Abstract
Purpose
Our purpose was to investigate the impact of lens status of corneal donors on the two-year course and clinical outcome of Descemet membrane endothelial keratoplasty (DMEK).
Methods
In 181 DMEK surgeries, 136 phakic and 45 pseudophakic donor corneas were grafted. In this retrospective audit we compared the lens status of corneal donors regarding the outcome measures best spectacle-corrected visual acuity (BSCVA), central corneal thickness (CCT), and endothelial cell density (ECD) at 1, 3, 6, 12, and 24 months, as well as intra- and postoperative complication rates and graft detachment rates requiring re-bubbling.
Results
Comparing the use of phakic and pseudophakic donor tissue in DMEK surgery, BSCVA results revealed no significant differences during the two-year course (p ≥ 0.087). CCT showed significantly lower values at 1 month (553.8 ± 56 vs. 625.2 ± 119 μm; p < 0.001) and 6 months follow-up (530.6 ± 49.9 vs. 557.3 ± 47 μm; p = 0.026) for phakic donor tissue recipients, but were comparable later (p ≥ 0.173). ECD values were statistically higher 6 (1915 ± 390 vs. 1565 ± 420 cells/mm2; p < 0.001) and 24 months postoperatively (1772 ± 384 vs. 1375 ± 377 cells/mm2; p = 0.030) in phakic donor tissue recipients. Mixed regression analyses demonstrated a significant association between ECD results and donor lens status (p = 0.029) and donor ECD (p = 0.028), but donor age did not show significant influence (p = 0.241).
Conclusion
ECD is higher in phakic corneal donors and appears to remain at a higher level during the course resulting in initially faster reduction of corneal edema compared to pseudophakic DMEK grafts. Nevertheless, pseudophakic transplants with high ECD seem to produce comparable functional results in recipients after a two year course.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endothelial pathologies, such as Fuchs endothelial dystrophy (FED) or pseudophakic bullous keratopathy (PBK) can successfully be treated by Descemet's stripping automated endothelial keratoplasty (DSAEK) or Descemet membrane endothelial keratoplasty (DMEK) [1,2,3]. Since the graft prepared for DMEK comprises corneal endothelium and Descemet's membrane only, the technique is known to be more challenging, including donor preparation, implantation and intracameral unfolding of the graft [4, 5]. Nevertheless, DMEK has achieved growing attention and has already replaced DSAEK as the standard procedure for posterior lamellar keratoplasty in some areas in Europe and within the United States of America (USA) [4, 6, 7].
The choice of the appropriate corneal transplant must be well-considered in advance to improve the success rate of DMEK preparation and surgery [4, 5, 7,8,9]. High endothelial cell density and the absence of corneal scars are desirable. Regarding donor age, most surgeons prefer donor corneas from older donors, since free floating DMEK grafts spontaneously form a roll, which is tighter in young donors, and, therefore, unfolding is suggested to be more challenging [5, 10, 11]. Especially due to donor deficiency, however, the demands on the perfect corneal transplant for posterior lamellar keratoplasty can often not be placed too high. It is of particular importance to further define important basic requirements of donor tissue for DMEK surgery. Due to a general increase in life expectancy, mean age of corneal donors increases steadily. Consecutively, mean endothelial cell count of a cornea donor button is likely to decrease on average and the incidence of corneal scars, especially due to previously performed cataract surgery rises. From our own experience, corneal surgeons prefer phakic corneal donors for DMEK in order to avoid preparation complications due to potential corneal scars and adhesions following cataract surgery, but pseudophakic donors are not excluded. However, there is insufficient evidence for a possible relation between donor lens status and outcome parameters in DMEK surgery. We therefore investigated whether pseudophakic corneal donors may influence the clinical outcome and intra- or postoperative complication rates of DMEK surgery.
Methods
In this retrospective study, we reviewed clinical records of 1,087 consecutively performed DMEKs between 1st July 2011 and 30th November 2015 at the Department of Ophthalmology, University of Cologne, Cologne, Germany. Furthermore, the database of the local eye bank was reviewed for corresponding corneal donor tissue parameters.
The study was approved by the local Institutional Review Board (15-301) and was conducted in adherence to the tenets of the Declaration of Helsinki.
Collection of donor graft data
Prior to donor graft distribution and transplantation, the following data were assessed: age (years) and gender of donor (male/female), lens status (phakic, pseudophakic or unknown), endothelial cell density (cells/mm2), and preservation time until grafting (days).
