Abstract
Purpose
To evaluate the correlation between contrast sensitivity (CS) and retinal nerve fiber layer (RNFL) in different areas of vision.
Methods
This report is a sub-analysis of a prospective, observational cohort study investigating changes in performance-based assessment, vision-related quality of life, and clinical measures in patients with moderate to advanced glaucoma. The study included 161 participants with at least a 2-year history of glaucoma who underwent annual testing for 4 years. Contrast sensitivity was measured using the Spaeth/Richman contrast sensitivity (SPARCS) test, while RNFL thickness (RNFLT) was measured using Cirrus optical coherence tomography (OCT). Statistical analyses were performed to determine correlations between CS and RNFLT; the correlations were calculated for each annual visit, totaling four correlation coefficients for each patient over the course of 4 years.
Results
The SPARCS score in the left upper area of vision correlated the most strongly with the RNFLT of the inferior quadrant for both eyes at each annual visit, specifically in the seven o’clock sector for the left eye and the six o’clock sector for the right eye (p < 0.05). There were no discernible trends for the correlations between the other areas of CS and RNFL quadrants or clock hours over the 4 years of the study. Linear regression between the SPARCS total score and average RNFLT showed a significant direct correlation at each visit (p < 0.01).
Conclusions
Contrast sensitivity in the left upper area of vision for both eyes correlated most strongly with the thickness of the inferior quadrant of the RNFL. These fibers project to the temporal portion of the right occipital lobe, implying a potential center for contrast perception in this area. The longitudinal nature of the study suggests that CS may be a predictive tool for changes in RNFL in patients with glaucoma. Despite this finding, retinal damage and its relationship to CS was diffuse. In addition, SPARCS was shown to predict RNFLT. Further research is warranted to understand how CS can be used as a tool in the clinical setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The correlation between sectors of the retinal nerve fiber layer (RNFL) damaged by glaucoma and the resulting functional deficits in visual field (VF) has been well studied [1,2,3,4]. However, the close association between vision-related quality of life (VRQoL) and contrast sensitivity (CS) has highlighted its importance in the progression of glaucoma [5, 6]. Further, Hu et al. and Crabb et al. concurrently found that glaucoma patients more often described their vision as poor image quality, noting a need for more light, rather than a mere limit in the scope of their visual field (i.e., “tunnel vision”) [7, 8]. These descriptions differ from the conventional beliefs regarding the pathomechanism of glaucoma, leading them to speculate that some of the symptomatology related to glaucoma may stem from an early loss in CS [7]. Decreased CS in glaucoma patients has been well documented, both via direct testing [9, 10] and activity-related testing [11, 12]; however, the relationship between decreased CS and concurrent loss in RNFL thickness (RNFLT) has not yet been fully elucidated.
Currently, three main tools are used to measure CS: (1) letter charts, such as the Pelli–Robson (PR), Mars, and Regan tests [13], (2) grating charts, such as the Arden, Cambridge, Ginsburg, and CSV-1000 tests [13], (3) digital testing, such as the mobile app Aston test [14], ClinicCSF app [15], and the Spaeth/Richman Contrast Sensitivity (SPARCS) test [13]. Most tests use a global score for CS, but SPARCS measures CS in five distinct areas of vision (one central and four peripheral areas). The SPARCS test is useful in detecting glaucoma, with similar sensitivity to and higher specificity than the more traditionally used PR test [16]. Furthermore, the SPARCS test showed better test–retest reliability when compared to the PR test [16, 17].
Optical coherence tomography (OCT) has proven accurate in diagnosing [18] and tracking the progression of glaucoma [19], even with a single protocol [20]. Although there is an abundance of information regarding the RNFLT (as measured by OCT) and CS (as measured by SPARCS) separately, we aimed to assess how these two diagnostic modalities would correlate in patients with glaucoma.
Methods
The present study is a subanalysis of a prospective, observational cohort study investigating the changes in performance-based assessment of daily activities, self-reported VRQoL, and clinical measures in patients with moderate to advanced glaucoma. All related research has been approved by the Institutional Review Board of Wills Eye Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The design and methods have been described in full in a previous publication [6].
Participants
Participants were between 21 and 85 years of age and had been diagnosed with moderate to advanced glaucoma (primary open-angle, secondary open-angle, or chronic angle-closure) at least 2 years prior to joining our study. Inclusion criteria included having a Disc Damage Likelihood Scale score between 5 and 8 in at least one eye. Participants were excluded for any condition that would influence their ability to perform activities of daily living, incisional eye surgery within the past 3 months, laser therapy within the previous month, or other visual impairment unrelated to glaucoma.
