Abstract
Purpose
The study objective was to compare dye angiography and optical coherence tomography angiography (OCTA) in detecting choroidal neovascuarization (CNV) in patients presenting with pachychoroid features and flat irregular pigment epithelial detachment (PED).
Methods
Nineteen eyes of 17 patients, presenting with flat PED and pachychoroid features, and without age-related macular degeneration or any other degenerative change, were analyzed. Fuorescein angiography (FA)/Indocyanine green angiography (ICGA) and OCTA were performed during the same visit. Subfoveal choroidal thickness was measured by enhanced depth imaging using spectral domain optical coherence tomography.
Results
The mean age of the patients was 59.1 years. Mean subfoveal choroidal thickness was 388 μm. FA revealed non-patognomic features including RPE alterations, window defects, leaking points and leakage from an undetermined source. ICGA revealed choroidal vascular plaque in eight eyes (42%) and suspicious plaque in five eyes (26%). Nonneovascular features, such as hyperpermeability or dilated choroidal vessels, were observed in six eyes (32%). OCTA showed choroidal neovascularization in 14 (74%). For all of the eyes, which ICGA was positive for presence of CNV, OCTA also showed CNV, and in one case it also revealed polypoidal characteristics of the neovascular network. OCTA was also able to detect CNV in all of the eyes with suspicious plaque, and in one eye without CNV appearance using ICGA.
Conclusions
OCTA demonstrated greater sensitivity in detecting type 1 CNV than conventional dye angiography in cases with pachychoroid spectrum disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pachychoroid-related macular disorders are a spectrum of diseases that share common features, such as increased choroidal thickness and dilated outer choroidal vessels [1]. This spectrum includes four different clinical entities; pachychoroid pigment epitheliopathy (PPE), central serous chorioretinopathy (CSCR), pachychoroid neovasculopathy (PNV), and polypoidal choroidal vasculopathy (PCV) [2].
PPE is the new term used to define a permanent and abnormal increase in choroidal thickness, often manifesting as dilatation of the large outer oval choroidal vessels (Haller’s layer), and which compress the overlying choriocapillaris and Sattler’s layer without causing subretinal fluid (SRF) [2,3,4]. Pachychoroid neovasculopathy is a recently proposed clinical entity in choroidal neovascularization (CNV) [5]. It has the features of type 1 neovascularization and choroidal thickening in the absence of characteristic age-related macular degeneration (AMD) or degenerative change.
Pachychoroid spectrum diseases may show pigment epithelium detachments (PED) in variable shape and size. Pigment epithelium detachments were seen in more than two thirds of the CSCR [6]. While acute CSCR usually reveals typical dome-shaped PEDs, flat irregular PED was generally observed in chronic CSCR and are defined as the irregular elevation of the retinal pigment epithelium (RPE), allowing visualization of the underlying Bruch’s membrane.
Complete or partial hyperreflectivity might be observed in the sub-RPE area in flat PED. Hyper-reflectivity of the material may be caused by neovascular tissue or optic material accumulation. It was reported that one third of flat, irregular PED in CSCR contained CNV [7]. If underlying type 1 CNV is present, the early detection of underlying neovascular tissue and the ability to make a distinction between these and AMD cases is essential to ensure accurate treatment planning.
Recently, the high sensitivity and specificity of optical coherence angiography (OCTA) in identifying CNV in eyes with chronic CSCR and pachychoroid spectrum disease was demonstrated in the literature [8,9,10,11,12,13]. Thus, the study objective was to detect the presence of a neovascular membrane underlying flat, irregular PED in patients presenting with pachychoroid features through the concurrent use of OCTA, fluorescein angiography (FA) and indocyanine green angiography (ICGA). The concurrent use of imaging techniques expedited an evaluation of the pathologic features without the time delay inherent in different follow-up visits and differences in the morphologic appearance. We hypothesized that the results of this comparison would help clinicians to more precisely detect which of the two imaging techniques had greater diagnostic sensitivity.
Method
The eyes of patients presenting with flat PED and pachychoroid features, and without age-related macular degeneration (AMD) or any other degenerative change, were analyzed in this observational consecutive case series. Nineteen eyes of 17 patients presenting with flat irregular PED at the Department of Ophthalmology, Ankara University, Ankara, Turkey, were included in the study. The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from the participants prior to enrolment.