Clinical information and collected data of recipients
Prior to surgery, 1, 3, 6, 12, and 24 months postoperatively standardized eye examinations, including best spectacle-corrected visual acuity (BSCVA), intraocular pressure (IOP), central corneal thickness (CCT; Pentacam HR, Oculus GmbH, Wetzlar, Germany), endothelial cell density (ECD; Tomey EM-3000 Specular Microscope; Erlangen, Germany), slit lamp biomicroscopy, and funduscopy were performed. Intraoperative rates of preparation failures and rates of postoperative Descemet's membrane detachments requiring air reinjection into the anterior chamber (re-bubbling) were documented.
Graft failure was defined as corneal edema and haze due to endothelial decompensation. In case of primary graft failure the cornea failed to clear up after surgery in the immediate postoperative period. Secondary graft failure was defined as endothelial decompensation over the time due to non-immunologic or immunologic reasons, i.e., graft-rejection or immune reaction.
Inclusion and exclusion criteria
All DMEK surgeries between 1st July 2011 and 30th November 2015 were reviewed. Only cases in which lens status of donor buttons was known and classified as "pseudophakic" or "phakic" were included. Donor lens status was provided by the supplying cornea banks. Furthermore, availability of corresponding donor tissue parameters (endothelial cell density, cultivation period, donor age, and gender) as well as preoperative and postoperative clinical data at 1, 3, 6, 12, and 24 months were considered as prerequisite for inclusion into the study. DMEK surgery alone in phakic or pseudophakic recipient eyes, as well as triple procedures (DMEK combined with phacoemulsification and posterior chamber lens implantation for co-existent cataract) were included.
Out of 1,087 cases, 900 cases had to be excluded due to unknown lens status of donor corneas. A further six cases were excluded due to the following reasons: complicated anterior segment pathologies (ICE Syndrome in n=1, buphthalmus in n=1), history of previously performed keratoplasty (n=2), Marfan syndrome (n=1), and history of perforating trauma (n=1).
For statistical evaluation of donor lens status related to clinical outcome after DMEK, all included datasets were divided into two groups: (1) Pseudophakic corneal donors and (2) phakic corneal donors.
For statistical analysis of BSCVA, eyes with extracorneal visual limitations such as age-related macular degeneration, amblyopia or advanced stage of glaucoma, were excluded.
Surgical technique and postoperative treatment
DMEK was performed under general anesthesia at the Department of Ophthalmology, University of Cologne.
DMEK surgery was performed by two experienced surgeons (CC, BOB) in a standardized fashion with some variations to methods described previously [12]. The DMEK graft was prepared by peeling of Descemet's membrane (DM) from the donor corneoscleral rim using forceps followed by trepanation prior to transplantation. For this purpose the edge of the DM was dissolved and the entire circumference of the peripheral DM edge was examined for existing tears, which then were transferred into curves. In case of focal adhesions or scarring areas, eccentric preparation and trepanation of the DM was preferably performed. Preparation failure was defined as larger tissue tearing during preparation, which centrally crossed the edges of the transplant despite eccentric trepanation. In case of a significant preparation failure, it was up to the surgeon whether the graft could be used or was rejected.
In eyes showing co-existent cataract formation, a combined procedure (triple DMEK) with phacoemulsification and posterior chamber lens implantation was performed directly before DMEK. Following descemetorhexis, a cataract-shooter was used to insert the graft into the anterior chamber. Then unfolding of the graft lamella was performed using a no-touch technique. When needed an air bubble was used to move the graft into the correct position. After centering and unfolding of the graft the anterior chamber was filled completely with air to secure the graft at the recipient's posterior corneal surface.
Prior to surgery, a neodymium-doped yttrium aluminium garnet (Nd YAG) iridotomy at 6 o’clock was performed to avoid postoperative angle block with intraocular pressure decompensation (Urrets-Zavalia syndrome). In cases in which the laser iridotomy was inadequate in size due to poor visualisation of the iris, the iridotomy was surgically extended during DMEK surgery using a cutter.
After DMEK surgery all patients were hospitalized for approximately one week and received standardized topical treatment in form of topical prednisolone acetate 1% in tapering doses over 12 months (first postoperative weeks 5 times a day tapered to one time a day after 4 months) and topical antibiotic eye drops for 1 to 2 weeks as well as lubricant eyes drops (five times a day) as long as needed. Pilocarpine 1% eye drops were applied three times a day as long as the anterior chamber was filled with air covering the pupil's bottom margin. Patients were instructed to keep a strict supine position postoperatively, at least for three days.
