Abstract
Purpose
Intravenously administered erythropoietin (EPO) was firstly commenced (phase 1) in patients with indirect traumatic optic neuropathy (TON) by this group in 2011. It was re-tested by another group (phase 2) in 2014. This multicenter clinical trial was designed to compare its effect with intravenous steroid and observation.
Methods
Included were TON patients ≥5 years of age and with trauma-treatment interval of ≤3 weeks. Follow-up visits were set at 1, 2, 3, 7, 14, 30, and at least 90 days after treatment. EPO and methylprednisolone were infused intravenously every day for three consecutive days. Primary outcome measure was change in the best corrected visual acuity (BCVA). Secondary outcomes included change in color vision and relative afferent pupillary defect (RAPD), side effects, and factors affecting the final visual improvement.
Results
Out of 120 patients, 100 (EPO: 69, steroid: 15, observation: 16) were finally included. All three groups showed a significant improvement of BCVA which was not significantly different between the groups (adjusted for pretreatment BCVA). Color vision was significantly improved in the EPO group. Late treatment (>3 days) (odds ratio = 2.53) and initial BCVA of NLP (odds ratio = 5.74) significantly worsened visual recovery. No side effect was observed in any group.
Conclusion
EPO, steroid, and observation showed a significant improvement of BCVA in patients with TON. Initial BCVA of NLP and late treatment (>3 days) were significant risk factors for visual improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic optic neuropathy is defined as a sudden reduction in visual function following head and neck trauma [1]. It results in variable degree of optic atrophy and loss of vision in which the prevalence of sever initial visual loss ranges from 43% to 56% [2].
While the direct type of traumatic optic neuropathy originates from a known cause, indirect traumatic optic neuropathy (TON), which is the more common type, cannot be attributed to any definite cause [1]. However, the shearing force of head trauma is assumed to cause small vessel injury around the optic nerve, which in consequence leads to ischemia, inflammation, oxidative damage, apoptosis, and finally ganglion cell death and optic atrophy [1].
Since the exact pathophysiology of TON is unclear, its management has remained controversial. Three common managements are observation, corticosteroid, and/or optic canal decompression [1, 2]. Corticosteroids and/or optic canal decompression surgery have not shown any significantly better visual outcome than observation in the literature [1, 2]. However, one meta-analysis concluded that treatment with corticosteroids, optic canal decompression, or both is better than no treatment [3]. The results of these medical and surgical interventions have shown to be uncertain [4, 5] with possible serious side effects or complications [6, 7]. Therefore, there has been no study, which could validate a particular approach to the management of TON.
The cytokine hormone erythropoietin (EPO), which is responsible for production of red blood cells by preventing apoptosis of erythroid progenitors [8], has been shown to be effective to reduce neural apoptosis and exert protective effect in animal models of brain hypoxic-ischemic injuries, retinal and spinal cord ischemia, spinal cord and peripheral nerve compression, and human study of ischemic brain injury [8]. Therefore, it has presented anti-ischemic, anti-inflammatory, anti-oxidative, and anti-apoptosis effects [8], all of which play role in presumed pathophysiology of TON. Intravenous administration (IV) of EPO at different dose (40,000 IU units/day for 3 days, [9] 33,000 units/day for 3 days, [10], 48,000 units/week for 6 weeks [11]) has also shown to be safe in different studies. Our team firstly commenced IV-EPO treatment for patients with TON [12] in 2011 in which patients in the EPO group showed a significantly better visual outcome than the observation group. EPO (10,000 units/day for 3 days) was found to be safe with no side effect [12]. Subsequently, a higher dose (20,000 units/day for 3 days) was also found to be effective and safe in patients with TON [13].
Since there is no clinical trial on comparing the EPO with the most common types of TON managements (corticosteroid and observation), this multicenter clinical trial was designed to compare the visual function and side effects at different time intervals between IV-EPO, IV corticosteroid, and observation in patients with TON and to assess the factors affecting the final visual outcome.
Methods
The study was an open label, phase 3, multicenter semi-experimental trial, which was carried out in four university based hospitals in Iran (two in Tehran, one in Shiraz, and one in Mashhad). The centers were masked to the data of the other centers. Ethics committee approval (90–01–124-12,972) was obtained, and the study protocol was registered with www.ClinicaTrial.gov (NCT01783847, 2013).