Inclusion criteria for participation in the study were the presence of flat, irregular PED, accompanied by dilated choroidal vessels, or increased choroid thickness, and/or a history of CSCR. Patients with a history of inflammatory ocular disease or macular drusen were excluded.
The participants underwent a thorough ophthalmic assessment that included best corrected visual acuity (BCVA), slit lamp biomicroscopy, and a dilated funduscopic examination. Spectral-domain optical coherence tomography (SD-OCT) (Spectralis®, Heidelberg Engineering Inc., Heidelberg, Germany), FA and ICGA (Heidelberg Retina Angiograph 2®; Heidelberg Engineering, Heidelberg, Germany), OCTA (Avanti RT Vue XR® with AngioVue® software; Optovue Inc., Fremont, CA, USA) were performed for all patients.
Subfoveal choroidal thickness (SFCT) measurement, defined as the distance from the outer portion of the hyper-reflective line (corresponding to the RPE) to the inner surface of the choroidal-scleral junction, was performed using enhanced depth imaging (EDI)-OCT scans. The diameter of the thickest choroid vessel under the flat PED was measured using a manual caliper to determine the widest distance between the two vessel walls. Fundus fluorescein angiography, ICGA and OCTA were concurrently performed on all the patients. According to the ICGA findings, the lesions were classified as choroidal vascular plaque, suspicious plaque, and ones with nonneovascular charactersitics, such as choroidal hyperpermeability or dilated choroidal vessels. Finally, imaging was performed using SD-OCT (OCTA software) on a commercially available device operating at 70.000 A-scans per second to acquire OCTA volumes for all patients concurrently with dye angiography. The OCTA scans were examined for the presence or absence of type 1 neovascular tissue. One retina specialist (SD) who was experienced in the interpretation of the OCTA, FA and ICGA read the images regarding the CNV presence in a blind fashion. The interpretation of the OCTA and FA-ICGA images were performed at different times in order not to be affected by image memory.
Statistical analysis was performed using SPSS® software for Windows® version 15.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean ± standard deviation for the continuous variables. A p-value of <0.05 was considered to be statistically significant.
Results
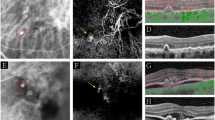
Nineteen eyes of seventeen patients were included in the study. Eleven of the patients (65%) were men and six (35%) were women. The mean age of the patients was 59.1 years (± 7.7). Before multimodal imaging tests, a preliminary diagnosis of CSCR had been given for 15 eyes (79%) and one of AMD for four eyes (21%). Concurrent EDI-OCT, FA, ICGA, and OCTA images were taken from the patients. The EDI-OCT images revealed flat, irregular PED and pachychoroid features, i.e., increased subfoveal choroidal thickness and the presence of pachyvessels. None of the patients were shown to have drusen or hemorrhage on clinical examination or imaging. Sixteen eyes contained SRF at the baseline examination (Table 1). FA revealed non-patognomic features including RPE alterations, window defects, leaking points and leakage from an undetermined source. Choroidal vascular plaque was shown on ICGA in eight eyes (42%) and suspicious plaque in five eyes (26%), as well as nonneovascular features, such as hyperpermeability and dilated choroidal vessels, in six eyes (32%). Choroidal neovascularization was shown using OCTA in 14 eyes (74%). For all of the eyes for which ICGA was positive for presence of CNV, OCTA also showed CNV, and in one case (case 4) it also revealed polypoidal characteristics of the neovascular network (Fig 1). OCTA was also able to detect CNV in all of the eyes with suspicious plaque, and in one eye without CNV appearance using ICGA (Fig 2). Five cases were found to be CNV negative using ICGA and OCTA (Fig 3). Overall, OCTA was able to identify CNV in six eyes in which apparent plaque appearance was not found with ICGA.