Statistical analyses
Descriptive data for donor tissue and recipients were collected and analyzed by SPSS (version 22.0 for windows; SPSS, Inc, Chicago, IL). The BSCVA was converted to the logarithm of Minimum Angle of Resolution (logMAR). For statistical significance testing for interval scale parameters, Student’s t-test (CCT, and BSCVA) as well as the Mann-Whitney U test (ECD, and frequency of re-bubbling) depending on normal distribution, and chi-Square test was used for ordinal scale parameters (overall re-bubbling rate, complication rates) for the phakic and pseudophakic donor group. Changes in clinical outcome parameters during the course were analyzed by the Wilcoxon signed-rank test. Correlations were calculated using the Pearson correlation coefficient. Mixed regression analyses were conducted to examine the relationship between the independent variables donor ECD, donor age, lens status, and time and the dependent variables BSCVA, CCT, and ECD, respectively, using the programming language RV 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria). The level of significance was defined as p < 0.05. Holm-Bonferroni procedure was performed to correct p values for multiple testing.
Results
DMEK surgery was performed in 181 eyes of 179 patients (mean age 69.1 ± 10.8 years; female-to-male ratio: 1.5:1) using 181 donor buttons, thereof 45 pseudophakic donor corneas (24.9%), and 136 phakic donor corneas (65.1%). Demographic data of DMEK recipient eyes and corresponding donor tissue details are summarized in Table 1.
Donor tissue parameters
Pseudophakic donors (55.6% females) were mean aged 71.6 ± 8.8 years and showed mean ECD of 2645 ± 200 cells/mm2. Phakic donors (44.9% females) were mean aged 63.0 ± 13.4 years and showed 2936 ± 262 endothelial cells/mm2 (Table 1).
Age and endothelial cell density differed statistically significant between both groups (p < 0.001).
Clinical outcome parameters
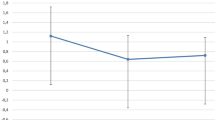
Clinical outcome parameters during the course are condensed in Fig. 1.
a Course of best spectacle-corrected visual acuity after Descemet Membrane Endothelial Keratoplasty. b: Course of central corneal thickness after Descemet Membrane Endothelial Keratoplasty. c: Course of endothelial cell density after Descemet Membrane Endothelial Keratoplasty. Clinical course of outcome parameters (a: best-spectacle corrected visual acuity; b: central corneal thickness; c: endothelial cell density) are presented for phakic and pseudophakic donor groups separately for several follow-up time points during 2 years of follow-up. Mean values and 95% confidence intervals are shown
Pseudophakic donor tissue
Endothelial cell density declined by 38% between presurgical level and one month follow-up for pseudophakic donor corneas (p < 0.001). Further ECD reduction during the course was statistically insiginificant (p ≥ 0.208).
Changes in BSCVA comparing pre- and postoperative results showed significant improvement for 6, 12 and 24 months follow-up (p ≤ 0.008). An early increase of BSCVA after surgery was statistically significant for the follow-up visit at month 3 (p = 0.019).
CCT was statistically significantly reduced at months 3, 6, and 12 postoperatively compared to preoperative values (p < 0.001).
Phakic donor tissue
Endothelial cell density declined by 39% between presurgical level and one month follow-up (p < 0.001) and recipients of phakic donor lamellae showed further significant ECD reduction between 12 and 24 months follow-up (p = 0.014).
Changes in BSCVA comparing pre- and postoperative results showed significant improvement for all follow-up visits for the phakic donor group (p < 0.001) with significant increase during the course for 1, 3, 6 and 12 months follow-up (p ≤ 0.036).
CCT was statistically significantly reduced for all follow-up time points compared to preoperative values (p ≤ 0.018) with significant changes during the course in between the first 3 months period (p ≤ 0.020).
Complication rate
In both donor groups all released donor buttons (100%) could be used for DMEK surgery; there was no tissue loss due to preparation failure or intraoperative complications.
Pseudophakic donor tissue
In four cases (8.9%) larger tears occurred intraoperatively due to adhesions or scarring. No relevant other intraoperative complications were documented.
During the postoperative course, re-bubblings were performed in 27 eyes that received pseudophakic donor tissue, thereof 13 following triple-DMEK, ten pseudophakic DMEK, and four phakic DMEK. Overall re-bubbling rate was at 60.0% independent of DMEK technique (p = 0.830). Twenty eyes (44.4%) needed only one re-bubbling, and five eyes (11.1%) had a second re-bubbling, and two eyes (4.4%) needed a third re-bubbling.