TON was defined as reduced best corrected visual acuity (BCVA), color vision, and positive relatively afferent pupillary defect (RAPD) with normal fundus and optic nerve examination and no evidence of direct trauma to optic nerve on spiral orbital and optic canal computer tomography (CT) scan [2]. Patients underwent comprehensive eye and systemic examination with the same setting in all centers.
BCVA was checked using a Snellen chart, which was available and used in all centers. If vision was less than 20/200, the chart was gradually brought closer up to 2.5 ft until the largest optotype was seen by the patient. BCVA was then recorded as the distance in feet (numerator) over the size of the letter (denominator). Counting fingers, hand motion, and light perception were consecutively used to record the BCVA if the patient was unable to see the largest optotype at 2.5 ft distance. Color vision was evaluated with Ishihara 14 plates test. RAPD was assessed using a swinging flash light and subjectively graded from +1 to +4. Complete ophthalmology examination was also performed for all the patients. Visual function tests were performed by or under direct supervision of four main investigators at four centers (MBK, ME, MER, and MRR). All the CT scans were reviewed by a radiologist and main investigator at each center paying special attention to possible subtle optic canal fractures.
All patients had systemic physical examination by an internist and underwent renal function test, liver function test, complete blood count, serum electrolytes, and fasting blood sugar before and 3 and 7 days after the treatment.
Included were patients with TON of ≥5 years of age and within 3 weeks after the trauma. Age limit was >5 years in the original protocol of study, which was changed into ≥5 years because we could reliably assess the visual function. TON causes axonal degeneration which in consequence leads to optic nerve atrophy in 4 to 8 weeks [14]. Since there might be no benefit from treatment in patients with long term TON, patients up to 3 weeks after their trauma were recruited in this study. Exclusion criteria were patients with direct optic nerve trauma, associated ocular, orbital or central nervous system (CNS) injury, decreased level of consciousness, concurrent CNS trauma requiring medical or surgical treatment, and any medical history which might be interfering with corticosteroid and EPO treatments (uncontrolled systemic hypertension, polycythemia, serum creatinine of ≥3 mg/dL, history of allergic reaction to recombinant human erythropoietin, serum electrolyte disturbance, history of malignancy, active peptic ulcer, gastrointestinal bleeding, active infection, immunodeficiency context, history of cerebrovascular accident or coronary artery disease, oral contraceptive consumption, pregnancy, or breast feeding).
Patients (adults) and their guardians (pediatric patients) were fully informed of different treatment options and then an informed consent was obtained. The study was in accordance with the tenets of the Declaration of Helsinki [15] and Iranian Declaration of Patients’ Rights [16].
Patients in EPO and steroid groups were admitted in the hospital. EPO (recombinant human erythropoietin, Pooyesh Darou Biopharmaceuticals Co., Tehran, Iran) was infused in 200 mL of normal saline intravenously over 2 h, every day for three consecutive days. According to our prior pilot study [12], 10,000 IU/day was infused into patients under 13 years of age and 20,000 IU/day for ≥13 years of age. Aspirin (80 mg tablet) was given 1 h before initiation of infusion. Steroid group received 250 mg methylprednisolone (Solu-medrol, Exir Pharmaceuticals Co., Boroujerd, Iran) intravenously over 30 min, four times a day for three consecutive days. The original study protocol was to be followed by oral steroid if there was improvement of vision. However, it was changed to just 3 days of intravenous steroid to achieve uniform treatment for all the participants. Blood pressure was measured on the left arm with the patient maintained in a 30° head up-tilt, just before and every 15 min during infusion in both groups. Both infusions were under supervision of an internist. Participants in observation group were daily followed as outpatients in the clinic for 3 days. All three groups were then planned to have four follow-up visits at week 1, week 2, month 1, and at least month 3 (final visit).
Main outcome measure was change in BCVA. The BCVA was presented in four different ways for the statistical analysis: 1) mean logMAR [12], 2) 0.3 change in logMAR (improvement, deterioration, and no change) [12, 17], 3) mean improvement percentage [3], which was calculated as:
× 20/13 is considered to be perfect vision [7], and 4) ordinal categorization of the BCVA as no light perception (NLP), light perception (LP) and hand motion (HM), count fingers (CF), and ≥20/200.
