders at 3M (early functional responders). The distribution of the baseline—3M SFCT of either group mostly overlaps, meaning that the decrease in the SFCT at 3M is not a useful tool to mark functional gain
Discussion
The aim of this study was to evaluate whether the mean baseline SFCT is a predictor of outcome in DME. Additional outcomes were to study how the CT changes with anti-VEGF agents and to correlate SFCT with other CT data (collected focally around the fovea or from an area underneath the fovea).
As previously reported, the choroid thinned under anti-VEGF treatment at variable degrees with time [4, 9]. The mean SFCT and the mean CRT decreased while the mean BCVA increased. Therefore, it is mathematically possible to find a correlation between the evolution of the mean SFCT and that of the mean CRT and the mean BCVA [5]. However, when the eyes are stratified by outcome as it was done in this work, that association is actually accidental, reflecting not a prognostic value but only the effectiveness of the anti-VEGF therapy.
A similar behavior was observed between SFCT, which is the CT marker most commonly used, and the other CT data collected at other locations as used in this study (Supplementary Fig. 1 and Fig. 4).
There were previous retrospective studies pointing out in different directions, while considering the value of SFCT as a marker of outcome [10,11,12]. All had considerable drawbacks: small samples, inclusion of both type 1 and type 2 diabetic patients, eyes with proliferative retinopathy included, short follow-up [10,11,12] the use of Snellen charts to assess BCVA, an uncommon definition of anatomical responder, and double organ bias [7, 12]. Snellen charts are not logarithmic and do not evaluate BCVA accurately bellow 20/50, and the choroid is actually thicker in type 1 diabetics [4].
About half of the eyes included had previous focal laser treatment, but focal laser does not seem to change CT [13]. In the population included, previous history of laser did not change the parameter profile with time and the differences found with laser-naives were not significantly different (Supplementary Fig. 2). As expected, the baseline SFCT decreased with age [14]. Nevertheless, the distribution was widespread and there were several outliers, indicating that small samples or double organ inclusion may mislead data when studying the choroid in cross sectional studies (Fig. 3).
A recovery of the mean BCVA and mean CRT that fluctuates over time was observed, as it is the hallmark of the reactive regimens such as PRN (in this study 4.6 ± 1.3 injections at 6M and 5.3 ± 2.0 injections at 12M). However, the evolution of SFCT did not mimic that profile (Fig. 2). The thickness of the retina (CRT) decreases more sharply than the thickness of the choroid (SFCT) (Fig. 2). In the retina, the edema is mostly composed of liquid material, at least in its early phases, and may increase the CRT up to two- or threefold. On the other hand, the choroid is mostly composed of medium and large vessels (Sattler and Haller layers), the choriocapillaris being less than 10% of the total choroid thickness. Therefore, the decrease in the thickness of the choroid mediated by the anti-VEGF use is probably due to liquid reabsorption, mainly from the stroma and from the lamellae of the suprachoroidal space [4]. Anti-VEGF agents act upon blood vessel permeability, decreasing retinal edema, by improving inner blood retinal barrier and probably by restoring outer retinal barrier homeostasis. RPE under homeostasis produces VEGF to assure the choriocapillaris fenestrations, needed to establish the routes for RPE and outer retina nutrition and for water clearance from the retina [4]. One may speculate that in diabetes, an overproduction of VEGF by a RPE under metabolic stress may lead to an increased permeability of the outer blood retinal barrier, contributing to DME, sometimes with subfoveal neuroretinal detachment (SND) in the acute phases. These changes can be reversed by the use of anti-VEGF agents. As the choroid thins under anti-VEGF administration, a link between the decrease in CRT, visual restoration, and a decrease in SFCT seemed unquestionable [5]. Indeed, the idea that the thinning of the choroid under the action of anti-VEGF may predict the homeostasis of the retina, the water clearance from the retina, resolution of the edema, and visual recovery, is appealing. Unfortunately, the thickening of the choroid in diabetes is controversial [4]. More importantly, DME behaves more like a group of diseases than as a single well-defined disease. As we pointed out in a previous review [4], and as stated by another group [15], the presence of SND probably signals an acute form of DME with outer blood retinal barrier dysfunction, very prone to be a good anatomic responder. It is possible that in such cases, the thinning of the choroid mirrors somehow the restoration of the outer blood retinal barrier homeostasis. It is doubtful, though, that this applies to all forms of DME, most notably those forms with persistent cysts or retinal thickening where there is only limited or poor response to anti-VEGFs and nevertheless the choroid thins. Furthermore, it is controversial that the SFCT decrease would differentiate those eyes that would recover vision or retinal edema from those that would not. When stratified by outcome, the mean SFCT decrease seems to be a side effect of the anti-VEGFs only. Nevertheless, data from area measurements seem to indicate that the percentage of the choroidal thinning may be an indicator of undertreatment in non-fixed regimens (Fig. 4).