Primary graft failure was observed in one case (2.2%) as was secondary graft failure in three eyes (6.7%). No case of immune reaction or graft rejection was documented. Re-grafting during the observation period was performed in four eyes (8.9%). All of those received a re-DMEK.
Phakic donor tissue
Larger tears occurred in nine cases during graft preparation (6.6%). In one case (0.7%) DMEK surgery had to be interrupted due to a strong anterior chamber hemorrhage from the iridectomy.
Re-bubblings were performed in 70 eyes that received phakic donor tissue, thereof 29 triple-DMEK, 28 pseudophakic DMEK, and three phakic DMEK. Overall re-bubbling rate was at 51.5%, independent of performed DMEK technique (p = 0.061). Sixty eyes (44.1%) needed only one re-bubbling, and seven eyes (5.1%) had a second re-bubbling, and in two eyes (1.5%) a third re-bubbling was performed.
No case of primary graft failure or graft rejection episodes occurred, whereas in seven eyes a re-DMEK was necessary due to secondary graft failure (5.1%). Three eyes developed an immune reaction (2.2%) which resolved during the course.
Differences between both groups regarding outcome parameters
ECD values were statistically higher six (p < 0.001) and 24 months (p = 0.030) postoperatively in phakic donor tissue recipients (Fig. 1). Donor endothelial cell density showed poor correlation to donor age in phakic donor eyes (r = -0.238; p = 0.005) and no significant correlation in pseudophakic donor eyes ECD (r = -0.093; p = 0.545). Mixed regression analyses revealed significant association between ECD results and donor lens status (p = 0.029) and donor ECD (p = 0.028), but donor age did not show significant influence on ECD outcome (p = 0.241; Table 2).
BSCVA results during the course were comparable between both groups (p ≥ 0.087). Donor ECD, donor age and lens status did not show significant influence (p ≥ 0.168; Table 2) by mixed regression analyses.
CCT showed lower values at 1 month (p < 0.001) and 6 months follow-up (p = 0.026) for phakic donor tissue recipients. Mixed regression analyses revealed significant association between CCT results and donor ECD (p = 0.013), whereas donor age (p = 0.069) and lens status (p = 0.237; Table 2) did not.
The overall re-bubbling rate was lower in the phakic donor group without statistical significance (p < 0.389). Concerning the frequency of needed re-bubblings, no difference could be revealed (p = 0.139).
Discussion
Donor tissue characteristics contributing to complications during donor preparation or graft implantation, such as longer preparation and transplantation times or tissue tearing during peeling may result in primary graft failure or in higher endothelial cell loss. Descemet's membranes from pseudophakic donors seem to be more fragile, especially in the areas of clear cornea incisions, which have been performed during cataract surgery. Therefore, surgeons in our department seem to prefer phakic donors for DMEK surgery. Several studies have been performed to evaluate possible correlations between corneal donor tissue characteristics and outcome or complication rates following DMEK surgery and difficulty of surgery [5, 6, 13,14,15]. Donor age, cultivation methods or cultivation times have been under debate several times. Yet, there are no further evaluations regarding success rate and clinical outcome of using pseudophakic donor lamellae for DMEK surgery. As reported before, up to now, surgeons’ preference for the ideal DMEK graft seems to be a donor age greater than 50-60 years with high endothelial cell density and without any scars [5, 6, 13,14,15]. Therefore, in most centers no, or only limited data, are available for outcome and complication rates following DMEK with pseudophakic corneal grafts.
In our cohort, pseudophakic donors were significantly older and showed lower endothelial cell densities than phakic donors. Nevertheless, in pseudophakic donor corneas, ECD did not correlate with age.
We could observe that recipient eyes of a phakic DMEK grafts resulted in lower CCT and higher ECD values. Corneal edema showed faster reduction in the phakic donor group, but results during the course were comparable. Regarding ECD values, significant association between postoperative ECD and lens status could be shown. Phakic donors showed higher ECD values and phakic donor grafts also appear to have a higher endothelial cell density during the course, independent of donor age. Nevertheless, both groups resulted in insignificant differences in visual function.