Secondary outcome measures were color vision (mean) and RAPD grading (mean and categorical). Color vision score was considered 0 in patients whose visual acuity was too low to see any plate in order to be able to compare the pre- versus post-treatment color vision mean scores. Visual field test had been considered one of the secondary outcome measures in the original study protocol. However, since visula acuity in most of the patients was too low to perform visual field test and they were mostly admitted when a visual field test and technician were not available prior to starting the medications, it was excluded from the study protocol. Impact of age, gender, trauma type, trauma-treatment time interval (for the EPO and steroid groups), concurrent anterior orbital or ethmoid/sphenoid sinuses fracture, baseline BCVA, and associated cranial nerve palsy on final visual function were finally assessed.
All the outcome measures were recorded based on the same protocol in all four centers. However, the records were not centrally assessed.
Randomization and masking
Included patients were randomized into EPO, steroid, and observation groups according to the simple digit number randomization, using a table of random numbers. Neither participants nor investigators were masked to the type of interventions. Patients were enrolled by the senior investigators in four centers (MBK, ME, MER, MRR), randomly allocated by one of the authors (FP) in the headquarters (IUMS), and assigned to interventions by four investigators in four centers (SY, ME, MS, MB). All the examinations were performed by two investigators at each center (MBK and SY at center 1, ME and ME at center 2, MER and MS at center 3, and MRR and MB at center 4). All participants, their nominal features such as gender and trauma type, and the type of intervention were coded by numbers on data entry to all of which the statistician (MN) was masked.
Majority of the patients or their parents did not accept going through randomization for three arms of the clinical trial and requested just EPO treatment. In fact, they had been referred or advised to receive just EPO treatment. Those patients were also included in the study because of uncommonness of the disease. Hence, the design of study was changed to semi-experimental clinical trial on the basis of “Convenient sampling and response adaptive randomization” [18, 19].
Statistical analysis
Considering 50% improvement in the observation group and to 85% improvement in EPO group [12], study power of 80%, effect size of 35%, and error type 1 of 5%, sample size was calculated to be 96 patients (32 in each group).
Participants who completed the protocol of study were finally included. Data were entered with SPSS software (Version 20, Chicago, IL, USA) at each center and then submitted to a masked statistician (MN) for the analysis. Chi-square test was used for comparing categorical variables across three groups. One-way analysis of variance (ANOVA) test was used to compare means between three groups. Baseline and final means of each group was separately compared by paired sample t-test. Wilcoxon signed rank test was used to compare categorical variables across baseline and final measures. To compare consecutive changes in continuous quantitative variables within and between groups, “repeated measures ANOVA” test was used. Pearson correlation test was also used to assess the association between nominal variables. Measurement of risks for predicting the final visual outcome was performed using odds ratio (OR). Level of significance was considered as 0.05. The study protocol was registered with www.ClinicaTrial.gov (NCT01783847). Data Sharing was entered with Mendeley.com, doi:10.17632/xgsxnz9ppj.1
Results
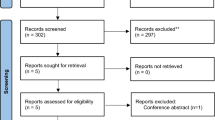
Out of 120 patients with initial enrollment, three were found to have minor optic canal fracture (two patients) and vitreous hemorrhage (one patient) that were consequently excluded. Therefore, 117 patients were included (Fig. 1). Seventeen patients with incomplete follow-up and not following the treatment protocol were also excluded during the study (Fig. 1). There were 100 patients (100 eyes) who completed the study protocol in whom two patients had direct optic neuropathy in their fellow eyes. Patients were recruited from.
March 1, 2013 to June 30, 2016. There were finally 69, 15, and 16 patients in the EPO, steroid, and observation groups, respectively. Seven patients in the EPO group who were under 13 years of age received 10,000 IU/day.
Demographics and pre-treatment variables including visual functions (BCVA, color vision, and RAPD) were not significantly different between three groups (Table 1).
Baseline BCVA ranged from NLP to 20/32. Mean follow-up time was 207.8 days (SD = 297.07, range: 90–1730). BCVA significantly improved in all three groups at the last follow-up time (Table 2).
None of the patients presented with a reduction of baseline BCVA at the final visit. Final mean (Table 2) logMAR of BCVA was not significantly different between three groups, even though EPO group showed a better visual outcome.
While steroid group showed the fastest recovery of vision within 1 month and no significant change afterward, the observation group showed almost no recovery up to 2 weeks after trauma when the improvement started and continued up to 2.5 months to reach plateau (Fig. 2). The EPO group, on the other hand, showed improvement from the starting point and had a steady increase up to and after month 3 (Fig. 2). While improvement of BCVA was significant in each group (repeated measurement of ANOVA, Tests of Within-Subjects Effects, Greenhouse-Geisser, P < 0.001), after adjusting the confounding effect of baseline BCVA on the final BCVA, mean improvement percentage was not significantly different between the groups (repeated measurement of ANOVA, Tests of Between-Subjects Effects, Greenhouse-Geisser, P = 0.16).