In Table 3, we see that there is a poor correlation of baseline CRT and functional outcome. The decrease in the CRT is not significantly different in functional responders and nonresponders. This poor correlation between anatomic response and functional response has been previously reported [16].
HR scan mode was used as it gives a higher quality image and allows a good visualization of the choroidoscleral border. Unlike the automatic segmentation modes that only include the choroidal vessels [17], this method includes the suprachoroidal space, most prone to change, and gives higher scores for the CT, even when dealing with type 2 diabetics only. Automatic segmentation was not used because it is not available in the EDI mode of the software version 6.9.4.0 we used. The central 1 mm cube automatic acquired volume for the choroid is not available either, even while using the normal non-EDI mode. However, as long as automatic segmentation does not overpass its most relevant shortcoming, that is, the need for manual correction of the border lines, its relevance is questionable.
The results indicating that the mean baseline SFCT does not predict outcome in DME do not exclude a role of the choroid in the pathogenesis of DME or DR, for which there is plenty of evidence [3, 4]..
The biggest limitation of this study is not being a randomized controlled trial or involving multiple centers and the small number of eyes in the longer follow-ups.
The strengths of this study are related to the prospective profile, the stratification of the eyes treated by outcome and the correlation of the SFCT with outcome, the inclusion of one eye per patient only [18], the inclusion of type 2 diabetics only, the use of high-resolution scans (HR mode), the multiple locations for CT evaluation, and the use of the ETDRS scale for evaluation of BCVA.
In conclusion, the choroid thins with anti-VEGF therapy but SFCT is not a predictor of outcome in DME. The amount of the choroid thinning may be an indicator of undertreatment in non-fixed regimens.
References
Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL III, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117:1064–1077.e35. https://doi.org/10.1016/j.ophtha.2010.02.031
Ashraf M, Souka A, Adelman R (2016) Predicting outcomes to anti-vascular endothelial growth factor (VEGF) therapy in diabetic macular oedema: a review of the literature. Br J Ophthalmol 100:1596–1604. https://doi.org/10.1136/bjophthalmol-2016-308388
Lutty GA (2017) Diabetic choroidopathy. Vis Res 139:161–167. https://doi.org/10.1016/j.visres.2017.04.011
Campos A, Campos EJ, Martins J, Ambrosio AF, Silva R (2017) Viewing the choroid: where we stand, challenges and contradictions in diabetic retinopathy and diabetic macular oedema. Acta Ophthalmol 95:446–459. https://doi.org/10.1111/aos.13210
Nourinia R, Ahmadieh H, Nekoei E, Malekifar P, Tofighi Z (2018) Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina 38:970–975. https://doi.org/10.1097/IAE.0000000000001645
Early Treatment Diabetic Retinopathy Study research group (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol 103:1796–1806
Esen F, Kostek M, Emekli AS, Eraslan M (2016) Double-organ bias in published randomized controlled trials of glaucoma. J Glaucoma 25:520–522. https://doi.org/10.1097/IJG.0000000000000369
Meng W, Butterworth J, Malecaze F, Calvas P (2011) Axial length of myopia: a review of current research. Ophthalmologica 225:127–134. https://doi.org/10.1159/000317072