Graft detachment seems to be the most common complication after DMEK surgery. Respective rates are reported to be up to 63% for partial graft detachments [16,17,18,19,20]. Rodríguez-Calvo de Mora et al. reported a possible association between donor age and graft detachment, following retrospective analysis of 334 DMEKs. Recipient eyes of grafts of younger donors seemed to show an increased risk for graft detachments (p = 0.049) [15]. Mean donor age in their investigation was high, at 65 years (range, 38 - 85), and it remains unclear how many younger donors were included [15]. Sáles et al. reported, that tighter scrolls are associated with younger donor age, while re-bubbling rate was not [21]. Very recently, we could not detect a negative association between donor age and re-bubbling rate. Even more reversed, the re-bubbling rate seemed to increase with increasing age [5]. Again, in none of these studies lens status of donor lamellae has been reported. The present study revealed a lower re-bubbling rate for phakic donor group (51.5% versus 60%) without statistical significance.
Alltogether, we evaluated 181 routine DMEKs performed with cornea donor tissue of 45 pseudophakic donors in comparison to 136 phakic donors. Our study revealed an insignificant negative association between pseudophakic donors and graft detachment postoperatively. Recipients of pseudophakic donor lamellae more often needed a re-bubbling for graft re-attachment. Clinical outcome including CCT and ECD values also tended to be influenced positively by phakic donor status, with faster reduction of corneal edema and higher endothelial cell density. Visual acuity and graft failure or re-grafting rates were comparable. While a larger number of DMEKs were carried out during the observation period, only in the minority the appropriate information regarding the lens status was available retrospectively. Due to the enormous donor shortage, we were forced to purchase the majority of our corneal donor tissue from other banks and to accept even donor tissue with lower quality features. We only included eyes in which donor lens status was provided by the supplying cornea banks. Many eyebanks do not provide this important information. Each cornea that is planed for transplantation in our department is additionally evaluated by slit-lamp biomicroscopy and checked for corneal scars. Thereby we try to choose corneas without scars for DMEK surgery to facilitate graft preparation. However, most of the DMEKs performed, which had been classified as "unknown" regarding the donors lens status might have been phakic. Furthermore, phakic, pseudophakic and triple-procedures have been included in our study. Even if there was no difference in the distribution of the surgical methods between the two groups, it would certainly be desirable to investigate especially pseudophakic DMEK surgeries in a subsequent study in order to rule out postoperative confounding factors including, i.e., decrease in visual acuity due to cataract progression. Major limitations of our study include the restrospective and non-randomized study design with a relatively small sample size. Endothelial cell densities, cultivation time and age differed significantly between both groups. Furthermore, significant association between lens status and ECD results could be revealed. It would be desirable to re-evaluate the influence of donor lens status in a cohort with comparable ECDs, cultivation times and donor ages.
Unfortunately we are not able to evaluate preparation or unscrolling times that may have added more insight in differences of preparation and implantation of phakic and pseudophakic DMEK grafts. In addition, we can not provide more detailed information regarding systemic diseases of corneal donors, including diabetes, hyperlipidemia, and obesity. It can be assumed that such comorbidities also have an impact on graft quality. Currently, comorbidities, which do not lead to exclusion of a deceased person from being a corneal donor, are not recorded by national or international eye banks routinely. This would certainly be an interesting and important question for a multicenter study involving eye banks delivering donor tissue for DMEK surgery.
These aspects should be addressed by further prospective analysis.
Up to now, pseudophakic grafts have been considered less suitable for DMEK surgery, since the risk of tearing during preparation has been estimated to be too high. Therefore, clinical results, especially long-term results following their use for DMEK surgery, are missing.
Intraoperative complications seemed to be comparable, while higher re-bubbling rates indicate that pseudophakic grafts may be more fragile, or even stiffer. Better ECD and CCT values during the first postoperative period seem to favor phakic grafts, independent of donor age. However, this advantage of phakic transplants in the early postoperative course appears to become subsequently relativized. Therefore, pseudophakic transplants with high endothelial cell count should not be excluded, especially since functional results seem to be not affected by donor lens status.