Clinical evaluation
Baseline measurements were collected at the time of enrollment, and the participants returned for repeat testing once per year for 3 years, totaling four visits. Participants underwent visual acuity testing, intraocular pressure measurement using Goldmann applanation tonometry, and slit-lamp examination of the anterior segment and fundus.
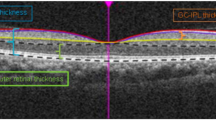
Peripapillary RNFLT was measured using Cirrus-HD OCT (spectral-domain technology with optic disc cube 200 × 200; Carl Zeiss Meditec Inc., Dublin, CA, USA) at baseline and at each annual follow-up visit. Images with signal strength greater than or equal to 7 were included in the analysis.
Contrast sensitivity testing was performed using the SPARCS test at baseline and at each annual follow-up visit. All SPARCS tests were conducted using the same computer monitor with 1024 × 768 display resolution in a dark room with standardized lighting conditions. Participants sit 50 cm from the screen and fixate their eyes on the center as gray bars flash in one of the five areas (Fig. 1). If seen, after fixation is resumed, the bars flash in a different area. The process is repeated with multiple steps of decreasing contrast until the subject cannot discern the bars further. Each of the areas of vision — four peripheral areas and one central area — received a score between 0 and 20, summing to a maximum total score of 100 [16].
Additional variables were assessed as part of the larger study (e.g., quality of life, ocular pain, and general physical health); however, we only analyzed data related to the parameters described above.
Statistical analysis
Research electronic data capture (REDCap) was used for data collection [21]. Statistical analysis was performed using R (R Core Team, Vienna, Austria) [22]. Each eye was analyzed separately for each visit. Pearson correlation coefficients were calculated to assess the relationships between the RNFLT and SPARCS scores, with significance level determined by F-test. For each eye at each visit, p-values were adjusted using the method of Benjamini and Hochberg to control the false discovery rate at 5%. To delineate all potential correlations between the RNFL sectors and CS areas, RNFL was analyzed as both quadrants and clock hours. All RNFL quadrants and clock hours were correlated to each of the five areas of the SPARCS test. To clarify the areas of the SPARCS test, the left upper area of SPARCS denotes the superior nasal quadrant of vision for the right eye and the superior temporal quadrant for the left eye. The right upper area of SPARCS is the superior temporal quadrant of vision for the right eye and the superior nasal quadrant for the left eye. The inferior areas of SPARCS follow in the same pattern.
A linear regression model was used to determine the relationship between total SPARCS score and the average RNFLT for each eye at each visit. A p-value less than 0.05 was considered statistically significant.
Results
One hundred and sixty-one participants with moderate to advanced glaucoma were enrolled at baseline. Table 1 summarizes their demographic characteristics at the beginning of the study and at each subsequent visit. At baseline, the average age was 64 years, and females comprised 53% of the participants. The majority (91%) of the study group was diagnosed with primary open-angle glaucoma. Other baseline characteristics have been documented in previous studies [5]. On average, 5% of patients were lost to follow-up each year, resulting in a total of 134 patients who attended all four visits. Nevertheless, statistical analysis found no significant differences between each visit regarding demographics or clinical characteristics. As OCT data with a signal strength less than 7 were excluded, a total of 95 to 121 patients were included in the final statistical analysis, depending on the visit. Table 2 displays the average RNFLT and CS scores in each area of vision over the four visits.
Table 3 displays the Pearson correlation coefficients between CS (as measured by SPARCS areas— right upper, left upper, right lower, left lower, central) and RNFLT (as measured by quadrant—superior, temporal, inferior, nasal) in each eye, for each visit. Table 4 reports the same relationship, but RNFL is divided into clock hours. These clock hours echo standard OCT reporting; for example, the one o’clock OCT sector spans from what would be 12:30 to 1:30 on an analog clock. All reported correlation coefficients were statistically significant (p < 0.05). The most consistent correlation was between the RNFLT in the inferior quadrant (RNFL clock hours 5:00–7:00) and the SPARCS score in the left upper area of vision. It appears that the RNFL seven o’clock sector may be driving this relationship, given the correlation coefficients for this clock hour (Table 4).
For the remaining SPARCS areas (right upper, right lower, left lower, and center), no trends were found in their relationships to specific RNFL quadrants or clock hours. All reported correlation coefficients in Tables 3 and 4 were statistically significant (p < 0.05); however, the correlations, despite their statistical significance, did not show any discernible patterns of association.