Imaging findings for cases 4, 5, 9, 10, 11 OD, 11 OS, 13 OD, and 13 OS. A, B, C, D, E, F, G, and H show multimodal imaging of cases 4, 5, 9, 10, 11OD, 11 OS, 13 OD, and 13 OS, respectively, which were characterized by choroidal neovascular plaque on indocyanine green angiography. All of the eyes had irregular, flat epithelial detachment and pachychoroid features. Subsequent optical coherence tomography angiography revealed prominent neovascular membrane in all the cases. In addition, it demonstrated polypoidal enlargement in case 4 (A). Both the eyes of patient 11 remained fluid free during the 34-month follow-up, and this was recognized to be silent choroidal neovascularization
Imaging findings for cases 1, 3, 6, 7, 8, and 12. Enhanced depth optical coherence tomography of five cases revealed pachychoroid, pachyvessels, and the absence of drusen. Fluorescein angiography revealed non-patognomic features including RPE alterations, window defect, and leaking points. Indocyanine green angiography showed suspicious plaque in cases 1, 3, 7, 8, and 12 (A, B, C, D, and E, respectively). It also showed dilated choroidal vessels in case number 6 (F). By contrast, optical coherence angiography successfully indicated type 1 choroidal neovascularization in all cases
A comparison of imaging findings in patients 14, 15, and 17. Spectral domain opitcal coherence tomography of cases 14 (A), 15 (B), and 17 (C) showed pachychoroid, subretinal fluid, and flat, irregular pigment epithelial detachment. Fluorescein angiography revealed leaking points in patients 14,15 and RPE alterations and window defect in pateint 17. Indocyanine green angiography revealed hyperpermeability or dilated choroidal vessels in these cases. Optical coherence tomography angiography also confirmed the absence of choroidal neovascularization, despite the presence of flat, irregular pigment epithelial detachment
Mean best-corrected visual acuity was 0.5 (0.05–1.0) snellen line at the baseline visit. Mean SFCT was 388 μm (a range of 186–558 μm) and mean choroidal vessel thickness was 224 μm (a range of 107–365 μm). The patients were treated with intravitreal injections of ranibizumab (Lucentis®; Novartis Pharma AG, Basel Switzerland) and/or aflibercept (Eylea®; Bayer Schweiz AG, Zurich, Switzerland), photodynamic therapy (PDT), subthreshold micropulse laser (yellow wavelength of 577 nm) (Supra Scan 577®; Quantel Medical, Clermont-Ferrand, France), or with a combination of these modalities. The mean follow-up time was 30.5 months (a range of 1–119 months). The mean BCVA at the last visit was 0.5 (0.1–1.0) snellen line. The mean SFCT was 330 μm (a range of 148–525 μm) and the mean choroidal vessel thickness was 171 μm (a range of 67–310 μm). The treatment modalities and outcomes of the patients are summarized in Table 2. Five (29%) of the cases responded well to single or two sessions of laser therapy or intravitreal aflibercept injections and remained fluid free for a prolonged time. Significant SFCT reduction was more effectively evident with PDT. However, seven (41%) of the cases were more resistant to monotherapy, and were controlled only by a long lasting combination therapy. Intravitreal ranibizumab injection was not as effective in these cases.
Discussion
Multimodal imaging data were compared with the data obtained through the concurrent use of OCTA in the current study on a series of 19 eyes with shallow, irregular PED within the pachychoroid clinical spectrum. We found the OCTA findings to be far superior and sensitive in detecting type 1 choroidal new vessels in these patients, compared to conventional dye angiography.
Irregular retinal PED in chronic CSCR was reported to give rise to an increased risk of CNV, when compared to regular retinal PED [14]. Recently, Bousquet et al. [7] evaluated 88 eyes of 61 chronic CSCR patients and reported the presence of flat irregular PED in 59 eyes of 51 patients. Previously, Hage et al. [15] evaluated the presence of flat, irregular PED in 172 eyes of 110 chronic CSC patients and reported that 53 of the eyes of 38 patients had flat irregular PED. Also, they showed the presence of type 1 CNV in 19% of the eyes with flat, irregular, retinal PED by multimodal imaging of the fundus. The authors indicated that although they used several multimodal imaging facilities, distinguishing between avascular, flat PED and the early stages of type 1 CNV was challenging. Conventional dye angiographies may be limited in visualizing CNV in CSCR. Fluorescein angiography usually reveals stippled early hyperfluoresence and ill-defined late staining, which may be seen in both CNV and wide spread RPE alterations of the CSCR. ICGA often reveals midphase patchy areas of choroidal hyperpermeability and discrete plaque of late hyperfluorescence corresponding to type 1 neovascular tissue. In such a case, differentiating neovascular plaque over the hyperfluorescent area may be challenging. Therefore, anti-VEGF treatment was suggested as a therapeutic option to rule out the presence of CNV, especially in patients in whom vision had worsened or who were nonresponsive to treatment [15].