References
Melles GR, Ong TS, Ververs B, der WJ V (2006) Descemet membrane endothelial keratoplasty (DMEK). Cornea 25:987–990
Melles GR (2006) Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 25:879–881
Maier P, Reinhard T, Cursiefen C (2013) Descemet stripping endothelial keratoplasty--rapid recovery of visual acuity. Dtsch Arztebl Int. 110:365–371
Cursiefen C (2013) Descemet membrane endothelial keratoplasty: the taming of the shrew. JAMA Ophthalmol. 131:88–89
Schaub F, Enders P, Zachewicz J, Heindl LM, Stanzel TP, Cursiefen C, Bachmann BO (2016) Impact of Donor Age on Descemet Membrane Endothelial Keratoplasty Outcome: Evaluation of Donors Aged 17-55 Years. Am J Ophthalmol. 170:119–127
Gorovoy IR, Cui QN, Gorovoy MS (2014) Donor tissue characteristics in preparation of DMEK grafts. Cornea 33:683–685
Weller JM, Tourtas T, Kruse FE, Schlotzer-Schrehardt U, Fuchsluger T, Bachmann BO (2015) Descemet membrane endothelial keratoplasty as treatment for graft failure after descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 159:1050–1057
Heindl LM, Riss S, Bachmann BO, Laaser K, Kruse FE, Cursiefen C (2011) Split cornea transplantation for 2 recipients: a new strategy to reduce corneal tissue cost and shortage. Ophthalmology. 118:294–301
Maier AK, Gundlach E, Schroeter J, Klamann MK, Gonnermann J, Riechardt AI, Bertelmann E, Joussen AM, Torun N (2015) Influence of the difficulty of graft unfolding and attachment on the outcome in Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. 253:895–900
Kruse FE, Schrehardt US, Tourtas T (2014) Optimizing outcomes with Descemet's membrane endothelial keratoplasty. Curr Opin Ophthalmol. 25:325–334
Steven P, Le Blanc C, Velten K, Lankenau E, Krug M, Oelckers S, Heindl LM, Gehlsen U, Huttmann G, Cursiefen C (2013) Optimizing descemet membrane endothelial keratoplasty using intraoperative optical coherence tomography. JAMA Ophthalmol. 131:1135–1142
Kruse FE, Laaser K, Cursiefen C, Heindl LM, Schlotzer-Schrehardt U, Riss S, Bachmann BO (2011) A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea. 30:580–587
Heindl LM, Bucher F, Caramoy A, Hos D, Matthaei M, Cursiefen C (2014) Safety of donor tissue preparation and use of descemetoschisis and torn tissue in descemet membrane endothelial keratoplasty. Cornea. 33:e7–e9
Heinzelmann S, Huther S, Bohringer D, Eberwein P, Reinhard T, Maier P (2014) Influence of donor characteristics on descemet membrane endothelial keratoplasty. Cornea. 33:644–648
Rodriguez-Calvo de Mora M, Groeneveld-van Beek EA, Frank LE, Van der WJ OS, Bruinsma M, Melles GR (2016) Association Between Graft Storage Time and Donor Age With Endothelial Cell Density and Graft Adherence After Descemet Membrane Endothelial Keratoplasty. JAMA Ophthalmol. 134:91–94
Ang M, Wilkins MR, Mehta JS, Tan D (2016) Descemet membrane endothelial keratoplasty. Br J Ophthalmol. 100:15–21
Guerra FP, Anshu A, Price MO, Giebel AW, Price FW (2011) Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 118:2368–2373
Melles GR, Ong TS, Ververs B, der WJ V (2008) Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 145:222–227
Price MO, Giebel AW, Fairchild KM, Price FW Jr (2009) Descemet's membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 116:2361–2368
Schaub F, Enders P, Snijders K, Schrittenlocher S, Siebelmann S, Heindl LM, Bachmann BO, Cursiefen C (2017) One-year outcome after Descemet membrane endothelial keratoplasty (DMEK) comparing sulfur hexafluoride (SF6) 20% versus 100% air for anterior chamber tamponade. Br J Ophthalmol. 101:902–908
Sales CS, Terry MA, Veldman PB, Mayko ZM, Straiko MD (2016) Relationship Between Tissue Unscrolling Time and Endothelial Cell Loss. Cornea. 35:471–476
Funding
This research was supported by German Research Foundation FOR 2240 “(Lymph) Angiogenesis And Cellular Immunity In Inflammatory Diseases Of The Eye” to CC and LMH (www.for2240.de) and EU COST BM 1302 “Joining Forces in Corneal Regeneration” to BOB and CC (www.biocornea.eu). The sponsor had no role in the design or implementation of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Schaub, F., Pohl, L., Enders, P. et al. Impact of corneal donor lens status on two-year course and outcome of Descemet membrane endothelial keratoplasty (DMEK). Graefes Arch Clin Exp Ophthalmol 255, 2407–2414 (2017). https://doi.org/10.1007/s00417-017-3827-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3827-2