Linear regression showed a direct relationship (p < 0.01) between total SPARCS score and the average RNFLT, with no difference between the two eyes (Fig. 2). This association was seen at each visit, throughout the entire course of the study.
Linear regression between average RNFLT and the SPARCS total score for each visit, with no difference in predictive value between each eye. The linear regressions in the figure represent visit 1 (a), visit 2 (b), visit 3 (c), and visit 4 (d). a p < 0.001, R2 = 0.18; b p < 0.001, R2 = 0.23; c p < 0.001, R2 = 0.11; d p < 0.001, R2 = 0.20
Discussion
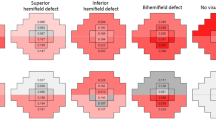
As SPARCS measures CS in multiple areas of vision, hypotheses regarding which CS areas would correlate with which RNFL sectors were based on Garway-Heath’s published map correlating areas of the VF and the optic nerve head [1]. David Garway-Heath, MD (Moorfields Eye Hospital, London, UK) advised us specifically, providing us with a hypothesized overlay of SPARCS on his visual field map. The degrees annotated within each SPARCS area indicate to which sector of the optic nerve head it would correlate. For the right eye, as seen in Fig. 3, the right upper area of SPARCS would correlate approximately to clock hours five through seven (285°–312°) and the left upper area would correlate to clock hour seven (253°–329°). The right lower area of SPARCS would correlate approximately to clock hour eleven (34°–78°) and the left lower area to clock hours ten and eleven (11°–95°).
Contrast sensitivity in the left upper area of the visual field correlated most strongly with the inferior quadrant of the RNFL in both eyes. When stratifying by clock hours, as seen in Table 4, this relationship seems to be driven by the seven o’clock sector for the left eye and by the six o’clock sector for the right eye. These findings are aligned with Garway-Heath’s map relating the VF to the optic nerve head. This correlation was maintained throughout the course of the study, and can probably be explained by the characteristic damage in discrete areas of vision in glaucoma patients [2]. The damaged areas tend to be more prominent in one area or the other (i.e., superior or inferior) [23].
Specifically, notching of the retinal neural tissue in glaucoma is most often seen in the inferior quadrant [18, 24]. The susceptibility of the inferior region of the RNFL to early glaucomatous damage could be a possible explanation for our finding a consistent pattern between CS and RNFLT in that area. Additionally, Mwanza et al. found that the thickness of the seven o’clock sector of the RNFL alone could differentiate between glaucomatous and healthy eyes [18]. This finding is in line with our results that the seven o’clock sector has the highest correlation with the left upper area of the SPARCS test (Table 4). As mentioned in the results, this specific clock hour is probably driving the consistent significant correlation between the RNFLT of the inferior quadrant (which is made up of clock hours five, six, and seven) and the SPARCS left upper area of vision. Extrapolating further, the nerve fibers from the left upper area of the visual field project through the brain to the temporal portion of the right occipital lobe, implying a potential center for contrast perception in this area of the brain.
However, for the remaining RNFL quadrants and clock hours, SPARCS did not find the expected correlations between CS and RNFLT. While structural and functional changes tend to be more pronounced in one area of the RNFL or visual field respectively, the damage from glaucoma is not limited to that area [2, 18]. Rather than the clusters of function loss that are classically found in VF testing for glaucoma, CS loss appears to be much more diffuse throughout the field of vision. This may help to explain why, despite having found many statistically significant correlations between individual SPARCS areas and RNFL quadrants, no clear patterns emerged from these correlations beyond the one discussed above.
There are several potential reasons for these findings. First, we speculate the lack of trends could be due to a threshold; that is, the nerve fibers may need to attain a certain level of damage before there are measurable changes in CS. Second, minute changes in CS may not be measurable with our current tools, in the same way small changes in RNFLT are quantifiable with OCT. Third, CS might pertain to a discrete aspect of vision, related to only a subpopulation of RNFL cells rather than the entire retina [25]. The ganglion cell and inner plexiform layers have been found to have comparable sensitivity in diagnosing glaucoma when compared to the average RNFLT [26]. It may very well be that our ability to discern contrast is a complex summation of the function of multiple cell types and layers in the retina, which OCT currently combines into one distinct layer.