Another controversy about the PNV is presently debatable as to whether or not pachychoroid neovasculopathy is distinct from neovascular AMD. This is because its characteristics have yet to be well described [16]. Miyake et al. [17] investigated the phenotypic/genetic differences of PNV and neovascular AMD. Genetic susceptibility to AMD was observed to be significantly lower than genetic susceptibility to neovascular AMD in patients with pachychoroid neovasculopathy, suggesting that the two conditions are distinct clinical entities. Since pachychoroid neovasculopathy may respond differently to photodynamic therapy or anti-vascular endothelial growth factor (VEGF) therapy, it is essential that a distinction is made between pachychoroid neovasculopathy and neovascular AMD. Current multimodal imaging techniques play the major rule in the process of definitive diagnosis.
Optical coherence tomography angiography (OCTA) is a novel imaging modality that provides high-quality images of the retinal and outer choroidal circulation of the choriocapillaris and retinal microvasculature without the need for intravenous dye or contrast injection [8]. OCTA was compared with FA regarding its ability to visualize CNV in a recent study. Ill-defined CNV lesions on FA, including late leakage from an undetermined source and fibrovascular PED, were shown to be type 1 CNV on OCTA imaging [18]. The specificity of OCTA in visualizing CNV in pachychoroid spectrum diseases has also been reported in many studies. In a cross-sectional study that evaluated 27 eyes of 23 patients with chronic CSCR, FA showed CNV in 30% of the eyes, while OCTA confirmed it in all of them [8]. Quaranta-El Maftouhi et al. showed distinct CNV, not visible on ICGA imaging, in 58% of CSCR patients using OCTA [9]. Similarly, Weng et al. reported that OCTA demonstrated definite abnormal vascularization that was undetectable using other conventional imaging techniques in the outer retina in 11% of eyes with CSCR [10]. In addition, Dansingani et al. [11] identified 95% of type 1 neovascular tissue with OCTA, in contrast to dye angiography (detection rates of 18% for polypoidal lesions and 29% for CNV). However, it was suggested that the results could either be owing to the time difference between the acquisition of the dye and the OCTA imaging, or to the quiescent nature of the new vessels, resulting in the low rate of detection. However, in our study, FA, ICGA and OCTA were performed concurrently. We used OCTA and dye angiography to confirm that the proportion of PED containing neovascularization was greater than that previously estimated using dye angiography alone. The lack of disparity in time between the use of these imaging modalities enabled a direct comparison of them.
The detection of CNV is important in determining the most appropriate treatment strategy. Although pachychoroid neovasculopathy exists on the spectrum of PPE and CSCR, there is a difference in the course of the diseases and associated treatment modalities. Left untreated, CNV can cause hemorrhage, exudation, macular atrophy, and fibrosis. It was reported in a recent study that 67% of PNV cases achieved dry macula following anti-VEGF therapy [19]. Photodynamic therapy for PCV, the last of these spectrum diseases, should also be combined with an anti-VEGF agent [20]. Therefore, the ability to recognize the true underlying pathology is essential to obtaining an optimal treatment response.
This study was limited because of its small sample size. Nevertheless, the diagnosis of PNV is frequently overlooked and this study will ensure that clinicians are aware of the appropriate diagnostic modality to use. It is likely that PNV patients have different phenotypes and treatment responses that vary from those of patients with type 1 neovascularization secondary to other causes. As a consequence, it is likely that PNV patients will follow a different natural disease course and response to treatment. Future studies that evaluate the diagnostic ability of dye angiography and OCTA in a larger series of patients are necessary to further characterize this disease and determine the optimum treatment approach.
In conclusion, this study highlights the diagnostic value of OCTA in diagnosing shallow, irregular PED seen on cross-sectional structural optical coherence tomography of the eyes with type 1 neovascularization. Conventional dye angiography may fail to show the presence of type 1 neovascularization in some cases and underestimate the presence of CNV in pachychoroid spectrum disease. OCTA may be particularly helpful in achieving an accurate diagnosis for cases with ill-defined dye angiography findings.