Lains I, Figueira J, Santos AR, Baltar A, Costa M, Nunes S, Farinha C, Pinto R, Henriques J, Silva R (2014) Choroidal thickness in diabetic retinopathy: the influence of antiangiogenic therapy. Retina 34:1199–1207. https://doi.org/10.1097/IAE.0000000000000053
Lee SH, Kim J, Chung H, Kim HC (2014) Changes of choroidal thickness after treatment for diabetic retinopathy. Curr Eye Res 39:736–744. https://doi.org/10.3109/02713683.2013.867064
Yiu G, Pecen P, Sarin N, Chiu SJ, Farsiu S, Mruthyunjaya P, Toth CA (2014) Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol 132:174–181. https://doi.org/10.1001/jamaophthalmol.2013.7288
Rayess N, Rahimy E, Ying GS, Bagheri N, Ho AC, Regillo CD, Vander JF, Hsu J (2015) Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol 159(85–91):e81–e83. https://doi.org/10.1016/j.ajo.2014.09.033
Adhi M, Alwassia AA, Duker JS (2013) Analysis of choroidal thickness in eyes treated with focal laser photocoagulation using SD-OCT. Can J Ophthalmol 48:535–538. https://doi.org/10.1016/j.jcjo.2013.05.010
Margolis R, Spaide RF (2009) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147:811–815. https://doi.org/10.1016/j.ajo.2008.12.008
Vujosevic S, Torresin T, Berton M, Bini S, Convento E, Midena E (2017) Diabetic macular edema with and without subfoveal neuroretinal detachment: two different morphological and functional entities. Am J Ophthalmol 181:149–155. https://doi.org/10.1016/j.ajo.2017.06.026
Diabetic Retinopathy Clinical Research, N, Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J, Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH (2007) Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 114:525–536. https://doi.org/10.1016/j.ophtha.2006.06.05217
Gerendas BS, Waldstein SM, Simader C, Deak G, Hajnajeeb B, Zhang L, Bogunovic H, Abramoff MD, Kundi M, Sonka M, Schmidt-Erfurth U (2014) Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am J Ophthalmol 158:1039–1048. https://doi.org/10.1016/j.ajo.2014.08.001
Armstrong RA (2013) Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt 33:7–14. https://doi.org/10.1111/n12009
Funding
This work is funded by the Portuguese Foundation for Science and Technology (Fundação para a Ciência e a Tecnologia, FCT), Strategic Project (UID/NEU/04539/2013), and COMPETE-FEDER (POCI-01-0145-FEDER-007440). EJC was financially supported by the FCT Postdoctoral Fellowship SFRH/BPD/93672/2013, through the European Union and National funds and co-funded by Human Capital Operating Programme (Programa Operacional do Capital Humano, POCH). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
AC: acquisition, analysis, interpretation of data, and drafting of the manuscript. EC: interpretation of data, administrative, technical, editorial, and submission support. Do Carmo: data collection, scoring, and statistical analysis. MP: mathematics and statistical analysis. JS: critical revision. AA: revision of the manuscript and interpretation of data. RS: concept, design, supervision, and revision.
Corresponding author
Ethics declarations
Conflict of interest
Rufino Silva is a member of the Advisory Boards for Bayer, Alcon, Alimera, Allergan, Novartis, and Thea. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 90 kb)
Rights and permissions
About this article
Cite this article
Campos, A., Campos, E.J., do Carmo, A. et al. Choroidal thickness changes stratified by outcome in real-world treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 256, 1857–1865 (2018). https://doi.org/10.1007/s00417-018-4072-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4072-z