Linear regression analysis showed a statistically significant relationship between the total SPARCS score and the average RNFLT in all four visits. This finding confirms the study hypothesis, in which we predicted that global RNFL thinning due to glaucomatous damage would correlate well with decreased CS. Although there was significant scattering of data points in the linear regression — probably the result of widespread RNFL damage — our results do suggest that SPARCS may be predictive of RNFL damage in glaucoma. The data regarding loss in CS did not show a ‘tipping point’, as Wollstein et al. found in their analyses of RNFL damage in the context of VF deficits [2]. It might be that different layers of the RNFL are responsible for CS, as we have speculated, or that this point could not be discerned by our data. Previous research has ascertained that glaucoma patients experience decreased sensitivity to contrast over time, consequently losing proficiency in activities of daily living that require contrast, such as facial and object recognition [12, 27]. Glaucoma patients also perform activities much less efficiently than their healthy counterparts in simulated low-contrast environments, such as reading and driving [28]. Our results support previous studies that have shown decreased CS in glaucoma [9, 11, 12] and suggest that this loss is due to RNFL thinning.
The exclusion of multiple OCT images due to poor signal strength — possibly due to poor fixation, hazy media, or inherent difficulties of the OCT machine — reduced the sample size of our study and may have limited the results. Furthermore, there was some loss to follow-up between each visit; however, on statistical comparison, there were no differences in demographics or clinical characteristics of these patients. Finally, a major limitation of the present study is the mismatch between areas of CS and RNFL; SPARCS areas are rectangular and centered over the macula, whereas OCT areas are pie-shaped and centered over the optic nerve. Nevertheless, we found significant correlations between SPARCS scores and RNFLT, regardless of the areas, pointing again to diffuse retinal damage.
In conclusion, further research is warranted to better understand how CS testing can help in the diagnosis of glaucoma, and how the decrease in CS relates to the progressive loss of neural tissue in specific areas of the retina. We aimed to determine if correlations could be found between CS and RNFLT throughout different retinal quadrants and clock hour sectors. We have shown that using SPARCS testing, a loss in CS in the left upper area of vision may reflect notching in the inferior quadrant of the RNFL, particularly the six- and seven o’clock sectors for the right and left eyes, respectively. This relationship was not found between the RNFLT and other SPARCS areas, supporting not only the idea that structural damage in specific sectors of the RNFL may be a driving force in functional vision deficits, but also that this damage may be more extensive than anticipated.
References
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA (2000) Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 107:1809–1815. https://doi.org/10.1016/S0161-6420(00)00284-0
Wollstein G, Kagemann L, Bilonick RA et al (2012) Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol 96:47–52. https://doi.org/10.1136/bjo.2010.196907
Malik R, Swanson WH, Garway-Heath DF (2012) “structure–function relationship” in glaucoma: past thinking and current concepts. Clin Experiment Ophthalmol 40:369–380. https://doi.org/10.1111/j.1442-9071.2012.02770.x
Harwerth RS, Wheat JL, Fredette MJ, Anderson DR (2010) Linking structure and function in glaucoma. Prog Retin Eye Res 29:249–271. https://doi.org/10.1016/j.preteyeres.2010.02.001
Ekici F, Loh R, Waisbourd M et al (2015) Relationships between measures of the ability to perform vision-related activities, vision-related quality of life, and clinical findings in patients with glaucoma. JAMA Ophthalmol 133:1–10. https://doi.org/10.1001/jamaophthalmol.2015.3426
Waisbourd M, Parker S, Ekici F et al (2015) A prospective, longitudinal, observational cohort study examining how glaucoma affects quality of life and visually-related function over 4 years: design and methodology. BMC Ophthalmol 15:91. https://doi.org/10.1186/s12886-015-0088-x
Hu CX, Zangalli C, Hsieh M et al (2014) What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. Am J Med Sci 348:403–409. https://doi.org/10.1097/MAJ.0000000000000319
Crabb DP, Smith ND, Glen FC et al (2013) How does glaucoma look?: patient perception of visual field loss. Ophthalmology 120:1120–1126. https://doi.org/10.1016/j.ophtha.2012.11.043