References
Dansingani KK, Balaratnasingam C, Naysan J, Freund KB (2016) En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 36:499–516. https://doi.org/10.1097/IAE.0000000000000742
Gallego-Pinazo R, Dolz-Marco R, Gomez-Ulla F, Mrejen S, Freund KB (2014) Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 3:111–115
Warrow DJ, Hoang QV, Freund KB (2013) Pachychoroid pigment epitheliopathy. Retina 33:1659–1672. https://doi.org/10.1097/IAE.0b013e3182953df4
Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F (2015) Pachychoroid: an inherited condition? Retina 35:10–16. https://doi.org/10.1097/IAE.0000000000000287
Pang CE, Freund KB (2015) Pachychoroid neovasculopathy. Retina 35:1–9. https://doi.org/10.1097/IAE.0000000000000331
Song IS, Shin YU, Lee BR (2012) Time-periodic characteristics in the morphology of idiopathic central serous chorioretinopathy evaluated by volume scan using spectral-domain optical coherence tomography. Am J Ophthalmol 154(366–375):e364. https://doi.org/10.1016/j.ajo.2012.02.031
Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A (2017) Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous Chorioretinopathy. Retina. https://doi.org/10.1097/IAE.0000000000001580
Bonini Filho MA, de Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, Reichel E, Duker JS, Waheed NK (2015) Association of Choroidal Neovascularization and Central Serous Chorioretinopathy with Optical Coherence Tomography Angiography. JAMA Ophthalmol 133:899–906. https://doi.org/10.1001/jamaophthalmol.2015.1320
Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM (2015) Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol 160(581–587):e581. https://doi.org/10.1016/j.ajo.2015.06.016
Weng S, Mao L, Yu S, Gong Y, Cheng L, Chen X (2016) Detection of Choroidal neovascularization in central serous Chorioretinopathy using optical coherence Tomographic angiography. Ophthalmologica 236:114–121. https://doi.org/10.1159/000448630
Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB (2015) Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol 160(1243–1254):e1242. https://doi.org/10.1016/j.ajo.2015.08.028
Azar G, Wolff B, Mauget-Faysse M, Rispoli M, Savastano MC, Lumbroso B (2016) Pachychoroid neovasculopathy: aspect on optical coherence tomography angiography. Acta Ophthalmol. https://doi.org/10.1111/aos.13221
Costanzo E, Cohen SY, Miere A, Querques G, Capuano V, Semoun O, El Ameen A, Oubraham H, Souied EH (2015) Optical coherence tomography angiography in central serous Chorioretinopathy. J Ophthalmol 2015:134783. https://doi.org/10.1155/2015/134783
de Carlo TE, Rosenblatt A, Goldstein M, Baumal CR, Loewenstein A, Duker JS (2016) Vascularization of irregular retinal pigment epithelial detachments in chronic central serous chorioretinopathy evaluated with OCT angiography. Ophthalmic Surg Lasers Imaging Retina 47:128–133. https://doi.org/10.3928/23258160-20160126-05
Hage R, Mrejen S, Krivosic V, Quentel G, Tadayoni R, Gaudric A (2015) Flat irregular retinal pigment epithelium detachments in chronic central serous chorioretinopathy and choroidal neovascularization. Am J Ophthalmol 159(890–903):e893. https://doi.org/10.1016/j.ajo.2015.02.002
Fung AT, Yannuzzi LA, Freund KB (2012) Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 32:1829–1837. https://doi.org/10.1097/IAE.0b013e3182680a66
Miyake M, Ooto S, Yamashiro K, Takahashi A, Yoshikawa M, Akagi-Kurashige Y, Ueda-Arakawa N, Oishi A, Nakanishi H, Tamura H, Tsujikawa A, Yoshimura N (2015) Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep 5:16204. https://doi.org/10.1038/srep16204
Malihi M, Jia Y, Gao SS, Flaxel C, Lauer AK, Hwang T, Wilson DJ, Huang D, Bailey ST (2017) Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br J Ophthalmol 101:45–50. https://doi.org/10.1136/bjophthalmol-2016-309094
Hata M, Yamashiro K, Ooto S, Oishi A, Tamura H, Miyata M, Ueda-Arakawa N, Takahashi A, Tsujikawa A, Yoshimura N (2017) Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 58:292–298. https://doi.org/10.1167/iovs.16-20967
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH (2012) EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32:1453–1464. https://doi.org/10.1097/IAE.0b013e31824f91e8
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Demirel, S., Yanık, Ö., Nalcı, H. et al. The use of optical coherence tomography angiography in pachychoroid spectrum diseases: a concurrent comparison with dye angiography. Graefes Arch Clin Exp Ophthalmol 255, 2317–2324 (2017). https://doi.org/10.1007/s00417-017-3793-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3793-8