Pre-treatment and last follow-up color vision test results were available in 86 patients (86/100, 86%). It was not available in 12 patients in the EPO group (12/69 (17.3%), one in the steroid group (1/15, 6.6%), and one in the observation group (1/16 (6.2%) from four centers. Color vision improvement was observed in all three groups even though it was just statistically significant (P = 0.02) in the EPO group (Table 3). This significance could be a bias resulting from the high number of patients in the EPO and low number of patients in the other groups.
RAPD (as categorical variable) was significantly improved in three groups without statistically significant difference between the groups (Table 3). However, mean RAPD improvement was only significant in the EPO (P < 0.001) and steroid group (P = 0.004) (Table 3). No side effect was observed in any of two treatment groups.
Since 41% of patients had presented with NLP vision, a subgroup analysis of those patients was performed in which there was no statistically significant difference between three groups with regard to the final recovery (Online resource 1).
Longer (>3 days) trauma-to-treatment time interval (odds ratio = 2.53, P = 0.04) and initial VA of NLP (odds ratio = 5.74, P < 0.001) were the only variables which significantly led to a worse final VA (Online resource 2).
Discussion
Following the primary insult from head injury, secondary mechanisms may cause further damage to the nerves. These mechanisms include ischemia, generation of oxygen free radicals, release of bradykinin and kallidin, and release of inflammatory mediators (infiltration of macrophages and polymorphonuclear cells), which may further damage the axon [1].
Parallel to previous reports [2], the majority of our patients were under 25 years of age (53/100, 53%) and male (88/100, 88%). Three types of TON managements (steroids, optic canal decompression, and observation) have been reported with inconsistent results. Although Chou et al. [20] reported a significantly higher visual recovery in patients receiving intravenous steroids or surgical decompression as compared with observation, others4 did not find any significant difference between high-dose IV corticosteroids and placebo. While IV methyl prednisolone showed a better visual outcome than observation, optic canal decompression, and oral prednisolone in one study [21], its effect was not significantly different from optic canal decompression in another [22]. Furthermore, there was no difference between IV dexamethasone and IV methyl prednisolone [23]. A systematic review by Cook et al. [3] on treatment of TON concluded that recovery of vision in treated patients (steroid and canal decompression) was significantly better than the recovery in patients receiving no treatment. However, no significant difference was found between corticosteroids alone, canal decompression alone, or both [3]. The international optic nerve trauma study [4] indicated that no significant benefit was provided by either steroid or surgical decompression as compared with observation. Not having a significantly better visual outcome after steroid or canal decompression persuaded us to look for a new treatment which could potentially address most or all primary and secondary mechanisms in the pathophysiology of TON.
The EPO/EPO receptor is highly prominent during fetal development, with very high levels of expression found in many tissues, diminishing rapidly after birth to generally low levels found in adults [24]. Its anti-inflammatory effect has been demonstrated in a model of experimental autoimmune encephalomyelitis disease [25]. EPO has also been used as an off label treatment of anemia in premature infants and severe orthostatic hypotension [26]. Furthermore, intravenous EPO has shown to be safe and effective in patients with stroke and multiple sclerosis [9,10,11]. Its intravenous administration was firstly introduced for treatment of TON (off label) in a pilot study [12] in 2011 in which its effect was significantly better than observation with no side effect. The second study on the effect of IV EPO in patients with TON confirmed its effectiveness and safety in 2014 [13]. However, a low number of patients and the design of studies (case series) were the limitations persuading us to design a multicenter RCT to compare its effect to IV corticosteroid and observation.
Significant improvement of BCVA was observed in all three groups at the last follow-up without a significant difference between three groups, even though EPO group showed an insignificantly better final BCVA (Table 2). This finding was not consistent with our pilot study [12] in which mean final LogMAR of BCVA was significantly better in the EPO than observation group.
The steroid group showed the fastest recovery of vision within first few weeks (Fig. 2) and then remained the same afterward, which was consistent with previous studies [27]. EPO, on the other hand, showed a steady recovery of BCVA up to 3 months, when the speed of recovery became slower, to the end of study period (mean = 208 days). Whether a second injection of IV EPO at 3 months might benefit the patients with TON would be the question of future studies.