Hawkins AS, Szlyk JP, Ardickas Z et al (2003) Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma 12:134–138
Breton ME, Wilson TW, Wilson R et al (1991) Temporal contrast sensitivity loss in primary open-angle glaucoma and glaucoma suspects. Invest Ophthalmol Vis Sci 32:2931–2941
Richman J, Lorenzana LL, Lankaranian D et al (2010) Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol 128:1576–1582. https://doi.org/10.1001/archophthalmol.2010.275
Burton R, Crabb DP, Smith ND et al (2012) Glaucoma and reading: exploring the effects of contrast lowering of text. Optom Vis Sci 89:1282–1287. https://doi.org/10.1097/OPX.0b013e3182686165
Richman J, Spaeth GL, Wirostko B (2013) Contrast sensitivity basics and a critique of currently available tests. J Cataract Refract Surg 39:1100–1106. https://doi.org/10.1016/j.jcrs.2013.05.001
Kingsnorth A, Drew T, Grewal B, Wolffsohn JS (2016) Mobile app Aston contrast sensitivity test. Clin Exp Optom 99(4):350–355. https://doi.org/10.1111/cxo.12362
Rodriguez-Vallejo M, Remon L, Monsoriu JA, Furlan WD (2015) Designing a new test for contrast sensitivity function measurement with iPad. J Optom 8:101–108. https://doi.org/10.1016/j.optom.2014.06.003
Richman J, Zangalli C, Lu L et al (2014) The Spaeth/Richman contrast sensitivity test (SPARCS): design, reproducibility and ability to identify patients with glaucoma. Br J Ophthalmol 99(1):16–20. https://doi.org/10.1136/bjophthalmol-2014-305223
Sun Y, Erdem E, Lyu A, et al (2016) The SPARCS: a novel assessment of contrast sensitivity and its reliability in patients with corrected refractive error. Br J Ophthalmol 100(10):1421–1426. doi: https://doi.org/10.1136/bjophthalmol-2015-307378
Mwanza JC, Oakley JD, Budenz DL, Anderson DR (2011) Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 118:241–248. https://doi.org/10.1016/j.ophtha.2010.06.036
Shin HY, Park HYL, Jung Y et al (2014) Glaucoma diagnostic accuracy of optical coherence tomography parameters in early glaucoma with different types of optic disc damage. Ophthalmology 121:1990–1997. https://doi.org/10.1016/j.ophtha.2014.04.030
Hood DC, De Cuir N, Blumberg DM et al (2016) A single wide-field OCT protocol can provide compelling information for the diagnosis of early glaucoma. Transl Vis Sci Technol 5:4. https://doi.org/10.1167/tvst.5.6.4
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
R Development Core Team R (2011) R: a language and environment for statistical computing. R Found Stat Comput. https://doi.org/10.1007/978-3-540-74686-7
Law SK, Choe R, Caprioli J (2001) Optic disk characteristics before the occurrence of disk hemorrhage in glaucoma patients. Am J Ophthalmol 132:411–413. https://doi.org/10.1016/S0002-9394(01)01009-1
Lloyd MJ, Mansberger SL, Fortune BA et al (2013) Features of optic disc progression in patients with ocular hypertension and early glaucoma. J Glaucoma 22:343–348. https://doi.org/10.1097/IJG.0b013e31824c9251
Ou Y, Jo RE, Ullian EM et al (2016) Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J Neurosci 36:9240–9252. https://doi.org/10.1523/JNEUROSCI.0940-16.2016
Mwanza JC, Durbin MK, Budenz DL et al (2012) Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology 119:1151–1158. https://doi.org/10.1016/j.ophtha.2011.12.014
Lenoble Q, Lek JJ, McKendrick AM (2016) Visual object categorisation in people with glaucoma. Br J Ophthalmol 100(11):1585-1590. doi: https://doi.org/10.1136/bjophthalmol-2015-308289
Tatham AJ, Boer ER, Rosen PN et al (2014) Glaucomatous retinal nerve fiber layer thickness loss is associated with slower reaction times under a divided attention task. Am J Ophthalmol 158:1008–1017. https://doi.org/10.1016/j.ajo.2014.07.028
Acknowledgements
We are grateful to both David Garway-Heath, MD (Moorsfields Eye Hospital, London, UK), who served as a scientific adviser and created the image we used for Fig. 3, and Michael Waisbourd, MD (Tel Aviv Sourasky Medical Center, Tel Aviv, Israel), who also served as a scientific advisor.
Funding
Merck Sharp & Dohme provided financial support in the form of a grant (IISP # 39138). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The primary investigator, George L. Spaeth, has developed and patented the Spaeth/Richman Contrast Sensitivity test. The remaining authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants in the study.
Rights and permissions
About this article
Cite this article
Amanullah, S., Okudolo, J., Rahmatnejad, K. et al. The relationship between contrast sensitivity and retinal nerve fiber layer thickness in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol 255, 2415–2422 (2017). https://doi.org/10.1007/s00417-017-3789-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3789-4