Initial VA of NLP was observed in 41% of patients. It is considered a poor prognostic factor for final visual recovery in several studies [19, 21], even though some improvement has been reported after EPO [12, 13], steroid [4], and observation [28]. All three groups showed some improvement of vision with no significant difference between the groups, even though EPO group showed an insignificantly better visual improvement percentage (Online resource 1). Similar to the others [4, 21, 22, 27], baseline VA of NLP was found to be a significant risk factor towards a worse final visual recovery (Online resource 1).
Dischromatopsia is one of the main signs of optic nerve function disturbance after trauma [13, 29]. Mean color vision differences between pre- and post-treatment were 0.83 and 0.86 in the EPO and observation group, respectively. While pre- vs. post-treatment mean color vision difference (0.83) was significant in the EPO group, such significance was not observed in the observation group in spite of having a bigger difference (0.86). We believe that this is because of low number of patients in the observation group. Entezari et al. [13] reported color vision improvement after IV EPO in patients with TON. There was also a significant improvement of RAPD in each group to which, to the best of our knowledge, no other study was found to be comparable.
Concurrent orbital fracture has been reported to result in significantly lower visual improvement [22]. Similarly, Cook et al. [3] indicated that posterior orbital fractures may have a worse prognosis than anterior orbital fractures. However, our trial showed that presence of anterior and posterior orbital fracture did not significantly affect the final visual recovery. This could be attributed to a few number of patients with anterior (23/100, 23%) and posterior (3/100, 3%) orbital fracture. While early treatment resulted in a better visual recovery in our study and others [27], Entezari et al. [13] showed no significant association between the time of treatment and final visual recovery. Similar to other studies [13, 22], age, gender, trauma type, and other variables did not have significant impact on final visual recovery (Online resource 2).
There was no significant side effect of steroid and EPO in this study. Different doses of IV EPO in different disorders have been reported to cause blood pressure rise, hyperkalemia, deterioration of renal function, and increased platelet count [26]. However, administration of 10,000 IU/day [12] or 20,000 IU/day [13] in patients with TON did not result in any side effects. Intravenous steroid has been associated with some side effects including sleep disturbance, mood change, stomach upset, facial flushing, and transient weight gain [30]. However, some reported no side effect of intravenous steroid in patients with TON [4, 5].
Change in the study design is the first limitation of this trial. The change was inevitable because some patients did not consent for randomization and requested only EPO treatment. Since TON is an uncommon disease, all the patients with TON were included, which resulted in an unequal number of patients in each group. Literature review on the issue showed that the study design could be changed on the basis of “Convenient sampling and response adaptive randomization” [19]. Another limitation was subjective grading of RAPD, which was because of unavailability of neutral density filter in all study centers. Furthermore, color vision test was not available in all patients, which might have influenced the analysis in this regard.
In conclusion, A statistically significant improvement of BCVA was observed in patients with TON who received either IV EPO, IV steroid, or observation. No significant difference was found between the groups, even though the EPO group ended with an insignificantly better final vision. No side effect was observed in two treatment groups. A significantly worse visual outcome was observed in patients with initial VA of NLP (all three groups) and longer (>3 days) time interval between trauma and treatment (EPO and steroid groups).
Since patients were recruited from different ethnic groups of four major universities in different geographic areas in Iran, we believe that the results could be applicable to other large populations.
Based on at least the same effect on BCVA recovery and no side effects in this and previous studies [12, 13], EPO could be recommended as an option in treatment of patients with TON. Future studies on multiple injections at different time intervals and or higher dose of IV EPO are recommended.
References
Steinsapir KD, Goldberg RA (1994) Traumatic optic neuropathy. Surv Ophthalmol 38(6):487–518
Singman EL, Daphalapurkar N, White H et al (2016) Indirect traumatic optic neuropathy. Mil Med Res 3:2
Cook MW, Levin LA, Joseph MP, Pinczower EF (1996) Traumatic optic neuropathy: a meta-analysis. Arch Otolaryngol Head Neck Surg 122(4):389–392
Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R, Group IONTS (1999) The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology 106(7):1268–1277
Entezari M, Rajavi Z, Sedighi N, Daftarian N, Sanagoo M (2007) High-dose intravenous methylprednisolone in recent traumatic optic neuropathy; a randomized double-masked placebo-controlled clinical trial. Graefes Arch Clin Exp Ophthalmol 245(9):1267–1271
Yu-Wai-Man P, Griffiths PG.(2005) Surgery for traumatic optic neuropathy. Cochrane Database Syst Rev. 19;(4):CD005024
Steinsapir KD, Goldberg RA, Sinha S, Hovda DA (2000) Methylprednisolone exacerbates axonal loss following optic nerve trauma in rats. Restor Neurol Neurosci 17:157–163
Ghezzi P, Brines M (2004) Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ 11(Suppl 1):S37–S44
Ehrenreich H, Weissenborn K, Prange H et al (2009) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40(12):e647–e656
Ehrenreich H, Hasselblatt M, Dembowski C et al (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8(8):495–505
Ehrenreich H, Fischer B, Norra C et al (2007) Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 130(pt 10):2577–2588
Kashkouli MB, Pakdel F, Sanjari MS et al (2011) Erythropoietin: a novel treatment for traumatic optic neuropathy—a pilot study. Graefes Arch Clin Exp Ophthalmol 249(5):731–736
Entezari M, Esmaeili M, Yaseri M (2014) A pilot study of the effect of intravenous erythropoetin on improvement of visual function in patients with recent indirect traumatic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 252(8):1309–1313
Kline LB, Morawetz RB, Swaid SN (1984) Indirect injury of the optic nerve. Neurosurgery 14(6):756–764
Association GAotWM (2014) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 81(3):14–18
Joolaee S, Nikbakht-Nasrabadi A, Parsa-Yekta Z, Tschudin V, Mansouri I (2006) An Iranian perspective on patients' rights. Nurs Ethics 13(5):488–502
Beck RW, Moke PS, Turpin AH, Ferris FL, SanGiovanni JP, Johnson CA et al (2003) A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol 135(2):194–205
Zelen M (1974) The randomization and stratification of patients to clinical trials. J Chronic Dis 27(7–8):365–375
Morabia A (2000) Survey Methods in Community Medicine: Epidemiological Research, Programme Evaluation, Clinical Trials. Fifth edition By JH Abramson and ZH Abramson. Am J Epidemiol 152(1):96
Chou PI, Sadun AA, Chen YC, Su WY, Lin SZ, Lee CC (1996) Clinical experiences in the management of traumatic optic neuropathy. J Neuroophthalmol 16(6):325–336
Chen HY, Tsai RK, Wang HZ (1998) Intravenous methylprednisolone in treatment of traumatic optic neuropathy. Kaohsiung J Med Sci 14(9):577–583
Wang BH, Robertson BC, Girotto JA et al (2001) Traumatic optic neuropathy: a review of 61 patients. Plast Reconstr Surg 107(7):1655–1664
Chuenkongkaew W, Chirapapaisan N (2002) A prospective randomized trial of megadose methylprednisolone and high dose dexamethasone for traumatic optic neuropathy. J Med Assoc Thail 85(5):597–603
Juul SE, Yachnis AT, Rojiani AM, Christensen RD (1999) Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol 2(2):148–158
Brines ML, Ghezzi P, Keenan S et al (2000) Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 97(19):10526–10531
Spivak JL (2001) Erythropoietin use and abuses: When physiology and pharmacology collide. Adv Exp Med Biol 502:207–224
Fujitani T, Inoue K, Takahashi T, Ikushima K, Asai T (1985) Indirect traumatic optic neuropathy--visual outcome of operative and nonoperative cases. Jpn J Ophthalmol 30:125–134
Mahapatra A, Tandon D (1993) Traumatic optic neuropathy in children: a prospective study. Pediatr Neurosurg 19(1):34–39
Bilyk JR, Joseph MP (1994) Traumatic optic neuropathy. Semin Ophthalmol 9(3):200–211
Roujeau JC (1996) Pulse Glucocorticoid Therapy: The'Big Shot'Revisited. Arch Dermatol 132(12):1499–1502
Funding
Iran University Eye Research Center provided financial support in the form of research funding. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Support
This study was funded by Iran (Tehran) University eye research center which had no role in design, collection, interpretation, analysis, execution of the study, and decision to submit the paper for publication.
Financial disclosures
No financial disclosures.
Additional information
Study protocol was registered with www.ClinicaTrial.gov (NCT01783847, 2013).
All the authors fulfill the criteria for authorship.
Rights and permissions
About this article
Cite this article
Kashkouli, M.B., Yousefi, S., Nojomi, M. et al. Traumatic optic neuropathy treatment trial (TONTT): open label, phase 3, multicenter, semi-experimental trial. Graefes Arch Clin Exp Ophthalmol 256, 209–218 (2018). https://doi.org/10.1007/s00417-017-3816-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3816-5